Introduction
Embolic stroke of undetermined source (ESUS) is defined as a not-lacunar ischemic stroke without significant extracranial or intracranial atherosclerosis, major-risk cardioembolic conditions, or other specified stroke causes. Reference Hart, Diener and Coutts1
Considering the possible etiologies implicated in the genesis of ESUS, the first and most important is paroxysmal atrial fibrillation (PAF). Reference Cotter, Martin, Ring, Warburton, Belham and Pugh2 Among heart rhythm monitoring systems, implantable loop recorders (ILRs) demonstrated the highest sensitivity for detecting post-stroke asymptomatic PAF. Reference Toni, Lorenzano and Strano3 Detection of PAF allows targeted anticoagulation, that it is known to reduce the risk of subsequent stroke. 4
Although the sensitivity of ILR in detecting PAF has been demonstrated in a series of studies, Reference Diederichsen, Haugan and Køber5,Reference Poli, Diedler and Härtig6 its impact on detection and targeted medical treatment on subsequent cerebrovascular events and death has not been evaluated, and current studies have not identified a high-risk population of ESUS recurrence that may benefit from anticoagulation therapy. Reference Kamel, Merkler, Iadecola, Gupta and Navi7
This would require a RCT, potentially excluding PAF patients from anticoagulation, and thus ethically not feasible.
This study aims to evaluate the clinical impact of a diagnostic-therapeutic pathway that includes ILR implant and subsequent anticoagulation therapy – if PAF is detected – by comparing two similar populations of patients, respectively, implanted (ILR group) and not implanted (control group).
Methods
Patient Enrolment
Between 2011 and 2016, patients with acute ischemic stroke presenting to ER division of Città della Salute (CdS) University Hospital were admitted to either the Stroke Unit or General Medicine Ward, mainly depending on bed availability. The destination was not completely random, as stroke neurologists tended to admit younger patients and in better general conditions to the Stroke Unit, in order to minimize the waste of medical resources and maximize clinical benefits.
To avoid misdiagnosis, we included in our analysis all patients with radiological features of cortical infarction that underwent a complete tests panel for a correct diagnosis of ESUS, as resumed in Table 1.
Table 1: Criteria for diagnosis of ESUS and tests panel*

CT indicates computed tomography, and MRI indicates magnetic resonance imaging.
* Requires minimum diagnostic evaluation that includes cardiac rhythm monitoring for >24 hours with automated rhythm detection.
† Lacunar defined as a subcortical infarct ≤1.5 cm (≤2.0 cm on MRI diffusion images) in largest dimension, including on MRI diffusion-weighted images, and in the distribution of the small, penetrating cerebral arteries of the cerebral hemispheres and pons.
‡ Permanent or paroxysmal atrial fibrillation, sustained atrial flutter, intracardiac thrombus, prosthetic cardiac valve, atrial myxoma or other cardiac tumors, mitral stenosis, recent (<4 weeks) myocardial infarction, left ventricular ejection fraction <30%, valvular vegetations or infective endocarditis.
In the General Medicine Ward, ILR implantation was not a routine procedure in stroke management before 2017 but, thanks to a specific pathway shared with cardiology division, ILR was implanted in patients aged 18–80 years old admitted to Stroke Unit.
A process of risk stratification for PAF incidence was used, as ILR was implanted in patients who had at least one risk factor for AF, including obesity, hyperthyroidism, atrial enlargement at transthoracic echocardiography (LA diameter >45 mm in parasternal axis), severe mitral valve disease, CHADS-VASc score ≥4, age >70, and diabetes. Reference Kamel, Longstreth and Tirschwell8
As a result, two different populations were available for comparison: ILR group and control group (Figure 1).
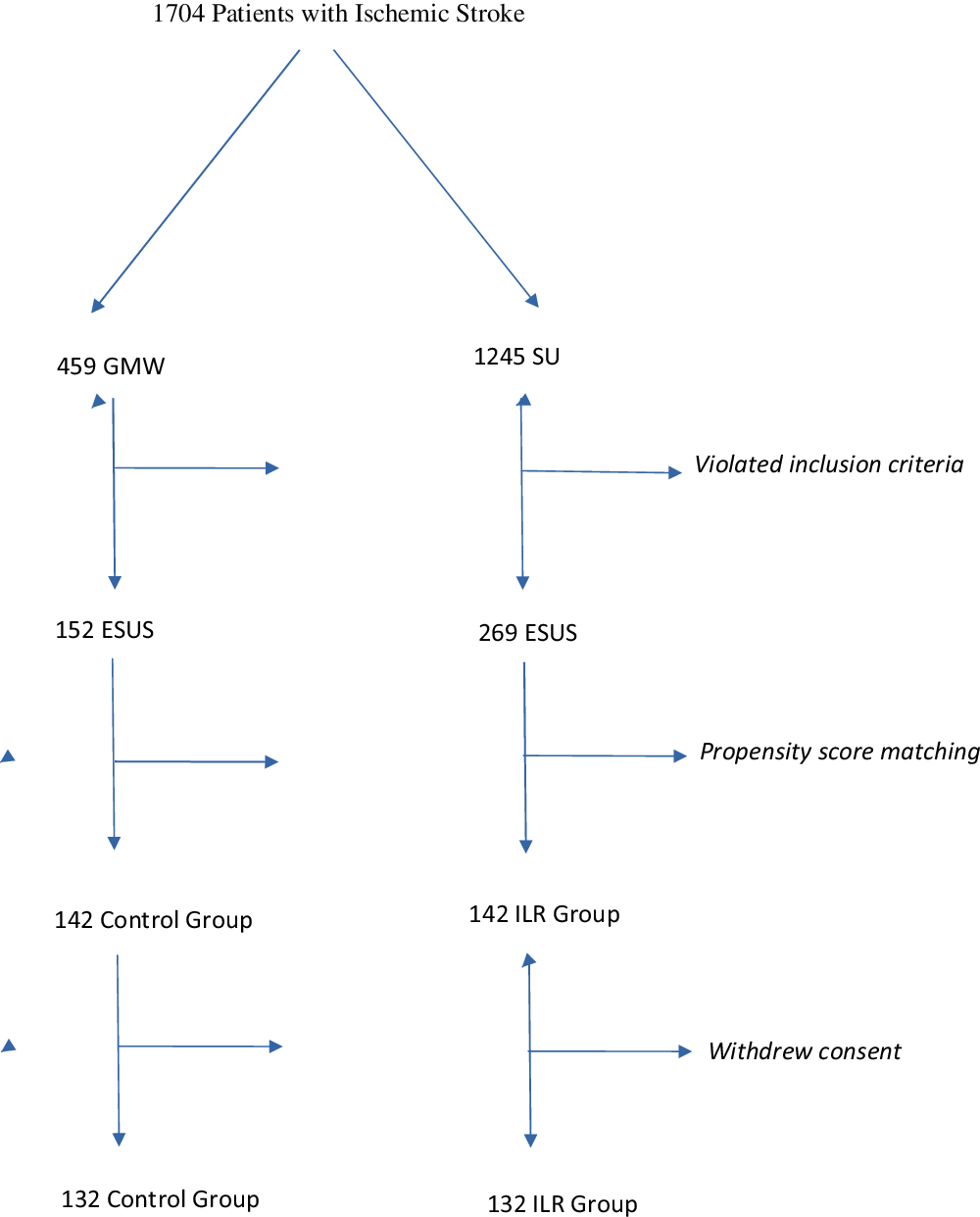
Figure 1: Selection of the patients. GMW: General Medicine Ward, SU: Stroke Unit.
During 2019, the clinical documentation of the above-mentioned patients with diagnosis of ischemic stroke at discharge was reviewed.
Patient Follow-up
In the ILR group, clinical examination, ILR control, and 12-lead ECG were performed on every patient at the time of enrollment, at the end of first year of follow-up, and in case of symptoms suggestive of PAF or PAF detection. Electrograms recorded by ILR were checked monthly with trans-telephonic data transmission (CareLink).
ILR was implanted an average of 3.5 months after the ischaemic event.
Long-term follow-up in both groups was performed with ambulatory clinical examinations or trans-telephonic interviews. Verbal consent was obtained from patients or relatives, if unable to express consent.
Statistical Analysis
To avoid potential selection bias, we used propensity score matching to extract paired patients from each group. Age, sex, diabetes status, CHADS-VASc score, and HAS-BLED score were used as baseline features to match patients (with IBM SPSS 27).
A difference was considered statistically significant for values equal to or less than 0.05.
We used recurrent stroke or TIA, death or composite (recurrent stroke or TIA and death) as primary endpoints.
Differences between groups were statistically evaluated by χ2 test.
Risk and protective factors were estimated for the primary endpoints and were evaluated with logistic regression univariate and multivariate analyses (supplementary material).
We searched the best subset for 2–4 variables (SAS 9) for predicting the clinical outcomes.
IRB approval was obtained (protocol number 0075807).
Atrial Fibrillation Diagnosis
The diagnosis of AF requires rhythm documentation with a 12-lead electrocardiogram (ECG) tracing showing AF. By convention, an episode lasting at least 30 s is diagnostic for clinical AF. Reference Steinberg, O’Connell, Li and Ziegler9
AF events recorded by ILR longer than five consecutive minutes were considered, as we considered this duration significant for thrombus formation and consequent potential embolic events. Reference Cotter, Martin, Ring, Warburton, Belham and Pugh2
Results
From 459 and 1245 patients admitted to General Medicine Ward and Stroke Unit, we selected 269 and 142 patients, respectively. Propensity score identified 142 pairs of patients, of which 132 accepted to participate in the study.
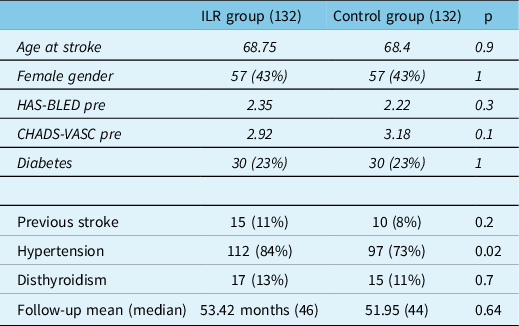
Table 2 resumes baseline features of the two groups. Variables used for matching (age, sex, diabetes, CHADS-VASc, and HAS-BLED) were similar in both populations; thyroid functionality and previous stroke prevalence were similar, therefore not used for the matching process; a greater incidence of hypertension was found in the implanted group.
Table 2: Baseline clinical characteristics of patients in the ILR and the control group. In italic style variables are used for the matching
Detection rate of PAF episodes in the ILR group during the first 12 months was significantly higher than in the control group (29% vs 6%, p < 0.001) (Figure 2).
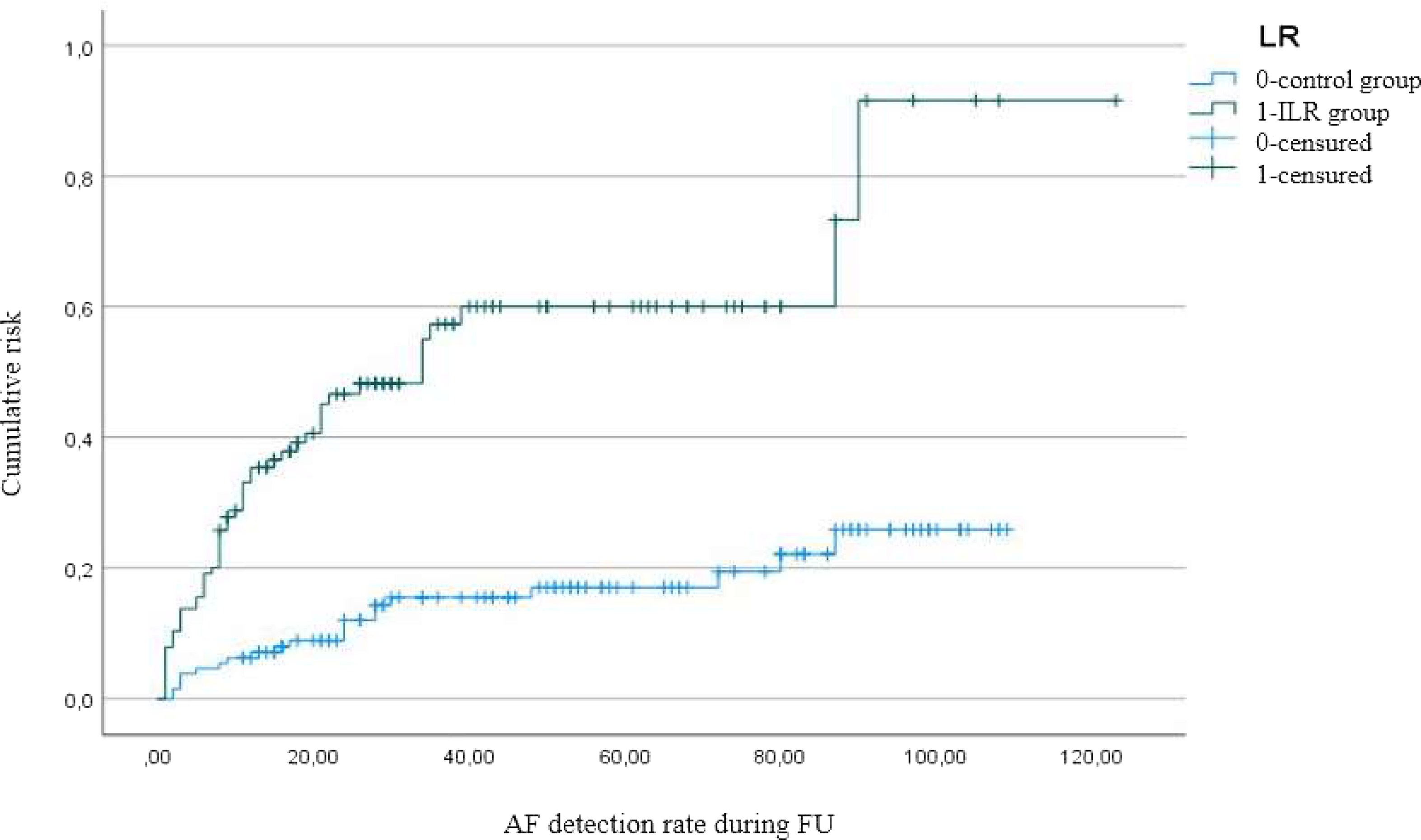
Figure 2: Kaplan-Meier representing rate of AF detection in the ILR and control group.
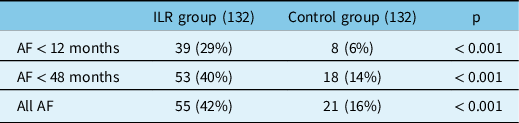
During the whole follow-up period, the detection rate was also significantly higher in the ILR group (42% vs 16%, p < 0.001), as resumed in Table 3 and indicated by the Kaplan-Meier curve.
Table 3: AF detection rate in the ILR and control group
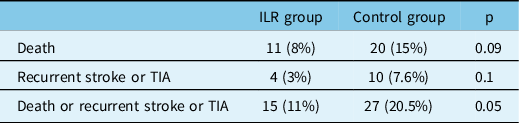
Death and recurrent TIA or stroke were not statistically different between groups. A significant difference was found in the composite outcome (p = 0.05) (Table 4).
Table 4: Clinical outcome in the ILR and control group
None of the recurrent stroke patients were on anticoagulation therapy at the time of the ischaemic event.
In all of these patients, AF was found to be the underlying cause of stroke at the admission.
No significant protective role of ILR for clinical endpoints was found on univariate analysis, although a trend towards significance has been pointed for the composite outcome of death and ischaemic events recurrence (OR 0.52, CI 0.26–1.04, p = 0.06).
A protective role of ILR was found for deaths (OR 0.4, CI 0.17–0.94, p 0.03) and for the composite outcome (OR 0.41, CI 0.19–0.87, p 0.02) on multivariate analysis in the best subsets.
Discussion
We found a higher detection rate of PAF in the ILR group at 12 months (29%) and at 4 years (42%), confirming the results already reported in literature. Reference Melis, Guido and Amellone10 Our detection rate was superior to that of the CRYSTAL-AF trial (12% at 12 months after ILR implantation, 30% at 3 years) and similar to other more recent series. 11 This may be due to the selection criteria we used; in literature, it is documented that stratification by risk factors for AF leads to an increase in the number of identified arrhythmias. Reference Poli, Diedler and Härtig6 The high prevalence of PAF obtained in our study population can be explained by the presence of at least one risk factor for AF as an inclusion criteria. 4
After PAF detection, anticoagulant therapy and rate or rhythm control therapy were proposed to all patients, following current guidelines.
Demonstration of the clinical impact of a policy including ILR implantation has never been directly demonstrated to be useful in patients with ESUS.
In our patients, ILR demonstrated a significant protective role in multivariate analysis for the composite endpoint of recurrent stroke or TIA and death, with an OR of 0.41. Death and stroke/TIA recurrences, isolated, were on the contrary not significant, probably due to the size of our samples.
It is known that anticoagulation is an effective treatment to avoid embolic events in patients with previous acute ischaemic stroke. On the other hand, the recent failure of the NAVIGATE ESUS and RE-SPECT ESUS clinical trials, 12,13 designed to demonstrate the efficacy of anticoagulant therapy in all patients with ESUS, highlights the importance of risk stratification for PAF and confirms the need for documentation of this arrhythmia to obtain clinical benefit from anticoagulant therapy.
Moreover, clinical benefits from AF screening with ILR in population at risk and treatment of PAF not already responsible for cerebrovascular events remain doubtful, as results from the LOOP and STROKESTOP studies suggest.
In the LOOP study, 14 ILR implantation and anticoagulation in patients with detected PAF lasting more than 6 minutes did not significantly reduce the risk of stroke or systemic arterial embolism compared with standard care. The STROKESTOP study 15 demonstrated that anticoagulation prescribed to patients with AF detected by twice-a-day standard ECGs screening for 2 weeks led to a significant reduction in vascular events. The difference between these trials may be explained by the different sensitivity in detecting PAF of these trials, ILR monitoring being far more sensitive than routine ECG, thus identifying a wider population with short-lasting PAF destinated to remain asymptomatic, and therefore without clinical benefit from anticoagulation.
Taken together, results of the above-mentioned trials suggest that selection of the patients is the key for effective anticoagulation in patients with ESUS.
Our selection criteria, that is patients with ESUS and at least one risk factor for AF, were selective enough to detect differences in outcome due to ILR implantation.
More recent studies have shown other possible features useful for risk stratification in AF patients that can potentially benefit from anticoagulation therapy (P-wave terminal force >5 mV × ms in ECG lead V1, serum NT-proBNP >250 pg/mL, left atrial diameter index ≥ 3 cm/m2 at echocardiography, Reference Kamel, Longstreth and Tirschwell8 and spontaneous echo contrast in left atrial appendage (LAA) and LAA flow velocity <20 cm/s.) Reference Geisler, Poli and Meisner16
In addition, a recent analysis of the aforementioned RE-SPECT ESUS demonstrated that patients older than 75 years and/or with CrCl 30–50 ml/min treated with dabigatran have significantly fewer stroke relapses and no significantly more bleeding compared with aspirin. This latter aspect may be due to the administration of dabigatran with a lower dosage (110 mg bid instead of 150 mg bid) to these two populations, as per the drug reduction dose criteria. As for the lower number of relapses in the elderly, it could be that age is a relevant risk factor for AF, so they would benefit more from anticoagulation, thus reducing relapses. Reference Diener, Sacco and Easton17
Several limitations may affect our results. The first is selection bias. ILR and control groups were identified retrospectively, and the differences were reduced as much as possible using statistical matching; this generated the best possible approximation of a randomly selected population, but some unavoidable biases may remain. In particular, patients’ clinical condition was used to select them for stroke unit admission, and this global evaluation could probably not be completely identified by clinical scores we used to match patients.
Second, statistical significance was not demonstrated for recurrent stroke/TIA or death, although both were trending toward significance, but only for the composite outcome. The limited size of our sample may explain these results; due to the progressive introduction of ILR as standard practice after demonstration of high efficiency in identifying AF, 12,13,Reference PérezRodon, FranciscoPascual, RivasGándara, RocaLuque, Bellera and MoyaMitjans18 it will not be probably possible to increase the size of a control group population. Moreover, in this setting, further studies on direct comparison of ILR and control groups are difficult to be done, given the clinical advantage granted from AF detection and subsequent treatment in patients with stroke.
Conclusions
This study demonstrated that a policy including ILR in patients with ESUS has an advantage in TIA or stroke recurrence and death with OR 0.41, although the limited size of the population and possible selection bias may limit the generalization of our results.
This finding further encourages the use of ILR, giving the first and actually best possible demonstration of its clinical advantage, which, at least in particular settings, has not yet fully entered the routine clinical practice, 19 despite its proven efficacy in the detection of AF with a low frequency of adverse effects events. Reference Toni, Lorenzano and Strano3
Author Contributions
Andrea Gambino – designed the analysis, analyzed the data, and wrote the paper
Emanuele Ravetti – collected data and wrote the paper
Andrea Naldi – reviewed paper
Riccardo Russo, Stefano Molinaro & Francesco Mistretta – contributed with the analysis
Marcella Jorfida, Davide Castagno, Gaetano Maria De Ferrari, Paolo Cerrato & Giovanni Bosco – contributed with data
Federico D’Agata & Alessandro Cicerale – contributed with statistical analysis
Mauro Bergui – wrote and reviewed the paper, supervised the research.
Funding
No funding.
Conflict of Interest
The authors have no conflicts of interest to declare.
Ethics
IRB approval was obtained with protocol number 0075807.








