Introduction
Acute ischemic stroke (AIS) represents the leading cause of disability in adults and is the second leading cause of death worldwide.Reference Hebert, Lindsay and McIntyre1–Reference Feigin, Nichols and Alam8 Since the publication of the major 2015 stroke trials, the gold standard treatment for large vessel occlusion (LVO) anterior circulation strokes is endovascular mechanical thrombectomy (MT). The goal of this intervention is to rapidly recanalize the occluded vessel and facilitate early reperfusion of the salvageable brain tissue contained within ischemic penumbra shown to improve the clinical and functional patient outcome.Reference Berkhemer, Fransen and Beumer9–Reference Goyal, Demchuk and Menon12
Posttreatment imaging combined with relevant clinical information is commonly utilized for prognostication and ultimately to predict clinical outcomes. These measures include National Institutes of Health Stroke Scale (NIHSS) 24-hour after treatment, modified Rankin Score (mRS) at discharge and post-revascularization infarct volume.Reference Kerr, Fulton and Lees13–Reference Kwah and Diong16 Regarding noncontrast CT, it has been suggested the Alberta stroke program early computed tomography score (ASPECTS) may be superior to clinical measures such as DRAGON in predicting outcome.Reference Baek, Kim and Lee17,Reference Raza and Rangaraju18 However, previous investigations showed that the degree of diffusion restriction on magnetic resonance imaging (MRI) at 5 days did not reliably predict the clinical outcome in the pre-endovascular era.Reference Johnston, Barrett, Ding and Wagner19
We hypothesized that post-revascularization diffusion-weighted imaging (DWI) may poorly estimate the severity of stroke in patients who undergo MT for emergent LVO. In this study, we specifically assessed whether post-revascularization DWI can qualitatively on interpretation or quantitatively with volume calculation, predict the discharge and 90-day clinical outcomes after endovascular therapy (EVT) reliably, as defined by the mRS.
The objective of this study was to determine if experienced readers could predict mRS at 90 days when blinded to these outcomes and given only the post-therapeutic DWI images.
Methods
We retrospectively analyzed all consecutive patients who underwent endovascular thrombectomy at our tertiary stroke center from March 2016 to January 2018. Research ethics board approval was obtained. We identified 80 patients with AIS who underwent endovascular reperfusion treatment. Inclusion criteria were: (1) acute stroke with LVO and (2) DWI obtained typically within 48 hours of reperfusion treatment. Twenty-nine were excluded due to lack of DWI following the reperfusion treatment.
Clinical Analysis
Stroke severity using NIHSSS at baseline and 24 hours was assessed by a stroke neurologist. Patients’ functional outcome was assessed using the mRS at baseline, discharge, and 90 days. The primary outcome was poor neurologic outcome using the mRS score 90 days after stroke onset defined as mRS > 2 and determined by a stroke neurologist.
Imaging Analysis
MRI sequences minimally included axial fluid-attenuated inversion recovery (FLAIR) TR (repetition time)/TE (echo time)/TI (inversion time) 8000/120/200, field of view (FOV) 22 cm, matrix 320 × 224, section thickness (ST) 5, 1 mm gap, gradient T2 (1100/35/20 TR/TE/FA (flip angle), FOV 24 cm, matrix 320 × 224, ST 5, 1 mm gap), DWI (8125/min (TR/TE), FOV 26 cm, matrix 128 × 128, ST of 5 mm with no gap and sagittal T1 FLAIR TR/TE/TI 2163/26/750, FOV 22 cm, matrix 320 × 224, ST 5, 1 mm gap.
For quantitative data, the final infarct volume was measured on the DWI or FLAIR on follow-up MRI study using Medical Image Processing, Analysis and Visualization (Center for Information Technology, National Institutes of Health, Version: 7.2.0) by an experienced neuroradiologist.
Three radiologists and one neurologist used the DWI to qualitatively predict clinical outcome at 90 days posttreatment based on infarct location and appearance. Readers were not restricted in the criteria they could use for estimation, although they were given the same DWI images only. A 90-day mRS scoring was predicted by a stroke neurologist, with all readers blinded to clinical, other imaging, and outcome data.
Treatment and Angiographic Recanalization Assessment
Three neurointerventionalists at our institution performed endovascular thrombectomy for all patients included in this study. Most patients (78.4%) underwent stent retriever thrombectomy as the primary treatment, with either the Trevo system (Stryker; Kalamazoo, Michigan, USA) or Solitaire AB device (ev3; Irvine, California, USA). The remaining minority of patients were treated with aspiration with the Penumbra separator (Penumbra Inc., Alameda, California, USA).
Statistical Analysis
Clinical and imaging baseline characteristics of the patients were analyzed. This study was reviewed and approved by our institutional research ethics board.
Categorical variables are reported as proportions. Continuous variables are reported as mean ± standard deviation (SD) or median [interquartile range (IQR)] as appropriate according to the distribution of the data. Comparison between patients who suffered a stroke and had a good neurological outcome vs. poor neurological outcome at 90 days measured with the mRS was undertaken. Categorical variables were compared using the Fisher’s exact test. Continuous variables were compared using either two-sample t-test or the Mann–Whitney U-test according to the normality assumption. Correlation analysis was performed using Spearman’s correlation, with 95% CIs. A multivariate logistic regression model for poor outcomes on last follow-up (mRS > 2) was performed. For the evaluation of the prediction of mRS based on the readers, the command rocreg was used. A p-value of <0.05 was considered significant. All analyses were performed using STATA 14 software (StataCorp, Texas, USA).
Results
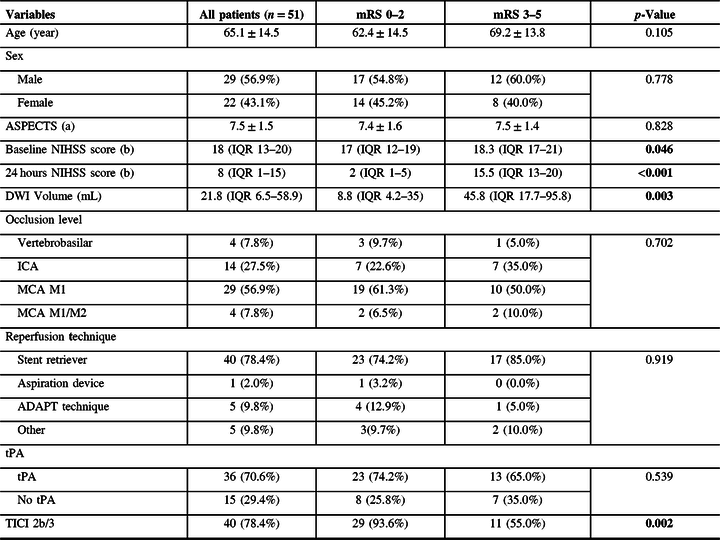
Fifty-one patients received endovascular treatment during the study period with posttreatment MRI/DWI (Table 1). The mean age was 65.1 ± 14.5 years and men comprised 56.9% (29/51). The median NIHSS score at admission was 18 (IQR 13–20) at admission. Mean infarct volume was 43.7 mL. Median time of post-MT MRI was 2 days (IQR 2–5).
Table 1: Baseline clinical and imaging characteristics, treatment, and predictors of 3-month mRS
n (%), p-Value: Fisher’s exact test.
Median (Interquartile range), p-Value: Mann-Whitney U-test.
Mean (± standard deviation), p-Value: Two-sample t-test.
Bold values: p-Value <0.05.
Missing data: (a) 4 (b) 2.
ICA, internal carotid artery; MCA, middle carotid artery.
Among the patients examined, 31 (60.8%) had a favorable outcome (mRS ≤ 2 at 90 days) and 20 (39.2%) a poor outcome (mRS ≥ 3). Mortality on last follow-up was 3 (5.9%). Thrombolysis in cerebral infarction (TICI) with 2b/3 (>50% of occluded territory/complete reperfusion) was achieved in (40/51) 78.4% of cases.
The demographics and treatment characteristics of those with mRS 0–2 were compared to cases with mRS 3–5 (Table 1). We found no differences in terms of mean age, sex, or ASPECTS scores. The baseline NIHSS scores, 24-hour NIHSS score, and DWI volume were lower for the mRS 0–2 group. No differences were found in terms of the occlusion level, occlusion territory (vertebrobasilar territory, basal ganglia/insula territory, cingulate/hippocampus territory, and lobar territory), reperfusion technique, or use of tPA. There was, however, a higher rate of TICI 2b/3 after treatment in the mRS 0–2 group.
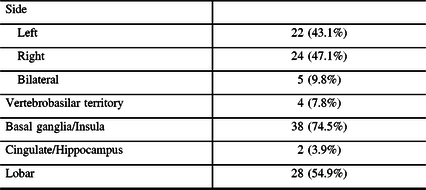
A multivariate analysis showed that a higher DWI volume was associated with poor neurological outcome (mRS > 2) at 90 days (OR 1.01; CI 95% 1.001–1.03; p = 0.04), even when adjusting for vessel occlusion location and TICI 2b/3 rates (Table 2).
Table 2: Infarction location characteristics
Diagnostic accuracy of the readers to predict mRS > 2 scores at discharge and 90 days, based on DWI volume, was poor as none had an area under curve (AUC) > 0.5 on receiver operator characteristic curves. There was also no significant difference between the readers in regard to the prediction of poor neurological outcome measured by mRS for both admission and 90-day follow-up rates (p = 0.61 and p = 0.45, respectively) (Table 3). Ability to predict mRS was not improved if the MRI occurred at any point on the IQR (1–5 days) (Table 4).
Table 3: Predictors of poor outcome (mRS > 2) at 90-day follow-up
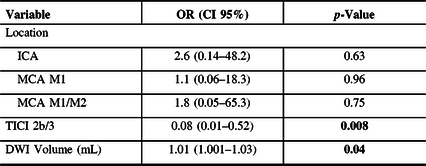
Bold values: p-Value < 0.05 (binary multivariable logistic regression)
ICA, internal carotid artery; MCA, middle carotid artery.
Table 4: Diagnostic accuracy prediction of mRS per reader based on DWI

Discussion
DWI can play an important role in the setting of emergent LVO and indeed acute stroke as a whole as regards patient selection for revascularization; however, it historically has not provided a relevant prediction for the 90 days clinical outcome in the pre-endovascular era.Reference Johnston, Wagner and Wang20,Reference Turc, Apoil and Naggara21
In the current study, we found that the physician’s impression of the DWI as a measure of clinical outcome for a given MT patient is poorly correlated (Figures 1–2) with actual clinical outcome, even with relevant training in the clinical neurosciences. In fact, the DWI appearance both frequently overestimated the severity of stroke for patients who achieved good recovery and underestimated the severity of stroke for poor outcome patients. Moreover, analysis failed to demonstrate any correlation between reader prediction of mRS based on DWI and the 90-day outcome (Figures 3–4).
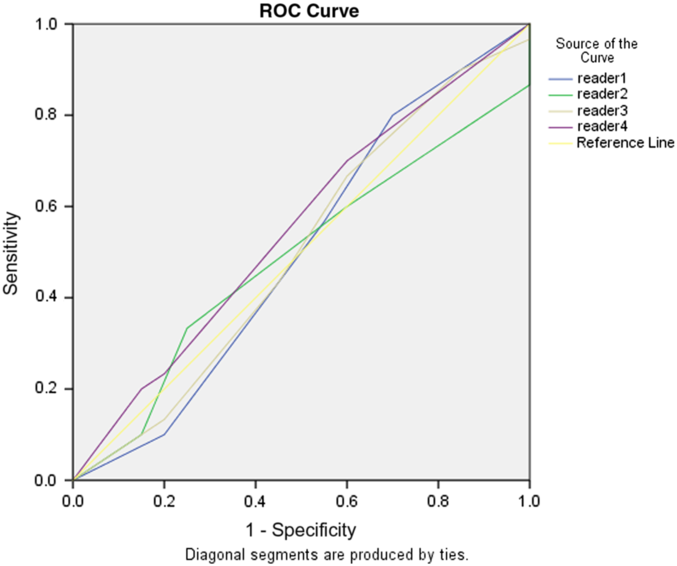
Figure 1. Performance plots for (A–B) readers, (C) mean DWI, and (D) infarct volume versus mRS across the MT for emergent large vessel occlusion cohort at our institution.
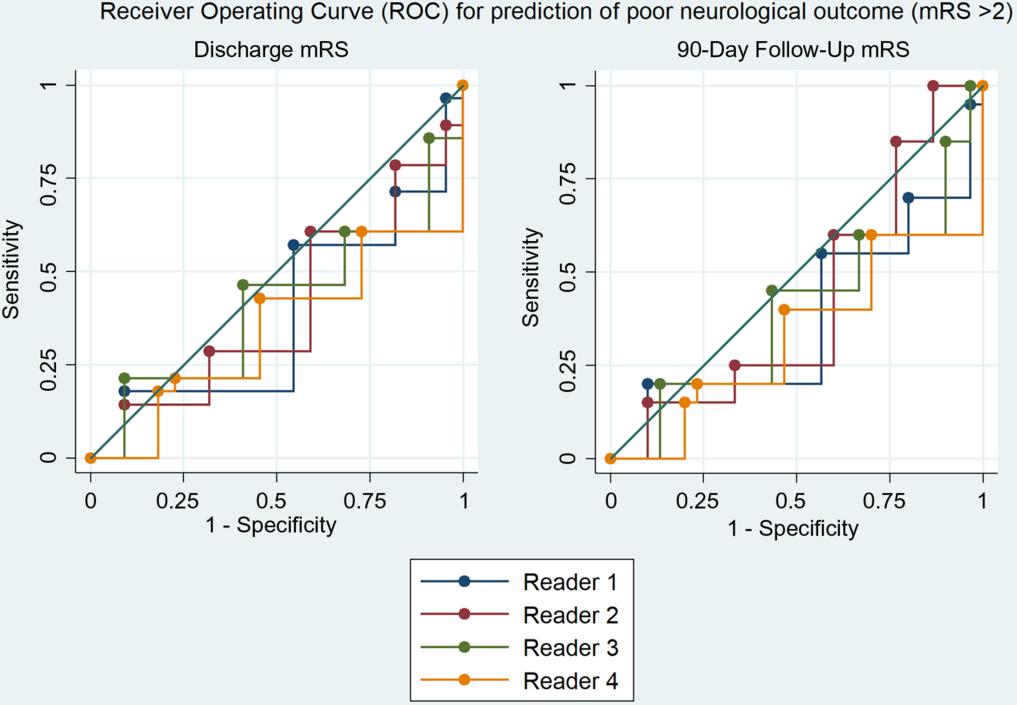
Figure 2. Receiver operating characteristic curve showing neither no correlation between rater prediction of mRS and true 90-day outcome.
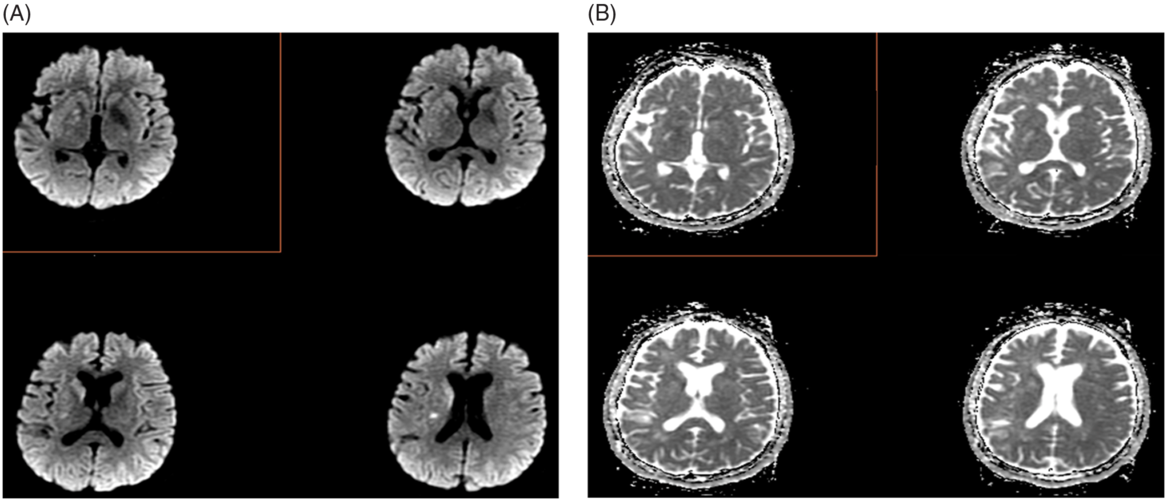
Figure 3. Post-MT case (A) DWI and (B) apparent diffusion coefficient (ADC) maps in which the mRS was rated to be high (mRS 4–5) by readers on the basis of DWI findings and was ultimately mRS 1 at 90 days.
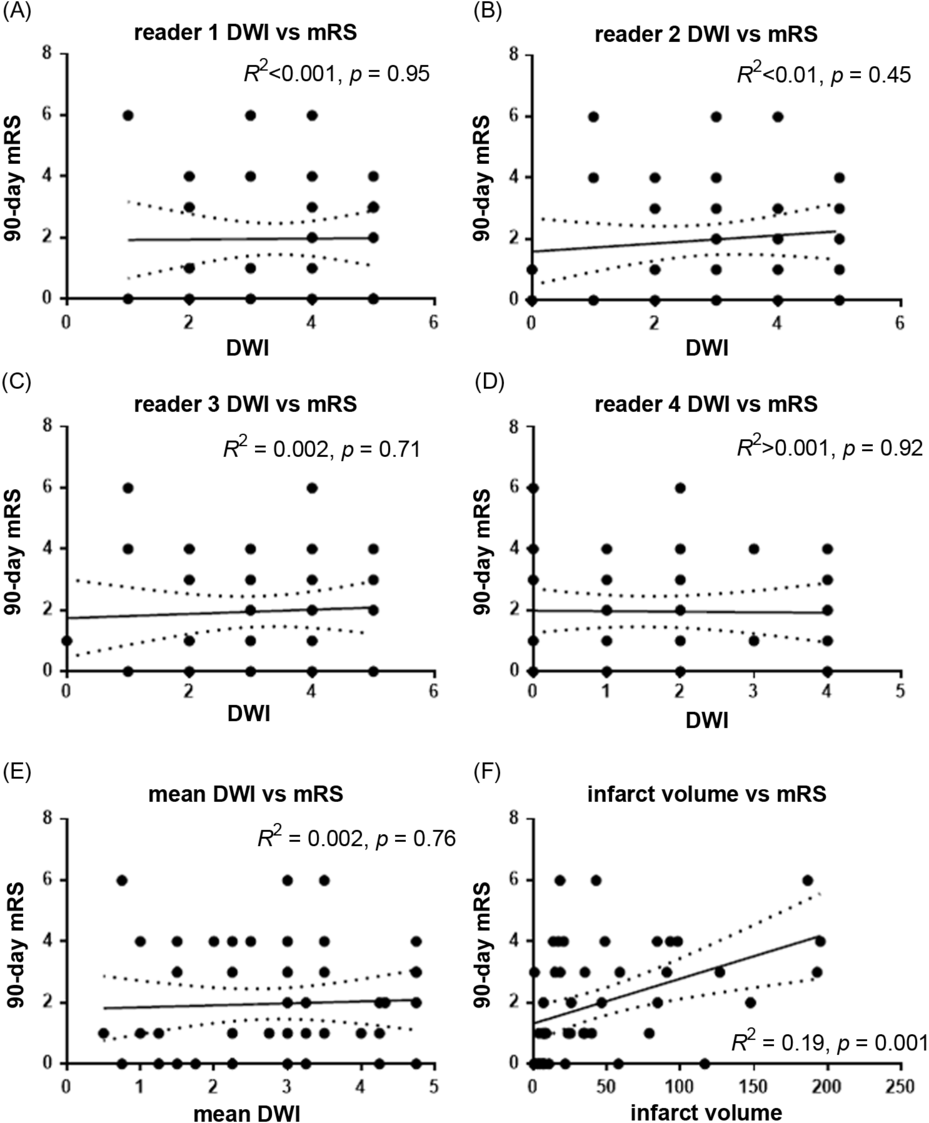
Figure 4. Post-MT case (A) DWI and (B) ADC maps in which the mRS was rated to be low (mRS 0–1) by readers on the basis of DWI findings and was ultimately mRS 4 at 90 days.
There are a number of reasons why prediction efforts may have failed. The problem with imaging prognostication is that both brain eloquence and crucial clinical variables are not necessarily considered in sufficient detail and may ultimately be of greater importance. For instance, a right middle carotid artery (MCA) cortical stroke that spares the deep motor fibers and the motor strip can be quite clinically benign but still >70 cc in volume. The same infarct in a patient with severe congestive heart failure and chronic obstructive pulmonary disease (COPD) may be deadly. Similarly, a small infarct in the right MCA territory involving the lenticulostriates but sparing the cortex after thrombectomy may result in complete and permanent hemiplegia with a small infarct volume. A small infarct volume post-thrombectomy in a mechanically ventilated patient due to the initial severity of symptoms with advanced COPD will undoubtedly not do well. Clinical prognostication is a multifaceted consideration of premorbid status, comorbidities, in addition to infarct volume. Controlling for clinical status as in this study, we must also entertain that it is possible that those with small infarcts in eloquent areas such as the internal capsule still did well because of neuroplasticity. Lastly, it may be that DWI is overly sensitive to ischemia and not predictive even or territorially appropriate deficits in the EVT era.
It is important that the infarct volume, which is more objective than the DWI appearance, correlated well with both discharge and 90-day mRS. Attempts to establish infarct core base on early DWI in acute stroke have been unreliable; however, large-scale studies such as Endovascular Treatment for Small Core and Anterior Circulation Proximal Occlusion with Emphasis on Minimizing CT to Recanalization Times (ESCAPE) have showed an inverse relationship between infarct volume and outcome in general.Reference Sajobi, Menon and Wang22 Randomized Trial of Revascularization with Solitaire FR Device Versus Best Medical Therapy in the Treatment of Acute Stroke Due to Anterior Circulation Large Vessel Occlusion Presenting Within 8 Hours of Symptom Onset (REVASCAT) subsequently replicated this finding, albeit with the caveat that NIHSS at 24 hours served as a superior predictor of 90-day mRS in their cohort. This is also precedented in the pre-endovascular era, in which NIHSS and mRS showed excellent correlation.Reference Kerr, Fulton and Lees13,Reference Govan, Langhorne and Weir23 Moreover, it has been shown that the effect of early recanalization is primarily dependent upon NIHSS in that the effect increases with severity by this clinical measure.Reference Skagen, Skjelland, Russell and Jacobsen24,Reference Todo, Sakai and Kono25 Our findings indeed suggest that infarct volume correlates with income. Even though DWI overestimates infarct size in the era of EVT and penumbra salvage, it likely does so in a somewhat-linear fashion making the quantitative finding less surprising. We believe that when an expert interpreting physician regards an MRI, they take into consideration their anticipation of eloquent cortex loss. It is possible that in the era of EVT, a neuroplasticity renders this less reliable than volume.
The ESCAPE trial investigators observed that patients with penumbral pattern fared much better than those who did not display this pattern on CTP, a wisdom that is now commonly accepted.Reference Wannamaker, Guinand and Menon26 A penumbral pattern is defined as an infarct core <70 mL, penumbral volume >15 mL, and a total hypoperfused volume:core volume ratio of >1.8. It may be that DWI hyperintensity should similarly regarded as at-risk tissue which is not reflective of the final functional insult despite longer-term MR findings, with perfusion-weighted imaging possibly strengthening prognostication.Reference Cheng, Forkert and Zavaglia27,Reference Kakuda, Lansberg and Thijs28 Furthermore, while it has been shown that FLAIR lesions at day 2–3 in characteristic motor control regions (i.e. corona radiata, internal capsule, and insula) correlated with functional outcome,Reference Cheng, Forkert and Zavaglia27 using this knowledge as a guide to predicting 90-day mRS on DWI was unhelpful. We conclude that DWI is at best useful for limited prognostication after deference to NIHSS score. It is not anecdotally uncommon to observe different outcomes than expected when using DWI as a predictor, and we thus urge caution in clinical practice. Instead, quantitative measures and clinical factors should be used preferentially.
Limitations
Limitations of our study include its retrospective nature during multiple endovascular epochs, which we sought to ameliorate through inclusion of all consecutive patients. Post-thrombectomy MRI was performed at a median of 2 days but a wider range, though readers were blinded to this information.
Conclusion
Our study suggests that that post-therapeutic DWI is an unreliable indicator for discharge and 90-day functional outcomes in the MT era for LVO patients. Overestimation of severity in patients with good outcome and underestimation of severity in patients with poor outcome were both common.
Acknowledgments
The authors acknowledge the imaging technologists for their hard work in the care of stroke patients.
Conflict of Interest
No relevant conflicts of interest.
Statement of Authorship
Each author contributed equally and meaningfully to the preparation of this manuscript. This reflects both time and effort invested. AAD: study design, participant data acquisition, interpretation and analysis; primary manuscript preparation and editing, critical review and intellectual contribution; approval of the final version. AA, AEM, SD: expert analysis; independent statistical analysis; manuscript preparation and editing, critical review and intellectual contribution; approval of the final version. GW, AR: participant data acquisition; data analysis; manuscript preparation and editing, critical review and intellectual contribution; approval of the final version. CH, AY, RIA: manuscript preparation and editing, critical review and intellectual contribution; approval of the final version. LDC, VXDY: expert neurointerventional care and analysis; expert neurosurgical interpretation and analysis; manuscript preparation and editing, critical review and intellectual contribution; approval of the final version.










