Long chain omega-3 polyunsaturated fatty acids (LC3PUFA) have been linked to healthy aging, health promotion and disease reduction throughout the life cycle(Reference Swanson, Block and Mousa1; Reference Shahidi2). Dietary surveys indicate that LC3PUFA are currently under consumed, particularly amongst vegetarians/vegans, adult men, pregnant/breast feeding women, infants, non-fish eaters and certain ethnic groups(Reference Bates, Lennox and Prentice3; Reference Ryan and Symington4; Reference Sanders5). New vegetarian food vehicles such as LC3PUFA micro algal oils have been developed to address this issue(Reference Ryan and Symington4).
Nanoemulsions are systems with droplet sizes in range of 20 to 500 nm(Reference Solans and Solé6). The incorporation of algal oil into foods using nanoemulsions has the potential to improve LC3PUFA bioavailability(Reference Lane, Li and Smith7). However, the creation of nanoemulsions may also affect the oxidation stability of LC3PUFA due to their high droplet surface areas(Reference Walker, Decker and McClements8).
The aim of the present study was to analyse the oxidation stability of an algal oil rich in docosahexaenoic acid (22:6 n−3; DHA) that provides a suitable alternative to fish oil(Reference Ryan and Symington4).
Peroxide value (PV) and anisidine value (AV) measurements were conducted to compare bulk oil (B), coarse emulsion (C) and nanoemulsions (N) stabilised with sunflower lecithin over a storage period of 37 days at temperatures of 4, 20 and 40 °C. Total oxidation values (TV) were then calculated by TV = 2PV + AV. Results were analysed using one and two-factor repeated measures ANOVA tests with Tukey and Duncan's tests and a Bonferroni correction at 5 per cent.
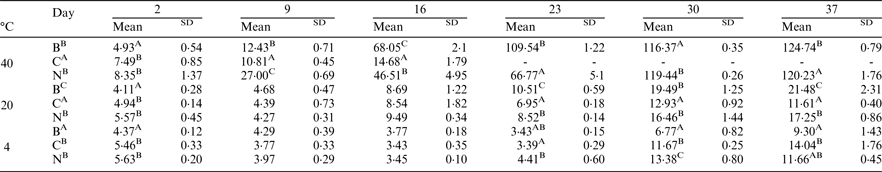
Different letters in sample and day columns represent statistically significant differences between samples or storage points. Significance is reported at P < 0·05 for each letter.
Increased storage and temperatures had a significant effect on oxidation stability for all samples (P < 0·05). For samples stored at 4 °C the bulk sample was significantly more stable to oxidation than the coarse emulsion and nanoemulsion samples (P < 0·05), however all samples remained within safe ranges at 4 °C (Reference Decker, Elias and McClements9). Further research is now warranted to investigate the oxidation stability of vegetarian LC3PUFA nanoemulsions using more sophisticated measures such as gas chromatography head space analysis. This will enable the analysis of volatile headspace compounds to further assess the impact of emulsion droplet sizes and food enrichment matrixes on oxidation stability.




