Article contents
Ink-jet-printed (ZnO)1−x(TiO2)x composite films for solar cell applications
Published online by Cambridge University Press: 15 October 2012
Abstract
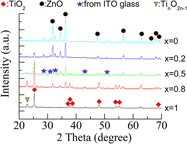
Ink-jet printing technique is used to prepare porous (ZnO)1−x(TiO2)x composite films on indium tin oxide-coated glass substrates. Dye-sensitized solar cells were fabricated using well-characterized printed films of thickness ∼20 and 30 μm, respectively. It is found that the photovoltaic performance of the cells is dependent on the film thickness and the concentrations of ZnO. The obtained results are compared with those of pure ZnO- and TiO2-based cells prepared by the same route to optimize the device efficiency. This study suggests that ink-jet printers promise an inexpensive and simple technology for manufacturing solar cell composite films.
- Type
- Articles
- Information
- Journal of Materials Research , Volume 28 , Issue 3: Focus Issue: Titanium Dioxide Nanomaterials , 14 February 2013 , pp. 502 - 506
- Copyright
- Copyright © Materials Research Society 2012
References
REFERENCES
- 9
- Cited by


