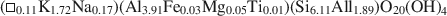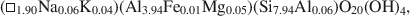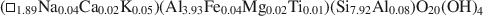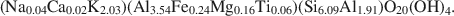Influence of crystal shape and orientation on the magnetic microstructure of bullet-shaped magnetosomes synthesized by magnetotactic bacteria
- Part of
-
- Journal:
- Geo-Bio Interfaces / Volume 1 / 2024
- Published online by Cambridge University Press:
- 25 September 2024, e1
-
- Article
-
- You have access
- Open access
- HTML
- Export citation
High Resolution Electron Microscopy of Feldspar Weathering
-
- Journal:
- Clays and Clay Minerals / Volume 28 / Issue 3 / June 1980
- Published online by Cambridge University Press:
- 01 July 2024, pp. 173-178
-
- Article
-
- You have access
- Export citation
Weathering of Enstatite to Talc Through a Sequence of Transitional Phases
-
- Journal:
- Clays and Clay Minerals / Volume 30 / Issue 1 / February 1982
- Published online by Cambridge University Press:
- 02 April 2024, pp. 11-20
-
- Article
-
- You have access
- Export citation
Transmission and Analytical Electron Microscopy of the Smectite-To-Illite Transition
-
- Journal:
- Clays and Clay Minerals / Volume 34 / Issue 2 / April 1986
- Published online by Cambridge University Press:
- 02 April 2024, pp. 165-179
-
- Article
-
- You have access
- Export citation
Acid Dissolution of Akaganiéite and Lepidocrocite: The Effect on Crystal Morphology
-
- Journal:
- Clays and Clay Minerals / Volume 36 / Issue 5 / October 1988
- Published online by Cambridge University Press:
- 02 April 2024, pp. 385-390
-
- Article
-
- You have access
- Export citation
Alternation of Clino- and Orthochrysotile in a Single Fiber as Revealed by High-Resolution Electron Microscopy
-
- Journal:
- Clays and Clay Minerals / Volume 32 / Issue 5 / October 1984
- Published online by Cambridge University Press:
- 02 April 2024, pp. 429-432
-
- Article
-
- You have access
- Export citation
Imogolite Synthesis at 25°C
-
- Journal:
- Clays and Clay Minerals / Volume 35 / Issue 5 / October 1987
- Published online by Cambridge University Press:
- 02 April 2024, pp. 379-384
-
- Article
-
- You have access
- Export citation
Ordered 1:1 Interstratification of Illite and Chlorite: A Transmission and Analytical Electron Microscopy Study
-
- Journal:
- Clays and Clay Minerals / Volume 33 / Issue 5 / October 1985
- Published online by Cambridge University Press:
- 02 April 2024, pp. 463-467
-
- Article
-
- You have access
- Export citation
Thermal Transformation of Talc as Studied by Electron-Optical Methods
-
- Journal:
- Clays and Clay Minerals / Volume 36 / Issue 4 / August 1988
- Published online by Cambridge University Press:
- 02 April 2024, pp. 289-296
-
- Article
-
- You have access
- Export citation
A Transmission Electron Microscopy Contribution to the Structure of Kaolinite
-
- Journal:
- Clays and Clay Minerals / Volume 35 / Issue 3 / June 1987
- Published online by Cambridge University Press:
- 02 April 2024, pp. 237-239
-
- Article
-
- You have access
- Export citation
Formation of Iddingsite Rims on Olivine: A Transmission Electron Microscope Study
-
- Journal:
- Clays and Clay Minerals / Volume 32 / Issue 1 / February 1984
- Published online by Cambridge University Press:
- 02 April 2024, pp. 1-11
-
- Article
-
- You have access
- Export citation
Effects of Dry Grinding and Leaching on the Crystal Structure of Chrysotile
-
- Journal:
- Clays and Clay Minerals / Volume 37 / Issue 5 / October 1989
- Published online by Cambridge University Press:
- 02 April 2024, pp. 439-445
-
- Article
-
- You have access
- Export citation
Factors That Govern the Formation of Multi-Domainic Goethites
-
- Journal:
- Clays and Clay Minerals / Volume 34 / Issue 5 / October 1986
- Published online by Cambridge University Press:
- 02 April 2024, pp. 557-564
-
- Article
-
- You have access
- Export citation
Illite/Smectite from Gulf Coast Shales: A Reappraisal of Transmission Electron Microscope Images
-
- Journal:
- Clays and Clay Minerals / Volume 37 / Issue 6 / December 1989
- Published online by Cambridge University Press:
- 02 April 2024, pp. 542-546
-
- Article
-
- You have access
- Export citation
The Fundamental Nature of Interstratified Illite/Smectite Clay Particles: A Reply
-
- Journal:
- Clays and Clay Minerals / Volume 35 / Issue 1 / February 1987
- Published online by Cambridge University Press:
- 02 April 2024, pp. 77-79
-
- Article
-
- You have access
- Export citation
Transmission Electron Microscopic Study of Coexisting Pyrophyllite and Muscovite: Direct Evidence for the Metastability of Illite
-
- Journal:
- Clays and Clay Minerals / Volume 38 / Issue 3 / June 1990
- Published online by Cambridge University Press:
- 02 April 2024, pp. 225-240
-
- Article
-
- You have access
- Export citation
Botryoidal Goethite: a Transmission Electron Microscope Study
-
- Journal:
- Clays and Clay Minerals / Volume 31 / Issue 5 / October 1983
- Published online by Cambridge University Press:
- 02 April 2024, pp. 392-396
-
- Article
-
- You have access
- Export citation
Weathering of Basalt: Formation of Iddingsite
-
- Journal:
- Clays and Clay Minerals / Volume 35 / Issue 6 / December 1987
- Published online by Cambridge University Press:
- 02 April 2024, pp. 418-428
-
- Article
-
- You have access
- Export citation
Transmission Electron Microscope Data for Rectorite: Implications for the Origin and Structure of “Fundamental Particles”
-
- Journal:
- Clays and Clay Minerals / Volume 34 / Issue 2 / April 1986
- Published online by Cambridge University Press:
- 02 April 2024, pp. 180-186
-
- Article
-
- You have access
- Export citation
Effect of Solution Conditions on the Proportion and Morphology of Goethite Formed from Ferrihydrite
-
- Journal:
- Clays and Clay Minerals / Volume 33 / Issue 5 / October 1985
- Published online by Cambridge University Press:
- 02 April 2024, pp. 424-432
-
- Article
-
- You have access
- Export citation