Article contents
Synthesis of nanosized zirconium dioxide and its solid solutions with titanium dioxide from the CO2 supercritical fluid
Published online by Cambridge University Press: 18 January 2018
Abstract
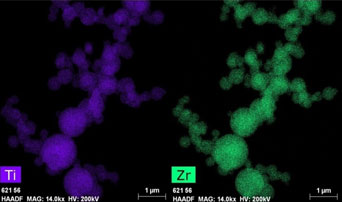
In this study, the formation solid solutions of titanium dioxide- zirconium dioxide (TiO2-ZrO2) system with the supercritical fluid method is described. The particles of solid solutions in the TiO2-ZrO2 system are spherical and form agglomerates, they are amorphous and have a size from 90 to 850 nm. The X-ray patterns of samples calcined above the temperatures of crystallization (450 °C) and phase transition (750 °C) demonstrate the decomposition of the solid solutions above the crystallization temperature and formation of phases in accordance with phase ratios in the TiO2-ZrO2 system at these temperatures. The formation solid solutions of the starting materials are observed in all region of concentrations.
- Type
- Research Letters
- Information
- Copyright
- Copyright © Materials Research Society 2018
References
- 5
- Cited by



