The presence of proteinuria is an independent risk factor for progression of chronic kidney disease, CVD and mortality(Reference Hemmelgarn, Manns and Lloyd1,Reference Li, Qin and Luo2) . As proteinuria is usually silent and requires laboratory tests for detection(Reference Levey, Becker and Inker3), the identification of modifiable risk factors to improve primary prevention has attracted great attention.
Neutrophils are the most abundant type of leucocyte in circulation and are essential for defense against pathogens. Neutrophil counts are used routinely as an indicator for acute and chronic inflammation(Reference Soehnlein, Steffens and Hidalgo4). Nevertheless, neutrophils are also involved in numerous pathologies including tumors and tissue damage by forming neutrophil extracellular traps, secreting pro-inflammatory cytokines, releasing reactive oxygen species and damaging endothelial cells(Reference Liew and Kubes5,Reference Gupta and Kaplan6) . Consistently, previous cross-sectional studies have reported that the presence of proteinuria is significantly related to leucocyte and neutrophil counts(Reference Muhlhauser, Verhasselt and Sawicki7–Reference Huang, Chen and Wu10). In addition, a prospective study has shown that elevated leucocyte counts were associated with an increased risk of proteinuria in Japanese men(Reference Sato, Hayashi and Harita11). However, data on the prospective association between neutrophil counts and proteinuria are limited.
Folate is a major component of one-carbon metabolism(Reference Depeint, Bruce and Shangari12). Studies have shown that folic acid supplementation reduces total homocysteine (tHcy) levels, improves endothelial function and has potent direct antioxidant and anti-inflammatory effects(Reference Doshi, McDowell and Moat13,Reference Qin, Li and Cui14) . Accordingly, a post hoc analysis of the renal substudy of the China Stroke Primary Prevention Trial (CSPPT) has suggested that folic acid therapy can significantly reduce the development of proteinuria in diabetic patients(Reference Li, Liang and Wang15). However, although higher neutrophil counts and lower folate levels seem to share some common mechanisms related to proteinuria and folic acid therapy may possibly counteract the detrimental effects of higher neutrophil counts on proteinuria, this hypothesis has not been thoroughly investigated in previous studies.
Therefore, we conducted a post hoc analysis of the renal substudy of the CSPPT in order to evaluate the relation of baseline neutrophil counts with the risk of new-onset proteinuria, and the modifying effect of neutrophil counts on efficacy of folic acid in prevention of new-onset proteinuria among hypertensive patients.
Methods
Study design and participants
The present study is a post hoc analysis of the renal substudy of the CSPPT. The design and major results of the CSPPT (ClinicalTrials.gov identifier: NCT00794885) and the renal substudy of the CSPPT have been previously described in detail(Reference Huo, Li and Qin16–Reference Qin, Li and He19). In brief, the CSPPT was a multi-community, randomised, double-blind, controlled trial conducted from May 2008 to August 2013 in 32 communities in China. Eligible participants were men and women aged 45–75 years who had hypertension, defined as seated, resting systolic blood pressure (SBP) ≥140 mmHg or diastolic blood pressure ≥90 mmHg at both the screening and recruitment visit, or who were on antihypertensive medication. The major exclusion criteria included history of physician-diagnosed stroke, myocardial infarction, heart failure, post-coronary revascularisation, and/or congenital heart disease, and/or current supplementation by folic acid, vitamin B12 or vitamin B6.
In the CSPPT, a total of 20 702 eligible participants were enrolled. Of those, a total of 15 104 eligible participants from twenty communities in Jiangsu province were enrolled in the renal substudy of the CSPPT, with an estimated glomerular filtration rate (eGFR) ≥30 ml/min per 1·73 m2. The present study was a post hoc analysis of the renal substudy of the CSPPT, where 8208 participants with complete measurements on baseline neutrophil counts, baseline and exit visit urine protein status, and without proteinuria (a urine dipstick reading of trace or ≥1+) at baseline were enrolled (Fig. S1).
The parent study (the CSPPT) and the present study were approved by the Ethics Committee of the Institute of Biomedicine, Anhui Medical University, Hefei, China (FWA assurance number: FWA00001263). All participants provided written informed consent. The data that support the findings of the present study will be available from the corresponding authors upon request, after the request is submitted and formally reviewed and approved by the Ethics Committee of the Institute of Biomedicine, Anhui Medical University.
Intervention and follow-up
Eligible participants were randomly assigned, in a 1:1 ratio, to one of two treatment groups: the enalapril–folic acid group, who received a daily oral dose of one tablet containing 10 mg of enalapril and 0·8 mg of folic acid, and the enalapril-only group, who received a daily oral dose of one tablet containing 10 mg of enalapril only. During the study period, if blood pressure was not properly controlled, other antihypertensive medications were allowed, but not B-vitamins.
Participants were scheduled for follow-up every 3 months. At each visit, seated blood pressure measurements were obtained; medication compliance, concomitant medication use and adverse events during the treatment period, as well as possible endpoint events, were recorded by trained research staff and physicians.
Laboratory assays
Complete baseline blood count, including the measurements of leucocytes, neutrophils and lymphocytes were obtained using a BC-3200 hematology analyzer (Mindray Medical). Fasting glucose, serum lipids, tHcy, uric acid and creatinine were measured using automatic clinical analyzers (Beckman Coulter) at the core laboratory of the National Clinical Research Center for Kidney Disease. Serum folate was measured at baseline by a commercial laboratory using a chemiluminescent immunoassay (New Industrial). Proteinuria was determined using a dipstick test (Dirui-H100) and classified as negative, trace, 1+, 2+ or 3+.
The eGFR was calculated with the use of the Chronic Kidney Disease Epidemiology Collaboration equation(Reference Levey, Stevens and Schmid20).
Diabetes was defined as self-reported diabetes, or under glucose-lowering therapy, or fasting glucose ≥ 7·0 mmol/l at baseline. Impaired fasting glucose was defined as fasting glucose 5·6–<7·0 mmol/l, and without the use of glucose-lowering drugs or self-reported diabetes at baseline.
Outcome
The study outcome was new-onset proteinuria, defined as a urine dipstick reading of ≥1+ at the exit visit.
Statistical analysis
The population characteristics, stratified by neutrophil counts (≥4·8 (quintile 5) v. <4·8 × 109/l) were presented as means and standard deviations for continuous variables and proportions for categorical variables by treatment group. The differences in population characteristics were compared using two-sample t tests or χ 2 tests, accordingly.
Variables known as traditional or suspected risk factors for proteinuria(Reference Gregg and Hedayati21,Reference Tsai, Wu and Peng22) , or those showed significant differences in different categories of neutrophil counts, were selected as covariables. The relation of baseline neutrophil counts with the risk of new-onset proteinuria in the enalapril-only group was evaluated using multivariate logistic models (OR and 95 % CI) without and with adjustments for age, sex, BMI, smoking status, fasting glucose, total cholesterol, TAG, HDL-cholesterol, eGFR, uric acid, folate, tHcy, methylenetetrahydrofolate reductase (MTHFR) C677T genotypes and SBP at baseline, as well as time-averaged SBP during the treatment period. Similarly, the OR and 95 % CI of new-onset proteinuria in response to folic acid treatment across each neutrophil count subgroup (≥4·8 or <4·8 × 109/l) were estimated and their interactions were tested.
As a post hoc analysis, correction for multiple hypothesis testing was not applied, and a two-tailed P < 0·05 was considered to be statistically significant in all analyses. R software, version 3.6.1 (http://www.R-project.org) was used for all data analyses.
Results
Study participants and characteristics
A total of 8208 participants (4107 in the enalapril-only group and 4101 in the enalapril–folic acid group) from the renal substudy of the CSPPT were included in the final analysis (Fig. S1). The mean age of the participants was 59·5 (sd, 7·4) years, 3088 (37·6 %) were male. And 1023 (12·5 %) of the participants had diabetes and 3260 (39·7 %) had impaired fasting glucose. For those participants excluded from the analysis due to missing neutrophil counts or proteinuria measurements, their baseline characteristics were similar to their counterparts who were included in the analysis (Table S1).
Characteristics of the participants in the enalapril-only group by quintiles of neutrophil counts are presented in Table S2. Participants with higher neutrophil counts were older and more likely to smoke, had higher SBP, total cholesterol, TAG, fasting glucose and tHcy levels, had higher leucocyte, lymphocyte counts and neutrophil to lymphocyte ratio, had higher usage of glucose-lowering drugs, had lower folate and eGFR levels at baseline, as well as had higher SBP levels during the follow-up period. However, almost all of the characteristics were comparable among the two treatment groups within each baseline neutrophil count stratum (≥4·8 (quintile 5) or <4·8 × 109/l) (Table 1).
Table 1. Characteristics of study participants by treatment groups for neutrophil count strata in the total population
(Mean values and standard deviations; numbers and percentages)

MTHFR, methylenetetrahydrofolate reductase; SBP, systolic blood pressure; DBP, diastolic blood pressure; eGFR, estimated glomerular filtration rate; NLR, neutrophil to lymphocyte ratio.
Treatment adherence and changes in serum total homocysteine and folate levels
Median treatment duration was 4·4 years. Mean treatment adherence, defined as percentage of the study treatment medication (enalapril or enalapril–folic acid tablet) that was actually taken during the trial, was approximately 80 % in both treatment groups within each baseline neutrophil count stratum. Rates of participant withdrawal from the study, defined as discontinuation of the use of study drugs for any reason for more than 180 d before termination of the study, ranged from 9·1 to 10·2 % within treatment groups among each baseline neutrophil count stratum (Table 1). All participants were included in the analysis irrespective of treatment withdrawal.
The baseline serum levels of folate and tHcy were comparable between the two treatment groups in the total population among each baseline neutrophil count stratum (Table 2). As expected, compared with the enalapril-only group, enalapril–folic acid treatment was associated with increased serum folate levels and decreased tHcy levels within each baseline neutrophil count stratum (Table 2).
Table 2. Serum folate and homocysteine levels at baseline and after treatment
(Mean values and standard deviations; numbers and percentages)

* Change was defined as the exit value minus the baseline value.
Neutrophil counts and new-onset proteinuria in the enalapril-only group
During a median treatment duration of 4·4 years of the treatment period, 160 (3·9 %) participants developed new-onset proteinuria in the enalapril-only group.
When neutrophil counts were assessed as quintiles, among the enalapril-only group, compared with those in quintile 1 (<2·7 × 109/l), the adjusted OR for participants in quintiles 2–4 and quintile 5 were 1·31 (95 % CI 0·78, 2·19) and 1·79 (95 % CI 1·02, 3·16), respectively. Accordingly, a significantly higher risk of new-onset proteinuria was found in quintile 5 (≥4·8 × 109/l, OR, 1·44; 95 % CI 1·00, 2·06) compared with participants in quintiles 1–4 (Table 3).
Table 3. Relationship of neutrophil counts with new-onset proteinuria in the enalapril-only group
(Odds ratios and 95% confidence intervals; numbers and percentages)
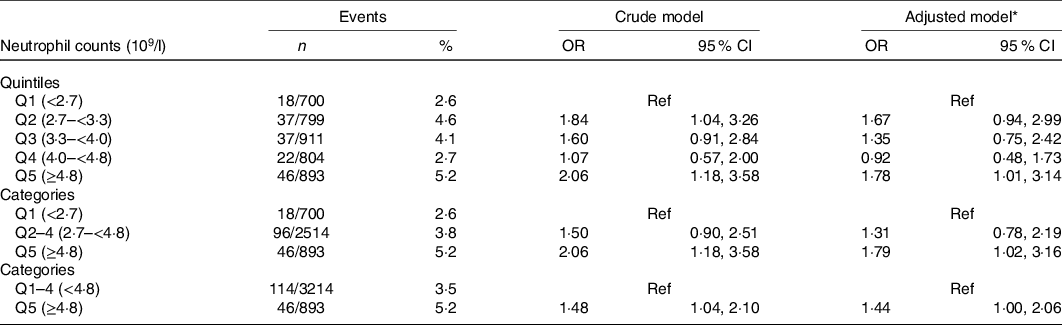
Ref, reference; MTHFR, methylenetetrahydrofolate reductase; SBP, systolic blood pressure.
* Adjusted for age, sex, BMI, smoking status, fasting glucose, total cholesterol, TAG, HDL-cholesterol, estimated glomerular filtration rate, uric acid, folate, total homocysteine, MTHFR C677T genotypes, SBP at baseline, as well as time-averaged SBP during the treatment period.
Similar, but non-significant trends were found for leucocyte counts (Table S3) and neutrophil to lymphocyte ratio (Table S4). Moreover, further adjustment for lymphocyte counts (Table S5) or excluding participants with an eGFR < 60 ml/min per 1·73 m2 (Table S6) did not affect the positive association between neutrophil counts and new-onset proteinuria.
In addition, during the treatment period, participants with higher neutrophil counts had a higher frequency in use of Ca channel blockers (Table S7). Nevertheless, further adjustment for Ca channel blocker usage during the treatment period also did not substantially change the association between neutrophil counts and new-onset proteinuria (Table S8).
Neutrophil counts and efficacy of folic acid on new-onset proteinuria among total population
Table 4 further quantifies the effect modification of neutrophil counts on efficacy of folic acid treatment in preventing new-onset proteinuria. Compared with the enalapril-only group, for those with enalapril and folic acid treatment, new-onset proteinuria incidence was reduced from 5·2 to 2·8 % (adjusted OR, 0·49; 95 % CI 0·29, 0·82) among participants with higher neutrophil counts (≥4·8 × 109/l). In contrast, new-onset proteinuria risk reduction in those with lower neutrophil counts (<4·8 × 109/l) was modest (adjusted OR, 0·93; 95 % CI 0·71, 1·22). The interaction between neutrophil counts and folic acid therapy on new-onset proteinuria was significant (P = 0·04) (Table 4).
Table 4. Effect modification of neutrophil counts on efficacy of folic acid in prevention of new-onset proteinuria
(Odds ratios and 95% confidence intervals; numbers and percentages)
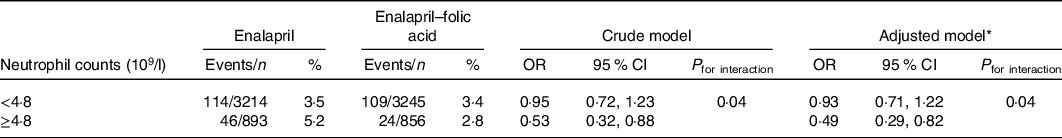
MTHFR, methylenetetrahydrofolate reductase; SBP, systolic blood pressure.
* Adjusted for age, sex, BMI, smoking status, fasting glucose, total cholesterol, TAG, HDL-cholesterol, estimated glomerular filtration rate, uric acid, folate, total homocysteine, MTHFR C677T genotypes, SBP at baseline, as well as time-averaged SBP during the treatment period.
Further adjustment for lymphocyte counts (Table S9), Ca channel blocker usage during the treatment period (Table S10) or annual rate of eGFR decline during the treatment period (Table S11) did not substantially change the effects.
Stratified analyses by important covariables
To further confirm that the beneficial effects associated with folic acid treatment among participants with higher neutrophil counts (≥4·8 × 109/l) are robust to potential confounders, we conducted stratified analyses by subgroups defined by major covariables, including age, sex, MTHFR C677T genotypes, diabetes, total cholesterol, eGFR, folate, tHcy, SBP at baseline, as well as time-averaged SBP during the treatment period.
Table 4 indicates a highly consistent pattern: among patients with higher neutrophil counts (≥4·8 × 109/l), regardless of subgroups, folic acid treatment was associated with a reduction trend in new-onset proteinuria (Fig. 1). Of note, the beneficial trends were consistent in participants with (adjusted OR, 0·47; 95 % CI 0·15, 1·49) or without baseline diabetes (adjusted OR, 0·50; 95 % CI 0·28, 0·92).

Fig. 1. Stratified analyses by important covariables on efficacy of folic acid in prevention of new-onset proteinuria among patients with higher neutrophil counts (⩾4·8 × 109/l). *Adjusted, if not stratified, for age, sex, BMI, smoking, fasting glucose, total cholesterol, TAG, HDL-cholesterol, estimated glomerular filtration rate (eGFR), uric acid, folate, total homocysteine, methylenetetrahydrofolate reductase (MTHFR) C677T genotypes, systolic blood pressure (SBP) at baseline, as well as time-averaged SBP during the treatment period.
Discussion
Our study is the first to demonstrate that hypertensive patients with higher neutrophil counts had increased risk of new-onset proteinuria, and this risk was reduced by 51 % with folic acid treatment.
Although there have been some previous cross-sectional studies that have reported inverse associations between leucocyte or neutrophil counts and proteinuria(Reference Muhlhauser, Verhasselt and Sawicki7–Reference Huang, Chen and Wu10), cross-sectional studies are not useful in determining temporal relations. A prospective study in Japanese men aged 40–55 years has shown that the highest quintile of leucocyte counts was associated with an increased risk of proteinuria incidence (hazard ratio 1·45, 95 % CI 1·23, 1·73) compared with the lowest quintile(Reference Sato, Hayashi and Harita11). However, all participants in the present study were registered employees from the same company; therefore, the results may not be representative of the general population. In addition, there was a lack of data on the association between differential leucocyte subtype counts and proteinuria. Our present study has several unique features. By utilising data from the CSPPT, we were able to not only evaluate the prospective association between neutrophil counts and new-onset proteinuria among hypertensive patients but also examine whether neutrophil counts modify the efficacy of folic acid in preventing new-onset proteinuria. Moreover, we adjusted for a large number of known covariables. It is by far the first and largest study of its kind, whose design and data are critically important for the development of primary prevention strategies for proteinuria.
Our study adds some new insights into this field. First, among this hypertensive population, participants with higher neutrophil counts (quintile 5) had a significantly increased risk of new-onset proteinuria. Moreover, there was no significant association between neutrophil counts and new-onset proteinuria among participants in quintiles 1–4 of neutrophil counts. We speculate that a possible threshold level of neutrophil counts exists at about 4·8 × 109/l, above which the risk of new-onset proteinuria significantly increases. Consistently, a previous cross-sectional study indicated a sharp increase in the presence of proteinuria only when neutrophil counts were more than 4·5 × 109/l(Reference Huang, Chen and Wu10). The presence of proteinuria usually reflects disrupted glomerular filtration barrier or tubular function(Reference Ou, Nakayama and Natori23,Reference van den Berg and Weening24) . Neutrophils may possibly increase the risk of new-onset proteinuria through the formation of neutrophil extracellular traps, synthesis of pro-inflammatory cytokines, release of reactive oxygen species and direct tissue damage(Reference Gupta and Kaplan6). Activated neutrophils could produce granules, including elastase 2, azurocidin 1 and myeloperoxidase, which may induce the expression of adhesion molecules, lead to endothelial dysfunction and increase vascular permeability(Reference Jickling, Liu and Ander25). Neutrophil extracellular traps are fibrillary networks of DNA, histones and cytotoxic proteases(Reference Noubouossie, Reeves and Strahl26). Neutrophil extracellular trap-associated histones and proteases, including cathepsin G, proteinase 3, neutrophil serine protease 4, matrix metalloproteinase 9 and neutrophil elastase, exert a pivotal role in the elimination of pathogens(Reference O’Donoghue, Jin and Knudsen27); however, they may also induce endothelial cell death and adhere to and damage the vascular wall, ultimately leading to vascular leakage, impairing endothelial monolayer integrity and promoting the development of proteinuria(Reference Pieterse, Rother and Garsen28–Reference Dejana, Orsenigo and Lampugnani30). Overall, while our findings seemed to be biologically plausible, the exact mechanisms regarding the relationship of neutrophil counts with new-onset proteinuria remain to be further investigated, and our findings and speculations also warrant further confirmation.
Second, folic acid treatment was associated with a significantly reduced risk of new-onset proteinuria among those with higher neutrophil counts. Participants with higher neutrophil counts had lower folate and elevated tHcy levels at baseline. Folic acid is effective in lowering tHcy levels(Reference Wang, Wu and Li31,Reference Zhao, Wu and Li32) . Elevated tHcy has been reported to be an independent determinant of the development of albuminuria(Reference Jager, Kostense and Nijpels33). Some evidence suggests that raised tHcy increases oxidative stress and inflammation and reduces the bioavailability of nitric oxide (NO), which could induce endothelial and mesangial cell dysfunction(Reference Spence, Yi and Hankey34,Reference Faraci and Lentz35) . Furthermore, folate has direct antioxidant and anti-inflammatory actions(Reference Verhaar, Stroes and Rabelink36,Reference Chen, Liu and Ji37) . It has been reported that folic acid supplementation can significantly enhance NO production, improve NO-mediated endothelial function and decrease superoxide production, independent of tHcy lowering(Reference Cianciolo, De Pascalis and Di Lullo38,Reference Capelli, Cianciolo and Gasperoni39) . As such, low folate, elevated tHcy and higher neutrophil counts appear to share some common mechanisms in the development of proteinuria. We speculated that folic acid therapy may counteract the detrimental effects of higher neutrophil counts by the decrease of tHcy and the increase of folate levels, resulting in a significant reduction of new-onset proteinuria among participants with higher neutrophil counts. However, more studies are necessary to confirm our results and further investigate the underlying mechanisms.
Several limitations of our study merit consideration. First, this was a post hoc analysis of the renal substudy of the CSPPT. Residual confounding cannot be completely eliminated. Second, proteinuria was only assessed at baseline and the exit visit. More frequent measurements would provide a more accurate evaluation of its progression. Third, proteinuria was measured by dipstick test and was reported as a qualitative rather than a quantitative variable. However, there was a graded relationship (P-trend <0·001) between dipstick proteinuria and the urinary albumin-to-creatinine ratio among participants in the CSPPT who had urinary albumin-to-creatinine values available (n 3225)(Reference Li, Qin and Luo2). Moreover, the dipstick test for proteinuria is a simple, widely available, instantaneous laboratory test. White et al.(Reference White, Yu and Craig40) further reported that urine dipstick readings of ≥1+ had a high sensitivity and specificity for detecting macroalbuminuria (albumin-to-creatinine ≥300 mg/g). Fourth, of note, mean folate levels in the enalapril-only group also had a substantially increase during the course of the study (Table 2). The explanation for this increase was still unclear. During the course of the trial, participants received nutritional health education, which may have led to improved dietary choices. Nevertheless, we did not have detailed food intake information at either baseline or the exit visit. Whatever the cause, this change may likely attenuate the beneficial effect of folic acid supplementation. Finally, our study was conducted in Chinese hypertensive patients. The generalisability of our results to other populations remains to be determined. Due to these limitations, our results are merely hypothesis-generating. Further confirmation of our findings in more studies is essential.
In summary, among hypertensive patients, those with higher neutrophil counts had increased risk of new-onset proteinuria, and this risk was reduced by 51 % with folic acid treatment. Obtaining neutrophil counts is relatively easy, rapid and economical and universally available in general clinical laboratories. If our results are further confirmed, neutrophil counts may serve as a biomarker to identify high-risk individuals who could particularly benefit from folic acid, a treatment that is simple, safe and inexpensive.
Acknowledgements
The study was supported by the National Key Research and Development Program (2016YFE0205400, 2018ZX09739010, 2018ZX09301034003); the Science and Technology Planning Project of Guangzhou, China (201707020010); the Science, Technology and Innovation Committee of Shenzhen (JSGG20170412155639040, GJHS20170314114526143, JSGG20180703155802047); the Economic, Trade and Information Commission of Shenzhen Municipality (20170505161556110, 20170505160926390); and the National Natural Science Foundation of China (81973133, 81730019, 81521003).
Author contributions were: Study concept and design: X. Q., Xiping Xu, J. N. and Z. Z.; Data collection: X. Q., Min Liang, B. W., J. L., Y. Z., Xin Xu, X. W., Y. H. and F. F. H.; Analysis of data: X. Q., Z. Z. and Mengyi Liu; Drafting of the manuscript: X. Q., Z. Z. and Y. Z.; Critical revision of the manuscript for important intellectual content: all authors.
Xiping Xu reports grants from the National Key Research and Development Program (2016YFE0205400, 2018ZX09739010, 2018ZX09301034003), the Science and Technology Planning Project of Guangzhou, China (201707020010), the Science, Technology and Innovation Committee of Shenzhen (JSGG20170412155639040, GJHS20170314114526143, JSGG20180703155802047), and the Economic, Trade and Information Commission of Shenzhen Municipality (20170505161556110, 20170505160926390). X. Q. reports grants from the National Natural Science Foundation of China (81973133, 81730019). J. N. reports grants from Nature and Science Foundation of China (81730019, 81521003). No other disclosures were reported.
Supplementary material
To view supplementary material for this article, please visit https://doi.org/10.1017/S000711452000505X








