Variability among estimates of serum 25-hydroxyvitamin D (25OHD) concentrations between different analytical methods and laboratories is well documented( Reference Roth, Schmidt-Gayk and Weber 1 ). There is evidence to suggest that commercial assays produce more unreliable estimates at the higher and lower ends of the distribution of 25OHD concentrations, due to higher limits of detection and cross-reactivity( Reference Lind, Chen and Byrjalsen 2 ). Liquid chromatography-tandem mass spectrometry (LC-MS/MS) is regarded as a gold standard reference method when NIST standard reference materials (SRM) are applied( Reference Yates, Bowron and Calton 3 ). As umbilical cord serum 25OHD concentrations are typically lower than adult values, the aim of this study was to compare three commonly used analytical methods to measure total 25OHD concentrations in 86 cord sera that were stored at −80 °C. Total serum 25OHD concentrations were quantified using an enzyme immunoassay (EIA), Immuno Diagnostic Systems (IDS), Boldon, UK, a direct competitive chemiluminescence immunoassay (LIAISON TOTAL Assay), Diasorin Stillwater, USA, and an LC-MS/MS method, which is traceable to the US National Institute for Standards and Technology (NIST) higher order reference measurement procedure( Reference Tai, Bedner and Phinney 4 ). Data were compared using the Bland-Altman method.
Both IDS EIA and DiaSorin Liaison correlated strongly with LC-MS/MS (r=0.945 and 0.976, both P<0.001). However, substantial bias was evident in the data from both methods, which ranged from −20% to 176% using IDS EIA and −20% to 193% with DiaSorin Liaison, respectively and the bias was highest at low 25OHD concentrations. The mean difference between IDS EIA and LC-MS/MS was 6.8 nmol/L and between DiaSorin Liaison and LC-MS/MS was 9.9 nmol/L (both P<0.001), see Table. IDS EIA and DiaSorin Liaison over-estimated total 25OHD by 35 and 41%, respectively, see Figure.
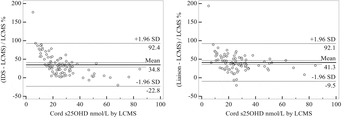

Large differences were observed in the concentrations of serum 25OHD in cord samples using both the IDS EIA and DiaSorin Liaison methods compared with LC-MS/MS. Reported levels of serum 25OHD concentrations in umbilical cord samples are not comparable between methods due to the positive bias observed and their validity is questionable, particularly at the low concentrations of 25OHD typically observed in cords. This study re-emphasises the need for international standardisation of serum 25OHD measurements, such as the ongoing Vitamin D Standardisation Program (VDSP)( Reference Sempos, Vesper and Phinney 5 ).
Supported by the Higher Education Authority Program for Research in Third Level Institutions (PRTLI Cycle 4)


