Introduction
Malignant tumours, also known as cancer, are extremely invasive and easily metastasized. Furthermore, they pose a major hazard to human health and the cause of millions of deaths worldwide each year (Ref. Reference Siegel1). For the time being, surgery, radiotherapy (RT) and chemotherapy remain significant ways of cancer treatment, but their therapeutic effects have the disadvantages of limited precision, low efficiency and high side effects (Refs Reference Vasan, Baselga and Hyman2, Reference Wang3, Reference Zhong4). Starvation therapy, which aims to block the nutrient supply pathway of tumours through drugs or other methods, leading to apoptosis of tumour cells and achieving the purpose of indirect treatment, has been widely developed and has begun to be used in clinical practice to meet the growing demand for clinical treatment (Refs Reference Li, Tong and Lin5, Reference Yang6). Currently, the main therapeutic mechanisms of current starvation therapies are as follows: (i) antineoplastic angiogenesis (Refs Reference Nakamura7, Reference Sheta8); (ii) blocking of tumour vascular oxygen and blood supply (Ref. Reference Li9); (iii) inhibition of tumour cell metabolic processes (Ref. Reference Ishak Gabra10). Nevertheless, starvation therapy still faces significant obstacles in the treatment of cancer, including its unsatisfactory efficacy, adverse reactions, etc. (Ref. Reference Willems11). Hence, researchers are committed to discovering suitable methods to improve the efficacy of starvation therapy. Recently, the continuous development of nanomaterials has brought new ideas to improve starvation therapy. Therefore, the use of nanomaterials with excellent properties in cancer treatment can improve the accuracy of cancer treatment and reduce the side effects of the treatment process (Refs Reference Cheng12, Reference Murugan13, Reference Raeisi-Kheirabadi, Nezamzadeh-Ejhieh and Aghaei14, Reference Raeisi-Kheirabadi, Nezamzadeh-Ejhieh and Aghaei15, Reference Raeisi-Kheirabadi and Nezamzadeh-Ejhieh16).
Compared to conventional nanomaterials, metal-organic framework (MOF), an organic–inorganic hybrid material with intramolecular pores formed by self-assembly of organic ligands and metal ions or clusters through coordination bonds, has been widely prepared and applied in drug delivery and cancer therapy with the advantages of controllable shapes, diverse compositions, adjustable pore sizes, extremely high porosities, high specific surface areas, high functionality as well as excellent physicochemical properties (Refs Reference Li17, Reference Tan18, Reference Furukawa19, Reference Liu, Zhao and Chen20, Reference Wu and Yang21, Reference Zimpel22). In the field of tumour therapy, the advantages of MOF are mainly in the following aspects: (i) the combination of metal ions/ion clusters and bridging ligands provides numerous options for constructing multifunctional MOF nanoplatforms for cancer therapy; (ii) the large specific surface area and high porosity of MOFs enable efficient loading of functional reagents such as chemotherapeutic drugs, phototherapeutic agents, proteins, enzymes and antigens; (iii) it is feasible to design appropriate pore sizes to enhance permeability and retention effects endowing the MOF nanoplatforms with the ability to passively target tumour sites; (iv) through covalent coupling of organic ligands on the MOF surface (like -COOH, -NH2, -N3) and coordination of metal nodes, some unique entities such as active targeting molecules, supramolecular nano-valves, biomolecules, polymers and cellular membranes were introduced onto the MOF surface to enhance targeting efficacy, colloidal/circulatory stability, biocompatibility and stimulus responsiveness in cancer therapeutics; (v) the degradability triggered by the tumour microenvironment (TME) promotes efficient MOF excretion (Refs Reference Huang23, Reference Li24, Reference Li, Song and Yang25, Reference Rao26, Reference Wang27, Reference Yang and Yang28, Reference Zhang29, Reference Zhou30, Reference Chen31). Not only that, MOF nanoplatform also shows unique advantages in some cancer therapies such as chemodynamical therapy (CDT), photodynamic therapy (PDT), photothermal therapy (PTT) and immunotherapy, which can deliver various therapeutic agents such as drugs, enzymes, proteins, photosensitizers, phototherapeutic agents and antibodies to the target site, improving the efficiency of tumour therapy and reducing side effects (Refs Reference Yang32, Reference Wu33, Reference Tan34, Reference Tan35, Reference Lin36, Reference Yang37, Reference Yang and Yang38). All these point to the significant potential of MOF-based nanoplatforms for treating cancer using many different kinds of therapeutic modalities including starvation therapy.
In some of the reviews published in the past on the progress of MOF research in tumour therapy, very few reviews have focused on the progress of MOF in a specific therapeutic application, especially starvation therapy (Refs Reference Li39, Reference Zeng40, Reference Zeng41). In this review, we focus on the recent progress and research findings regarding representative MOF-based nanoplatforms for tumour starvation therapy or combination therapy of starvation therapy with other therapeutic approaches such as RT, chemotherapy, CDT, PTT, PDT and immunotherapy. In addition, by reviewing and summarizing representative research, the necessity and efficacy of MOF-based nanoplatforms in the therapy of tumour starvation are stressed. Furthermore, this review anticipates the prospects and challenges of MOF-based nanoplatforms in tumour starvation therapy and explores the potential applications of MOF nanoplatforms in cancer clinical therapy to further enhance the development of MOF-mediated cancer therapy.
MOF in cancer starvation therapy
Due to the advantageous features of MOF, the development of MOF-based nanoplatforms in the field of medicine is garnering ever more attention, while they are extremely promising prospects for future tumour therapies (Ref. Reference Huang42). Interestingly, starvation therapy is one of the important prospects in cancer therapy, and the use of MOF-based nanoplatforms to mediate cancer starvation therapy can compensate for the drawbacks of traditional cancer starvation therapy that is more susceptible to drug resistance and mutation (Ref. Reference Yu43). Besides, MOF can mediate the combination of starvation therapy with other tumour treatments (Ref. Reference Gao44).
Currently, many studies have demonstrated that MOF-based nanoplatforms can be ideal candidates in cancer starvation therapy. (Fig. 1) In this section, we will focus on two perspectives: (i) single starvation therapy strategy; (ii) combination treatment strategies of starvation therapy and other therapies, outlining the recent applications of MOF-based nanoplatforms in the field of cancer starvation therapy and summarizing the mechanisms of action as well as the relevant properties (Table 1).

Figure 1. (By FigDraw) We can construct MOF-based nanoplatforms, which use MOF materials to package substances like GOD, PLT, Anti-VGEF2, etc., to enable targeted treatment of tumour cells, causing apoptosis through starvation therapy.
Note: MOF, metal-organic framework; GOD, glucose oxidase; PLT, platelet; Anti-VGEF2, anti-vascular endothelial growth factor receptor-2.
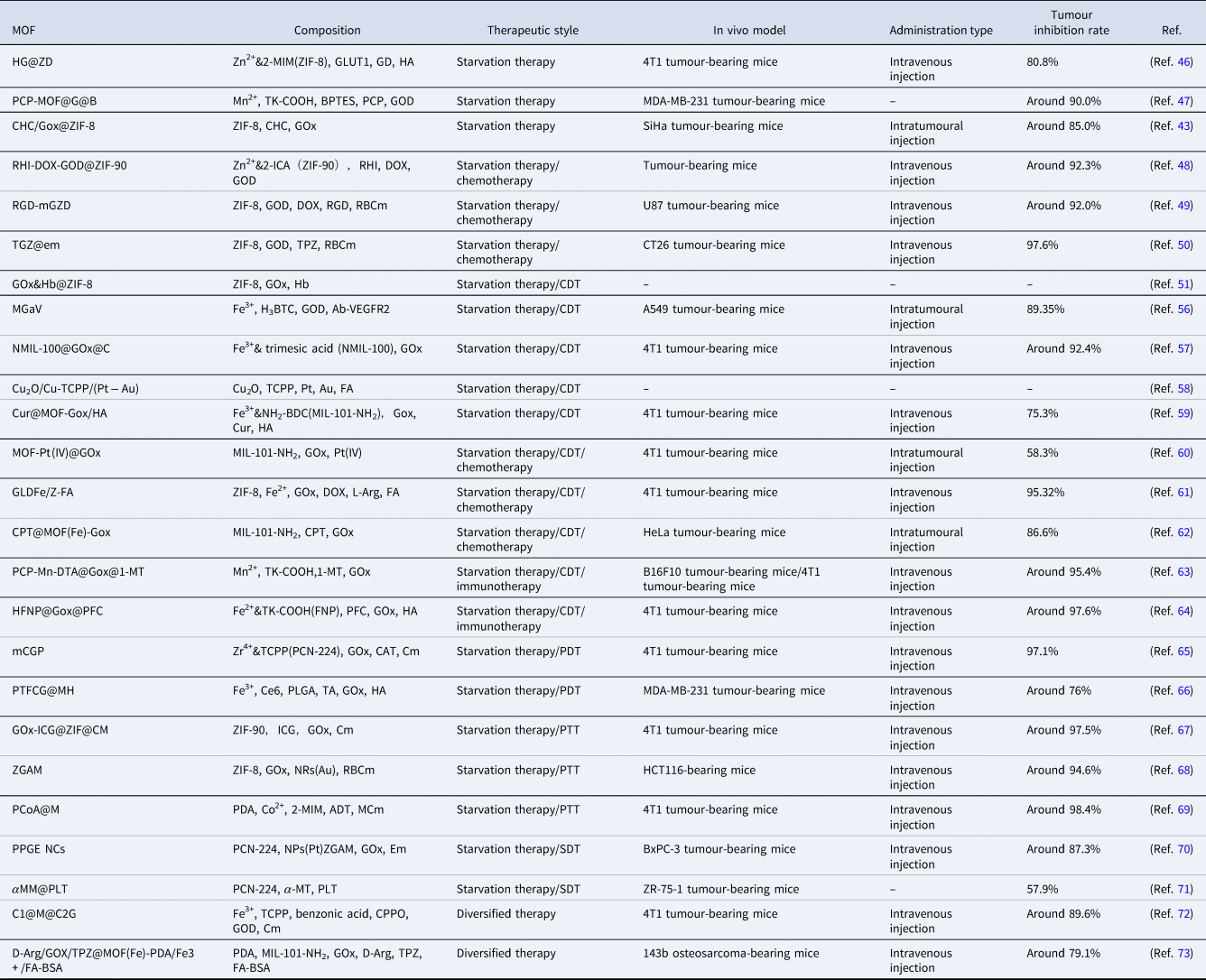
Table 1. Examples of MOF-based nanoplatforms used in tumour starvation therapy
Single starvation therapy based on MOF
According to the Warburg effect, the main source of energy for tumour cells is the glycolytic pathway, which also allows tumour cells to obtain energy mainly by consuming large amounts of glucose (Ref. Reference Vander Heiden, Cantley and Thompson45). Hence, inhibition of tumour cell glycolytic metabolism is one of the significant ways to achieve tumour starvation therapy. For example, Wu et al. rationally constructed a dual-gated controlled nano-energy circuit breaker, HZ@GD, which contains an MOF material, ZIF-8, glucose transporter protein 1 (GLUT1) DNAzyme (GD) and a tumour-targeting hydrophilic shell (hyaluronic acid, HA) (Ref. Reference Wu46). The ability of HZ@GD to actively target tumour cells resulted in accurate recombination and efficient endocytosis of nanoparticles (NPs), which led to the degradation of ZIF-8 in the acidic environment of tumours and the ability of ZIF-8, which consists of Zn2+ junctions and 2-MIM ligands, to release both Zn2+ and GD into the cytoplasm. The sudden elevation of Zn2+ achieves a tumour-specific ‘Zn2+ interference’ effect and then an effective blockade of glycolysis is achieved through the reduction of nicotinamide adenine dinucleotide (NAD+) and inactivation of glyceraldehyde-3-phosphate dehydrogenase (GAPDH) caused by the interference in Zn2+. At the same time, the ‘Zn2+ interference’ can activate the catalytic shear effect of GD, which reduces the expression of GLUT1 and cuts off the glucose supply and thereby achieves the systematic energy depletion in the tumour.
Without doubt, inhibition of the glycolytic pathway alone may not be sufficient, because tumour cells can vicariously metabolize other substances like lactate and glutamine as nutrients (Ref. Reference Vander Heiden, Cantley and Thompson45). Interestingly, Du et al. devised a functional MOF-based core-shell nanoreactor – PCP-MOF@G@B, which consisted of a ph-responsive separable copolymer shell, PEG-CDM-PEI (PCP) and a reactive oxygen species (ROS)-sensitive degradable substance, while the glucose oxidase (GOD or GOx) and glutaminase (GLS1) targeting inhibitor bis-2-(5-phenylacetamido-1,2,4-thiadiazol-2-yl) ethyl thioether (BPTES) were encapsulated inside (Ref. Reference Du47). In TME, PCP-MOF@G@B not only significantly breaks the biological barriers of tumour penetration and phagocytosis and improves the delivery efficiency, but also flexibly self-amplifies the degradation of MOF and release of cargoes and generates H2O2 on its own. It was demonstrated that PCP-MOF@G@B nanosystems synergistically cut off the energy supply to tumours by simultaneously restricting glycolysis and compensatory glutamine metabolic pathways, inducing significant mitochondrial damage and cell cycle arrest, which is highly effective in dual starvation therapy used for triple-negative breast cancer. Aside from that, Yu et al. synthesized a novel nanomedicine, CHC/GOx@ZIF-8, which is capable of removing lactic acid and glucose simultaneously by co-loading hydrophobic α-cyano-4-hydroxycinnamate (CHC) and hydrophilic GOx into ZIF-8 molecular sieves (Ref. Reference Yu43). CHC/GOx@ZIF-8 utilizes CHC to inhibit monocarboxylate transporter 1 (MCT1) mediated inward flow of lactic acid, which not only promotes energy shortage, but also blocks lactate-driven respiration and alleviates hypoxia in solid tumours to enhance catalytic activity of GOx, consume excess glucose and produce more cytotoxic H2O2 for enhanced tumour killing effect. By cellular and in vivo experiments, CHC/Gox@ZIF-8 could enhance the effect of starvation therapy by depriving lactate and glucose simultaneously.
In brief, these studies demonstrated that starvation therapy based on MOF can effectively cut off the energy supply of tumour cells and induce mitochondrial damage and cell cycle arrest by inhibiting glycolytic metabolism, compensatory glutamine metabolism and lactate metabolism pathways, thus achieving significant results in starvation therapy.
Combined treatment strategies for starvation therapy and other therapies based on MOF
The application of MOF-based nanoplatforms to a single starvation therapy strategy has demonstrated positive therapeutic effects, but single tumour therapy can have limitations after all. However, compared with a single treatment, if two or more treatments are integrated for synergistic oncology treatment, the cumulative therapeutic effect of ‘1 + 1 > 2’ can often be achieved, even the dosage of therapeutic drugs and side effects can be reduced (Ref. Reference Gao44). Fortunately, through the MOF-based nanoplatform, tumour starvation therapy can create a good synergistic therapeutic effect with a variety of other tumour treatments.
Combination of starvation therapy and bio-chemotherapy based on MOF
To start with, MOF can mediate the use of starvation therapy in combination with traditional chemotherapy (Fig. 2). For example, Chen et al. synthesized RHI-DOX-GOD@ZIF-90 MOF-based NPs by encapsulating the near-infrared fluorescent dye RhI and the anticancer drug adriamycin (DOX) within the framework of an MOF material, ZIF-90, then loading GOD onto the surface of ZIF-90 (Ref. Reference Chen48). Remarkably, RHI-DOX-GOD@ZIF-90 NPs are exclusively broken down in cancer cells due to their exceptional ATP-detection sensitivity and selectivity as well as the increased ATP level in cancer cells compared to normal cells. After degrading materials within cancer cells, RhI is released to provide significant NIR emission, achieving controlled drug delivery. Subsequently, DOX and GOD are released to kill cancer cells through chemicals and starvation mechanisms. Interestingly, Ke et al. also opt for synergistic starvation and chemotherapy using GOD and DOX. NP bioreactor RGD-mGZD was obtained by embedding GOD and DOX in tumour-targeting ligand (RGD)-modified red blood cell membranes (RBCm) camouflaged with metal-organic framework ZIF-8 (Ref. Reference Ke49). With its remarkable bio-interfacial properties, RGD-mGZD not only improves blood retention time through immune evasion properties, but also selectively targets tumour sites by specifically recognizing overexpressed integrin receptors on the tumour cell membranes. Once the bioreactor reaches the desired area, GOD rapidly depletes glucose and oxygen from the tumour, starving cancer cells and initiating potent starvation therapy. More importantly, the study reveals that the deterioration of the acidic microenvironment in the tumour region induces the breakdown of MOF structures, which triggers the release of DOX and enhances the effectiveness of chemotherapy. Similarly, Zhang et al. also developed an erythrocyte membrane (EM)-encapsulated MOF biomimetic nanoreactor, TGZ@em, which likewise chose ZIF-8 that decomposes under acidic condition but stabilizes under physiological condition as a nanocarrier to encapsulate GOx and the prodrug tirapazamine (TPZ) for starvation-activated colon cancer treatment (Ref. Reference Zhang50). Based on the bionic properties of EM, TGZ@em can effectively accumulate in tumour tissues with immune escape and prolong blood circulation. Afterwards, GOx in the nanoreactor effectively consumes endogenous glucose and O2 to starve tumour cells. Meanwhile, the aggravation of the hypoxic microenvironment within the tumour induced by the nanoreactor can convert the prodrug TPZ released by the nanoreactor in the acidic ‘lysostaphin/inclusion bodies’ environment into highly cytotoxic free radicals, which can enhance the chemotherapeutic effect of TPZ. In brief, all three nanomedicines have been demonstrated to achieve good synergistic therapeutic effects of chemotherapy and starvation therapy through in vivo and in vitro experiments.

Figure 2. (By FigDraw) (A) The types of bio-chemotherapy. (B) MOF-based nanoplatforms mediated starvation therapy combined with physical therapy to kill tumour cells.
Note: MOF, multifunctional metal-organic framework; CDT, chemodynamical therapy.
Fenton response (Fig. 3) based therapy (FBT) is an important pathway for CDT in recent years (Ref. Reference Ranji-Burachaloo51) and the process of utilizing GOx for starvation therapy happens to enhance FBT. For instance, Ranji-Burachaloo et al. prepared novel NPs, GOx&Hb@ZIF-8, by encapsulating GOx and haemoglobin Hb in pH-sensitive ZIF-8 NPs (Ref. Reference Ranji-Burachaloo51). In the micro acidic environment of cancer cells, GOx is released, which consumes D-glucose and O2 and produces gluconic acid and H2O2, respectively, to achieve starvation therapy. And the produced gluconic acid increases the acidity of the TME, leading to complete destruction of the NPs and increasing the release of Hb and GOx. In the presence of endogenous and produced H2O2, Fe2+ from the haeme group of haemoglobin is also released, undergoing the Fenton reaction, and producing ⋅OH. Reactive free radicals represented by ⋅OH can rapidly oxidize surrounding biomolecules in biological systems to treat cancer cells (Refs Reference Ghattavi and Nezamzadeh-Ejhieh52, Reference Mirsalari and Nezamzadeh-Ejhieh53, Reference Ghattavi and Nezamzadeh-Ejhieh54, Reference Rezaei and Nezamzadeh-Ejhieha55). The in vitro experiments demonstrated the cytotoxicity of this novel NP at very low concentrations (<2 μg/ml) against HeLa cervical cancer cell line and MCF-7 breast cancer cell line. Similarly, Zhou et al. developed a multifunctional nanoplatform, MGaV for efficient CDT and anti-angiogenesis, which was constructed using the bioconjugation of GOD and anti-vascular endothelial growth factor receptor-2 (anti-VEGFR2) on top of a peroxidase-mimicking Fe-MOF (Ref. Reference Zhou56). GOD catalyses intratumoural glucose decomposition and triggers tumour starvation, while providing abundant H2O2 as a substrate, which undergoes the Fenton reaction catalysed by Fe-MOF, generating enough highly ⋅OH to enhance CDT, instantly attacking the tumour vascular endothelium, and destroying existing blood vessels, while the anti-VEGFR2 directs the nano-heterotrophs to target the blood vessels and block the VEGF-VEGFR2 linkage to prevent angiogenesis. Results of in vitro and in vivo studies showed that the nanoplatform led to apoptosis and vascular disruption of tumour cells and promoted tumour regression. Apart from that, Wan et al. designed a cancer cell membrane-covered cascade nanoreactor, NMIL-100@GOx@C, based on Fe-metallic-organic skeleton and glucose oxidase modification, which used Fe-based MOF (NMIL-100) as a Fe source for FBT and a carrier for loading GOx, while it was encapsulated on the cancer cell membrane (Ref. Reference Wan57). The nanoreactors, benefiting from the homologous targeting and immune escape ability of the cell membrane, are more inclined to accumulate at the tumour site and are efficiently internalized by cancer cells. Once internalized, NMIL-100@GOx@C generates the following cascade of reactions: (i) high levels of glutathione (GSH) at the tumour site reduce Fe3+ in NMIL-100 to Fe2+, leading to structural collapse of NMIL-100 and complete release of Fe; (ii) GOx catalyses the oxidation of glucose to generate gluconic acid and H2O2, which depletes glucose at the tumour site, cutting off the nutrient supply for starvation therapy; (iii) Fe2+ undergoes a Fenton reaction with the H2O2 generated from the first two reactions to produce ⋅OH, leading to FBT treatment. In this way, this cancer cell membrane-camouflaged nanoreactor could achieve enhanced synergistic effects of tumour-targeted FBT and starvation therapy, thereby effectively inhibiting tumour growth in a more efficient and safer manner. Interestingly, Gao and Song et al. proposed a new strategy for the synthesis of porphyrin-based metal-organic compounds using Cu2O nano-cubes and Cu-TCPP nanosheets as a Cu source. These were utilized as a nanoplatform to prepare multifunctional nanomedicines by sequential loading and functionalization (Cu2O/Cu-TCPP/(Pt-Au)/FA) (Ref. Reference Gao58). Among them, Cu+ originating from the reduction of Cu2+ by Cu2O or GSH can trigger the Fenton reaction to generate ⋅OH for effective CDT. Then, Pt NPs with catalase (CAT) mimetic activity can catalyse the conversion of H2O2 to O2 in tumour cells, modulating the hypoxic atmosphere of the tumour and enhancing the O2-dependent glucose oxidation reaction. In addition, gold NPs with GOx mimetic activity can accelerate glucose consumption and cut off the nutrient supply for inducing starvation therapy while generating additional H2O2. And folic acid (FA) is incorporated to enhance drug targeting. This study demonstrates that multifunctional Cu2O/Cu-TCPP/(Pt-Au)/FA nanomedicines exhibit high specificity, significant activity, and negligible systemic toxicity. Importantly, they enable synergistic tumour starvation therapy with CDT, as evidenced by in vitro experiments.
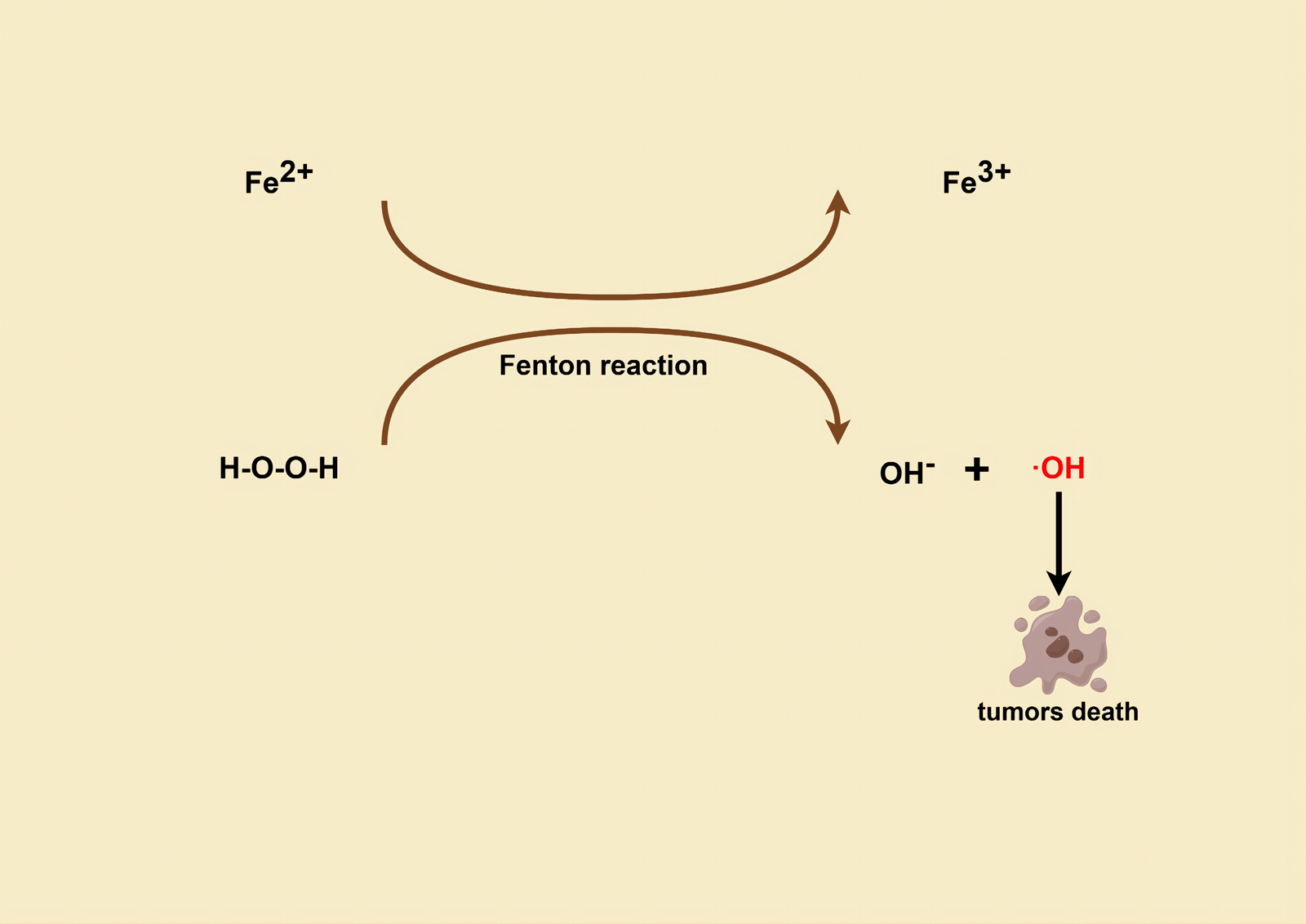
Figure 3. (By FigDraw) Mechanism of tumour cell killing with ⋅OH produced by the Fenton reaction.
Autophagy is a self-protection mechanism exerted by tumour cells under starvation stress, in which tumours phagocytose degraded and inactivated proteins, organelles and fragmented nuclei, etc. This mechanism is also a critical factor that reduces the efficacy of starvation therapy (Ref. Reference Yao59). Interestingly, studies have shown that over-activated autophagy not only improves the effectiveness of starvation therapy, but also leads to autophagic death. Yao et al. designed Cur@MOF-GOx/HA, a nano-enzyme that treats tumours by inducing autophagy hyperactivation (Ref. Reference Yao59). When the nano-enzymes were internalized by the tumour cells, the HA shell of the nano-enzymes was degraded by the HAase. Then, exposure to GOx triggers starvation while activating self-protective autophagy to resist the starvation treatment. Moreover, GOx successively catalysed the generation of H2O2 gluconic acid from glucose to feed the MOF-mediated Fenton reaction, while releasing a powerful autophagy inducer, curcumin (Cur), which converted GOx-induced pro-survival autophagy into pro-death autophagy, leading to autophagic death. Both in vivo and in vitro experiments showed that the nano-enzymes could manipulate tumour cells to produce cytotoxic ROS and autophagic cell death.
Interestingly, Wu et al. successfully synthesized a nanoreactor, MOF-Pt(IV)@GOx, which can synchronously perform iron oxidation, starvation therapy, and chemotherapy via a synergistic cascade reaction (Ref. Reference Wu60). High concentrations of GSH in the TME can decompose the MOF-Pt(IV)@GOx nanoreactor by reducing Fe(III) in the MOF centre and Pt(IV) in the grafted prodrug, resulting in the simultaneous release of Fe(II), cisplatin and GOx. Fe(II) is responsible for the generation of highly toxic ROS via the Fenton reaction, in which the required H2O2 is produced by GOx-catalysed glucose oxidation and cisplatin-mediated activation of NADPH oxidase (NOX). Glucose oxidation not only cuts off the glucose supply, starving the cancer cells, but also provides the acidic environment needed for the Fenton reaction. Moreover, cisplatin creates Pt-DNA adducts, hindering both DNA replication and transcription. Similarly, Li et al. have developed a Fe-doped ZIF-8 (Fe/Z) nano-delivery system, denoted as GLDFe/Z-FA, featuring synergistic effects and FA receptor targeting. This system involves the co-loading of GOx, L-arginine and DOX (Ref. Reference Li61). Notably, Fe/Z not only facilitates efficient loading of therapeutic agents but also provides protection for GOx from degradation by substances in the physiological environment, while FA imparts drug targeting capabilities. When the nano-systems are successfully internalized by tumour cells and disintegrate in the micro-acidic environment of the tumour, loaded drug molecules are released, triggering a cascade reaction. First, GOx consumes glucose to produce H+ and H2O2 in cancer cells, disrupting energy and nutrient supply and providing a substrate for the subsequent production of ⋅OH. Second, H2O2 catalyses the Fenton reaction to generate ⋅OH, leading to tumour CDT. Third, L-arginine is oxidized to produce NO, which serves as an intracellular signalling molecule at low concentrations and inducing cancer cell death at high concentrations. Both in vitro and in vivo experiments have shown that GLDFe/Z-FA can lead to glutathione peroxidase 4 (Gpx4) inactivation and lipid ROS accumulation, which ultimately results in mitochondrial dysfunction, cellular Fe death and nutrient-poor cancer cell death. Other than that, Liu et al. initially synthesized MOF(Fe) (NH2-MIL-101(Fe)) using 2-aminoterephthalic acid (BDC) and FeCl3, and then covalently modified the outer surface of MOF(Fe) with GOx to create MOF(Fe)-GOx. Afterwards, the drug CPT was loaded into the channels of MOF(Fe) to obtain CPT @MOF(Fe)-GOx (Ref. Reference Liu62), which can simultaneously deliver GOx, Fe3+ and drugs capable of starvation/ROS-mediated/chemotherapy in a cascade manner. Once the prepared CPT@MOF(Fe)-GOx particles enter the cancer cells, GOx catalyses glucose in the cytoplasm to gluconic acid (H+) and H2O2. The depletion of glucose starves cancer cells for starvation therapy. Next, the particles were degraded in the H+-enhanced acidic environment to release CPT and Fe3+ for tumour chemotherapy. Finally, the H2O2 produced in the initial step of the reaction is catalysed by Fe3+-activated ROS-mediated therapy into ⋅OH, inducing cancer cells death for CDT. Both in vitro and in vivo experiments demonstrated the outstanding therapeutic efficacy of CPT@MOF(Fe)-Gox, confirming the effectiveness of the cascade response in enabling starvation/ROS-mediated/chemotherapy.
The immune checkpoint protein indoleamine 2,3-dioxygenase (IDO), which is highly expressed in tumours, inhibits the proliferation of effector T cells by catalysing the generation of kynurenine (Kyn) from tryptophan (Trp) and induces the expansion of T regulatory (Treg) cells, thus becoming an attractive immunotherapeutic target for alleviating the immunosuppressive microenvironment (Ref. Reference Dai63). For example, Dai et al. constructed a pH/ROS dual-sensitive degradable MOF nanoreactor nano-system (denoted as PCP-Mn-DTA@Gox@1-MT) for tumour starvation/CDT/IDO blockade immunotherapy (Ref. Reference Dai63). The nano-system is rapidly decomposed in tumour cells triggered by abundant ROS and acidic environment to form polyethylene glycol imine(PEI)-coupled cationic nuclei, which significantly improves the depth of penetration and phagocytosis of the system into the tumours. Apart from this, the nano-system are further converted into highly toxic hydroxyl radicals (⋅OH) through Mn2+ mediated Fenton-like reactions. And the IDO-specific competitive inhibitor, 1-methyltryptophan (1-MT), contained within the nano-system can effectively alleviate immune evasion. By in vivo and in vitro experiments, the PCP-Mn-DTA@Gox@1-MT nano-system effectively inhibited tumour growth and metastasis with these mechanisms. Differently, Zheng et al. achieved immunotherapy from the perspective of tumour-associated macrophages (TAMs) (Ref. Reference Zheng64). They synthesized ROS-responsive degradable Fe-MOF using 2,2′-[propane-2,2-diylbis(thio)] diacetic acid (TK-COOH) as a ROS-sensitizing agent. Subsequently, GOx as an exogenous H2O2 supplier and starvation inducer and perfluorocarbon (PFC) as an oxygen supplier were co-loaded into Fe-MOF. Finally, the tumour-targeting molecule HA was introduced onto the surface of the loaded Fe-MOF to obtain the functional nano-system (HFNP@Gox@PFC) (Ref. Reference Zheng64). The nano-system offers several advantages: (i) it can specifically aggregate at tumour sites via the HA receptor-ligand-mediated targeted endocytosis pathway; (ii) its uptake by tumour cells can be catabolized in response to ROS in the tumour cell cytosol, leading to the release of PFC, GOx and Fe2+; (iii) PFC provides O2 and promotes the initiation of GOx-mediated catalytic reactions, while GOx can react competitively with intracellular glucose and O2 to starve tumours and produce abundant H2O2, which further facilitates its disassembly, drug release and oxidative tumour killing; (iv) cascade amplification of CDT by Fe2+ reaction with H2O2 induces effective tumour damage; (v) the ROS generated from the above reaction further enhance the tumour immune response by re-inducing TAMs through the activation of the nuclear factor NF-κB and mitogen-activated protein K signalling pathways, resulting in a better tumour therapeutic effect. In vitro and in vivo studies have shown that the nano-system combines starvation, CDT and immunotherapy with good stability, biocompatibility and anti-tumour effects.
From the above synergistic treatment of starvation therapy with various biochemical therapies we can see that starvation therapy plays an important role and mutually reinforces with various therapies in terms of therapeutic mechanisms. However, there is no specific synergistic treatment programme that has a clear advantage.
Combination of starvation therapy and physiotherapy based on MOF
Physical therapy mainly includes PDT, PTT and sonodynamic therapy (SDT) (Fig. 4).

Figure 4. (By FigDraw) (A) The types of physiotherapy. (B) MOF-based nanoplatforms mediated starvation therapy combined with physical therapy to kill tumour cells.
Note: MOF, multifunctional metal-organic framework; PDT, photodynamic therapy; PTT, photothermal therapy; SDT, sonodynamic therapy.
Starvation therapy + PDT. In 2017, Li et al. used porphyrin-based Zr-MOF, PCN-224, as a nano-sensitizer (PS) and as a carrier loaded with both GOx and CAT. Finally, PCN-224 loaded with GOx and CAT was further coated with a cancer cell membrane to obtain a cancer cell membrane camouflaged tandem bioreactor mem@catalase@GOx@PCN-224 (mCGP), which was used for tumour-targeted starvation therapy and PDT (Ref. Reference Li65). According to the study, mCGP will preferentially accumulate in tumour tissues and selectively internalize into cancer cells due to the immune escape and isotype targeting ability of cancer cell membranes. Besides, CAT not only regulates the hypoxic microenvironment of tumours by catalysing the catabolism of endogenous H2O2, but also reduces O2 consumption for glucose catabolism by GOx. In addition, the produced O2 catalysed by PCN-224 also promotes the production of highly toxic single-linear state oxygen (1O2), which will further enhance the therapeutic efficiency by synergizing starvation therapy and PDT. And in 2021, Liu et al. synthesized MOF core-shell nano-assemblies using poly(D,L-lactic-glycolic acid) (PLGA) and Fe3+, in which GOx and trichloroethane (Ce6) were introduced, to construct a MOF-based targeted nanoreactor, PTFCG@MH, with hyaluronic acid deposited on the surface HA-stabilized MnO2 (Ref. Reference Liu66). Because of its surface shape, the nanoreactor can target tumour cells specifically. Upon absorption, it initiates a series of processes that modulate the TME. Firstly, GOx depletes glucose, hindering ATP synthesis. Subsequently, MnO2 catalyses the generated H2O2 to produce O2, alleviating hypoxia and reducing photodynamic sensitization of tumours. Concurrently, MnO2 effectively depletes GSH to prevent the tumour from establishing an antioxidant defence. These two nano-systems have been demonstrated to exhibit a robust synergistic therapeutic effect involving PDT and starvation therapy, effectively inhibiting tumour growth without significant side effects.
Starvation therapy + PTT. In addition to PDT, the MOF nanoplatform also enables synergistic treatment with PTT and starvation therapy. For example, Gao and Shi with their team developed a MOF-based biomimetic nanoreactor, GOx-ICG@ZIF@CM, for tumour therapy by simultaneous inhibition of intracellular protective proteins and defence systems (Ref. Reference Gao67). To be specific, GOx and the photothermic agent indocyanine green (ICG) were loaded into MOFs, which were further camouflaged with homotypic cancer cell membranes for tumour targeting. Nanoreactor-mediated hyperthermia down-regulated intracellular CAT and MCT, while the starvation effect of GOx could effectively inhibit the expression of heat shock protein 90 (HSP90, a defence protein protecting intracellular proteins from thermal inactivation), further exacerbating the thermal vulnerability of intracellular CAT and MCT. These mechanisms ultimately synergistically inhibit the intracellular defence system, trigger cellular acidic stress and oxidative stress, activate the apoptotic cascade response and induce tumour apoptosis. This work explores the protective role of MOF against heat-inactivated enzymes for the first time, with the realization of synergistic treatment with starvation therapy and PTT. Later in 2021, Zhu et al. obtained a biomimetic multifunctional nanoreactor, ZGAM, by coating EM onto MOF-based molecular sieve imidazole skeleton-8 (ZIF-8) containing gold nanorods and GOx (Ref. Reference Zhu68). By camouflaging its surface with a red blood cell membrane, this nanoreactor can effectively accumulate in tumour tissue after intravenous injection without being recognized by the immune system. The gold NPs can trigger a photothermal effect when irradiated with near-infrared light, while the released GOx effectively consumes endogenous glucose, limiting the energy supply to tumour cells. Significantly, the expression of HSPs in tumour cells is inhibited in the absence of ATP and thereby the efficacy of PTT is enhanced. In vivo studies have shown that ZGAM has a significant therapeutic effect on HCT116 hormonal mice. Interestingly, Cheng et al. designed a biomimetic functional nanoplatform, PCoA@M, using macrophage membrane as a carrier in 2022 (Ref. Reference Cheng69). In this nanoplatform, polydopamine (PDA) was used as the core and Co-MOF as the shell layer to construct the multiphase structure, after which the precursor for H2S release, Anethole Trithione (ADT), was loaded into the pores of the MOF and the interstices of the heterostructures by a one-pot method. Finally, modification of macrophage membranes improves probe biocompatibility, reduces phagocytosis of the nanoplatform by immune cells, and achieves target recognition on tumour cells. The study found that after degradation of Co-MOF in the acidic TME, free Co2+ downregulated the expression of HSP90, which inhibited the heat resistance of HSP and enhanced the effect of PTT. In addition, a high concentration of H2S was released from the premedicine to kill the tumour. ADT also significantly alters the level of reduced coenzyme I (NADH) at the tumour site, affecting the NADH/flavin adenine dinucleotide (FAD) balance, which results in impaired ATP synthesis in the tumour cells and a reduction in the energy supply to the cells to achieve starvation therapy. In vivo experiments have demonstrated that the application of this nanoplatform can effectively enhance targeted PTT, synergistic gas starvation therapy and inhibit lung cancer cell metastasis.
Starvation therapy + SDT. SDT is also an important physical therapy for tumours and has recently been applied in synergy with starvation therapy. For instance, Bao et al. developed a MOF-based biomimetic nanocomposite-PPGE NCs for loading PCN-224 NPs and GOx as acoustic sensitizers (Ref. Reference Bao70). Among them, platinum nanoparticles (Pt NPs) on PCN-224 facilitated the catalytic conversion of overexpressed H2O2 to oxygen at the tumour site, combined with ultrasound (US) irradiation to generate highly toxic ROS via SDT. Simultaneously, GOx can effectively reduce the glucose content at the tumour site and locally generated additional H2O2 can be further utilized by Pt NPs and through a cascade reaction to regenerate O2, which in turn enhances SDT, thereby realizing the combined treatment of starvation therapy with SDT. In addition, the EM artefacts on the surface of PCN-224 carriers could enhance their biocompatibility. In vitro and in vivo studies have shown that PPGE NCS can result in an increase in apoptosis and a decrease in the proliferation rate of tumour cells. Interestingly, Zhou et al. reported that they constructed adaptive acoustic-sensitive platelet (PLT) cassettes to mediate the cascade delivery engineering of porphyrin MOF nanostructures loaded with the selective amino acid transporter protein blocker α-methyl-DL-tryptophan (α-MT) and overlaid with MnO2 unblocking layers to obtain new integrated bio-nanoplatforms (denoted as αMM@PLT) (Ref. Reference Zhou71). When αMM@PLT reaches the tumour site, US stimulates ROS production from porphyrin MOFs loaded with PLTs, leading to morphology changes of PLTs and release of NPs. Subsequently, the outer MnO2 layer is broken down by excess GSH in the TME, which triggers the release of encapsulated α-MT, thereby preventing glutamine addition and interfering with glutamine metabolism. The research revealed that US stimulation mediated the transformation of de-extended pseudopods of circulating PLT into dendritic PLT and thrombus formation, effectively blocking the blood supply to the tumour. Simultaneously, MnO2-induced depletion of intracellular glutathione and α-MT-induced lack of raw materials for glutathione synthesis resulted in a significant increase in the sensitivity of stereotactic US treatment of tumours to achieve a synergistic treatment with SDT and starvation therapy.
According to Table 1, it can be observed that the experiments in which starvation therapy was synergistically treated with PTT generally achieved better tumour suppression rates, which may be attributed to the fact that the two are mutually reinforcing in terms of therapeutic mechanisms. In contrast, the effect of starvation therapy with PDT or SDT that mutually promotes each other in the mechanism may not be obvious, which leads to the fact that its efficacy may not be as desirable as that of the former.
Diversified therapy with starvation therapy as one of the adjuvant therapies based on MOF
MOF can also provide diversified therapies, which means that starvation therapy, biochemistry therapy and physical therapy can be realized simultaneously on a single nanoplatform (Fig. 5). Currently, Yin et al. developed an MOF-based cascade biomimetic therapeutic nanoplatform, C1@M@C2G, by utilizing Fe-based porphyrin MOF wrapped with bis(2-carbocyclooxy-35,6-trichlorophenyl) oxalate (CPPO), GOD and further encapsulated with a cancer cell membrane on the outside, which could simultaneously realize chemical light-induced photodynamic therapy, Fenton-responsive CDT-based and GOD-mediated starvation therapy to synergistically enhance cancer treatment (Ref. Reference Yin72). The cancer cell membrane encapsulated on the NPs enables the nanoplatform to accumulate at the tumour site in a targeted manner. Local glucose is then consumed by GOD-catalysed oxidation reaction to activate starvation therapy, while the produced gluconic acid and H2O2 can cause a significant decrease in the pH of the TME. Subsequently, additional elevated H2O2 undergoes a low pH-accelerated Fenton reaction, converting it into OH for CDT. Meanwhile, some other hydrogen peroxide can react with CPPO to form a high-energy state that excites the porphyrin photosensitizer, leading to in situ generation of 1O2 for PDT. Moreover, the nanoplatform featuring CAT activity continuously generates O2, effectively mitigating tumour hypoxia, thus enhancing the catalytic effect of GOD and the therapeutic efficacy of PDT to achieve diversified combined therapy. Recently, Wang et al. developed a multifunctional therapeutic platform integrating CDT, radiation therapy, starvation therapy, gas therapy, magnetic resonance imaging and chemotherapy, D-Arg/GOX/TPZ@MOF (Fe)-PDA/Fe3+/FA-BSA (Ref. Reference Wang73). The obtained NPs demonstrated the capability to undergo a Fenton reaction, utilizing Fe3+ to produce reactive oxygen species for CDT. Additionally, GOD effectively depleted glucose, the energy source for starvation therapy, while D-Arg produced NO to alleviate tumour hypoxia and promote radio-sensitization. Apart from that, these combined therapies, along with TPZ, down-regulated hypoxia-inducible factor, vascular endothelial growth factor, platelet endothelial cell adhesion molecule-1 and Ki67, up-regulated histone γ-H2AX expression, as well as reduce non-targeted side-effects on healthy tissues. More importantly, the NPs exhibited T1-weighted MRI contrast properties, enabling rapid and efficient MRI imaging, while maintaining an optimal effective X-ray dose. The results of the in vitro and in vivo studies suggest that the NPs can control tumour volume to a greater extent and reduce damage to healthy cells through diversified combined therapy.

Figure 5. (By FigDraw) MOF-based nanoplatforms mediated diversified therapies to kill tumour cells.
Note: MOF, multifunctional metal-organic framework.
According to Table 1, it is evident that the tumour inhibition rate of diversified therapies including starvation therapy mediated by MOF nanoplatforms is not high, which may indicate that although multiple therapeutic overlays can be achieved at the same time by MOF, their efficacy may not necessarily be significantly enhanced.
Current challenges and drawbacks
Significantly, many studies, with a few exceptions utilizing PLT, anti-VEGFR2, etc., predominantly relied on glucose oxidase (GOD or GOx) for starvation therapy, acknowledging its inherent advantages. Yet, it is a crucial point to consider whether better bio-enzymes or agents other than GOD may be discovered to carry out starvation therapy. Furthermore, there are a few disadvantages to MOF-based nanoplatforms. For instance: (i) MOFs must be prepared under extremely strict guidelines and take a long time to prepare (Ref. Reference Chen74). As a result, lowering the cost of MOF preparation and subsequently lowering the cost of any medications that may be used with them is a significant challenge. (ii) MOF has been shown to have water instability, which may lead to early dissociation of its backbone structure before it can be useful, thereby affecting its targeting and biosafety (Ref. Reference Guo75). Moreover, a lot of research has been done to lower the environmental emissions of nanomedicines to lessen pollution (Refs Reference Arabpour and Nezamzadeh-Ejhieh76, Reference Foroughipour and Nezamzadeh-Ejhieh77, Reference Mohammadyari and Nezamzadeh-Ejhieh78, Reference Rahmani-Aliabadi and Nezamzadeh-Ejhieh79, Reference Saberian and Nezamzadeh-Ejhieh80, Reference Salesi and Nezamzadeh-Ejhieh81, Reference Soleimani and Nezamzadeh-Ejhieh82, Reference Vahabirad, Nezamzadeh-Ejhieh and Mirmohammadi83, Reference Yousefi and Nezamzadeh-Ejhieh84). Nevertheless, no studies have been done to examine the effects of MOF-related medications on the environment, so it is possible that the metabolized emissions of MOF-based nano-drug delivery systems will have a negative environmental impact.
Conclusion and outlook
In summary, we have outlined the research progress in the field of cancer starvation therapy using MOF-based nanoplatforms, encompassing both the standalone application of starvation therapy and its synergistic combination with other therapeutic modalities. As highlighted, researchers widely favour starvation therapy for its advantages, such as low side effects and non-invasiveness. MOF-based nanoplatforms offer a robust foundation for the precise implementation of starvation therapy, benefitting from their diverse compositions, versatile structures, facile functionalization, and excellent biocompatibility. To date, numerous researchers have devised and developed smart MOF-based nanoplatforms, catering to the realization of cancer starvation therapy, exploring single treatment strategies and synergistic approaches combining starvation therapy with other therapeutic interventions.
According to our summary, the prevalent use of intravenous administration in numerous experiments prompts researchers to considerate if intravenous administration is always better than other administrations or if we can develop an entirely new method in the future. Furthermore, while these studies primarily advanced to the stage of animal experiments, and in some instances lacked them, a crucial future avenue involves extensive exploration of the efficacy, side effects, etc., of MOF nanoplatform-based starvation therapeutic drugs in human clinical settings. Moreover, it is evident that the tumour inhibition rate with bionic nanoplatforms is generally higher. Therefore, we assume that once MOF nanoplatforms have been synthesized and one of the three substances – red blood cell membranes, cancer cell membranes or macrophage membranes – has been post-synthesized to modified to create bionic nanoplatforms, it will be possible to assist MOFs in overcoming their water instability and enhancing their therapeutic efficacy in the future. In addition, it is necessary to carry out experiments on the environmental impact of MOF emissions in the future, which will be beneficial in reducing pollution.
To sum up, MOF-based nanoplatforms in cancer starvation therapy have marked significant progress, notwithstanding the challenges outlined. It is anticipated that ongoing advancements in MOF-based nanoplatforms will deliver breakthroughs in cancer starvation therapy, offering promising prospects for clinical cancer treatment.
Data availability
No data were used for the research described in the article.
Author contributions
First, Jinghan Cai and Fei Liao conceived the idea for this review. Moreover, Jinghan Cai performed literature searches and drafted the manuscript, who also created the figures and tables. Finally, Fei Liao and Yan Xu assisted in improving the manuscript.
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Competing interests
All authors read and approved the final manuscript. They declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.









