Article contents
Intercalation of ε-caprolactam Ions into Inorganic Hosts
Published online by Cambridge University Press: 31 January 2011
Abstract
The intercalation reaction of ε-caprolactam anion (ε–CL-) into hydrotalcite heated up to 500 °C to expel 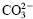 in its double hydroxide gallery and also that of ε-caprolactam cation (ε–CL+) into mica were successfully performed in their aqueous solutions. The intercalation reaction of ε–CL- into 500 °C heated hydrotalcite was completed within 1 h at 60 °C, and the resultant intercalation compound had the interlayer spacing of 0.77 nm. The intercalation of ε–CL+ into mica, on the other hand, proceeded rather slowly in two steps, which was due to the presence of two species, Na+ and hydrated Na+ in the mica gallery, and gave the intercalation compound with the interlayer spacing of 1.47 nm.
in its double hydroxide gallery and also that of ε-caprolactam cation (ε–CL+) into mica were successfully performed in their aqueous solutions. The intercalation reaction of ε–CL- into 500 °C heated hydrotalcite was completed within 1 h at 60 °C, and the resultant intercalation compound had the interlayer spacing of 0.77 nm. The intercalation of ε–CL+ into mica, on the other hand, proceeded rather slowly in two steps, which was due to the presence of two species, Na+ and hydrated Na+ in the mica gallery, and gave the intercalation compound with the interlayer spacing of 1.47 nm.
- Type
- Articles
- Information
- Copyright
- Copyright © Materials Research Society 1999
References
REFERENCES
- 8
- Cited by


