INTRODUCTION
The UK is a low endemicity country with regard to hepatitis A [1] and has a low incidence; between 1998 and 2007 the peak incidence was 4/100 000 per annum in those aged between 20 and 25 years and was shown to decline with increasing age [2]. As a result the sero-prevalence is low rendering the general population highly susceptible to infection [Reference Morris3]. With such a high level of susceptibility outbreaks can readily occur and the burden of disease is high as the symptoms and severity of the disease are generally worse with increasing age with 75–90% of adults presenting with clinical jaundice [1]. Immunization is recommended for at-risk groups such as travellers to areas of high or intermediate prevalence, patients with chronic liver disease, patients with haemophilia, men who have sex with men, injecting drug users and individuals at occupational risk [4]. Although onward transmission of hepatitis A can be prevented by good hygiene and immunization [4] it would seem prudent to limit introduction of hepatitis A virus (HAV) into the UK.
Recently, the Health Protection Agency (HPA) has reported that the number of travel-related hepatitis A cases is decreasing in the UK [5]; this is in part due to the decreasing completeness of reporting of travel history [2]. In 1998, 131 (11% of the total) cases of hepatitis A were reported to be associated with travel abroad compared to 21 (5%) in 2006 and 15 (4%) in 2007. Outbreaks linked to importation from abroad have posed public health challenges in the UK in recent time [Reference Edelstein6, Reference Stefcu7]. Introduction of the virus into a susceptible populations is normally via a ‘seeding’ event which is often a traveller returning from an endemic area. In The Netherlands and Sweden annual epidemics and outbreaks have coincided with children of non-Dutch/Swedish origin returning from visiting their native countries [Reference van Gorkom8, Reference Christenson9]. Children are an important factor in transmission of infection within the households and community outbreaks as 80–95% of them are asymptomatic [1] and they may also continue to excrete the virus for longer than an adult [Reference Robertson10].
In 2007, 18% of all foreign travel by UK residents was to visit friends and relatives (VFR). A significant number of these visitors travelled to countries with a high prevalence of tropical diseases [5]. The majority of the hepatitis A cases were reported in people of South Asian origin and travel to the Indian subcontinent was an important risk factor [5, Reference Gungabissoon, Andrews and Crowcroft11]. Here we describe an outbreak of hepatitis A in an extended family after a visit to Pakistan which re-emphasizes the importance of correct preventive measures including immunization to prevent travel-associated hepatitis A infection.
Outbreak description
All cases were reported to the Thames Valley Health Protection Unit (TVHPU) in South East England. The TVHPU is responsible for the management of outbreaks of communicable disease within the count-ies of Berkshire, Buckinghamshire and Oxfordshire.
On 11 September 2008 a case of hepatitis A was diagnosed in a 29-year-old female after presenting with a flu-like illness and jaundice on 10 September (case 1). She had returned to the UK on 3 August 2008 after visiting Pakistan. Case 2 was diagnosed with hepatitis A on 18 September after presenting with jaundice; he was an 18-year-old male who was a brother of case 1. The cases had both visited Pakistan at the same time for a series of family weddings and returned to the UK on 3 August. At this time cases 1 and 2 were dealt with on an individual basis and immunization was offered to their immediate household contacts.
The investigation of an outbreak was initiated following notification of a third case (index case) that was part of the same family. The index case was a 23-year-old female and was a cousin of cases 1 and 2; she was diagnosed with hepatitis A on 3 November 2008 after presenting with diarrhoea, vomiting and jaundice. On 5 November 2008 the sister-in-law of the index case, a 22-year-old female, presented with jaundice and was diagnosed with hepatitis A (case 4). The index case and case 4 had also attended the same series of family weddings in Pakistan but as they both became symptomatic over 11 weeks after returning from Pakistan it was considered unlikely that they had acquired their infections in Pakistan or directly from cases 1 or 2. After notification of the index case a risk assessment was performed and the entire extended family was offered immunization.
This extended family consisted of 19 adults and 12 children living in six separate households in Buckinghamshire. The households were all within a few miles of each other and were all registered with the same General Practitioner (GP). There had been a lot of social interactions within the extended family while in Pakistan and on their return to the UK as a result of the three weddings in the family. The index case and her three sisters were very close and they frequently visited each other to help each other out. In addition, their mother had died in October 2008 which increased interaction between them and other family members supporting each other during the mourning period. During the index case's illness the sisters all cared for her in her room and spent time together and shared food there. Sharing of food was a common activity for the sisters and the index case had cooked food for her sisters at their houses before she became symptomatic. None of the family members had any history of immunization against hepatitis A prior to the outbreak.
Epidemiological investigation
For the purpose of investigation a confirmed case was defined as a person whose serum was positive for IgM anti-HAV, who had either attended the weddings or was exposed to a confirmed case related to the weddings. A probable case was defined as a contact of a confirmed case with clinical symptoms. A contact was defined as household member or visitor who shared food and/or stayed overnight at a home of a case. Contacts were identified through interviewing confirmed cases and liaising with their GP.
Microbiological investigation of the cases
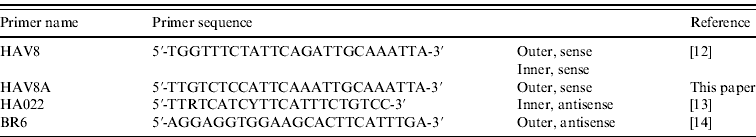
Initial blood samples were collected by the patient's GP and were tested for anti-HAV IgM at Stoke Mandeville Hospital (Buckinghamshire). These samples were forwarded to the Virus Reference Department of the HPA for HAV RNA detection and genotyping. Nucleic acid was extracted from 200 μl serum using the QIAamp Ultrasens Virus kit (Qiagen, UK) followed by generation of cDNA by random hexamers. A 445-bp fragment covering the VP1/2PA junction was amplified by a hemi-nested PCR using the primers detailed in Table 1. PCR products were sequenced and genotype assignment was preformed by alignment and comparison with sequences of known genotype in MegAlign (DNASTAR, USA) followed by confirmation using BLAST (http://www.ncbi.nlm.nih.gov/blast/).
Table 1. Primers used to amplify the VP1/2PA junction of hepatitis A virus
Anti-HAV IgM and HAV RNA detection from dried blood spots (DBS)
DBS taken by finger prick were used to investigate some of the children within the family. DBS testing for HAV infection was chosen as it is less invasive than taking a venous blood sample and there was no rapid access to salivary antibody testing. DBS sampling also has the advantage of being suitable for HAV RNA detection. In addition viral RNA would be present in plasma before anti-HAV IgM during the incubation phase of the virus. Anti-HAV and HAV RNA were tested using 6-mm DBS spots as previously described [Reference McFarland15]. In brief, HAV IgM was tested on 20 μl DBS eluate the Bioelisa HAV IgM kit (Biokit, UK) and nucleic acid was extracted from DBS which had undergone pre-digestion in ATL buffer with proteinase K (Qiagen) prior to extraction followed by detection of HAV RNA using the Artus HAV RT–PCR kit (Qiagen) which has a sensitivity of 56 IU/ml. HAV RNA-positive DBS eluates were amplified and sequenced where possible.
RESULTS
Between August and November 2008 a total of nine hepatitis A infections were identified within this extended family with two additional probable cases. The four adult cases all presented to their GP with clinical symptoms including jaundice. The remaining five cases were in children who were tested for anti-HAV IgM and HAV RNA using DBS: three children were found to be anti-HAV IgM positive in the absence of HAV RNA indicating a recent resolved HAV infection, one child was found to be anti-HAV IgM positive and HAV RNA positive indicating current HAV infection and one child was found to be anti-HAV IgM negative but HAV RNA positive indicating that the child was incubating the virus at the time DBS sampling was performed. Sequencing of the HAV RNA-positive samples (four patients from serum and one patient from DBS) showed that all individuals had been infected by the same HAV strain. This was a genotype IIIA virus (Fig. 1) unlike any HAV isolate previously sequenced at the Centre for Infections, London, and served to link all cases. One HAV RNA-positive DBS sample could not be amplified as the RNA was at too low a level.
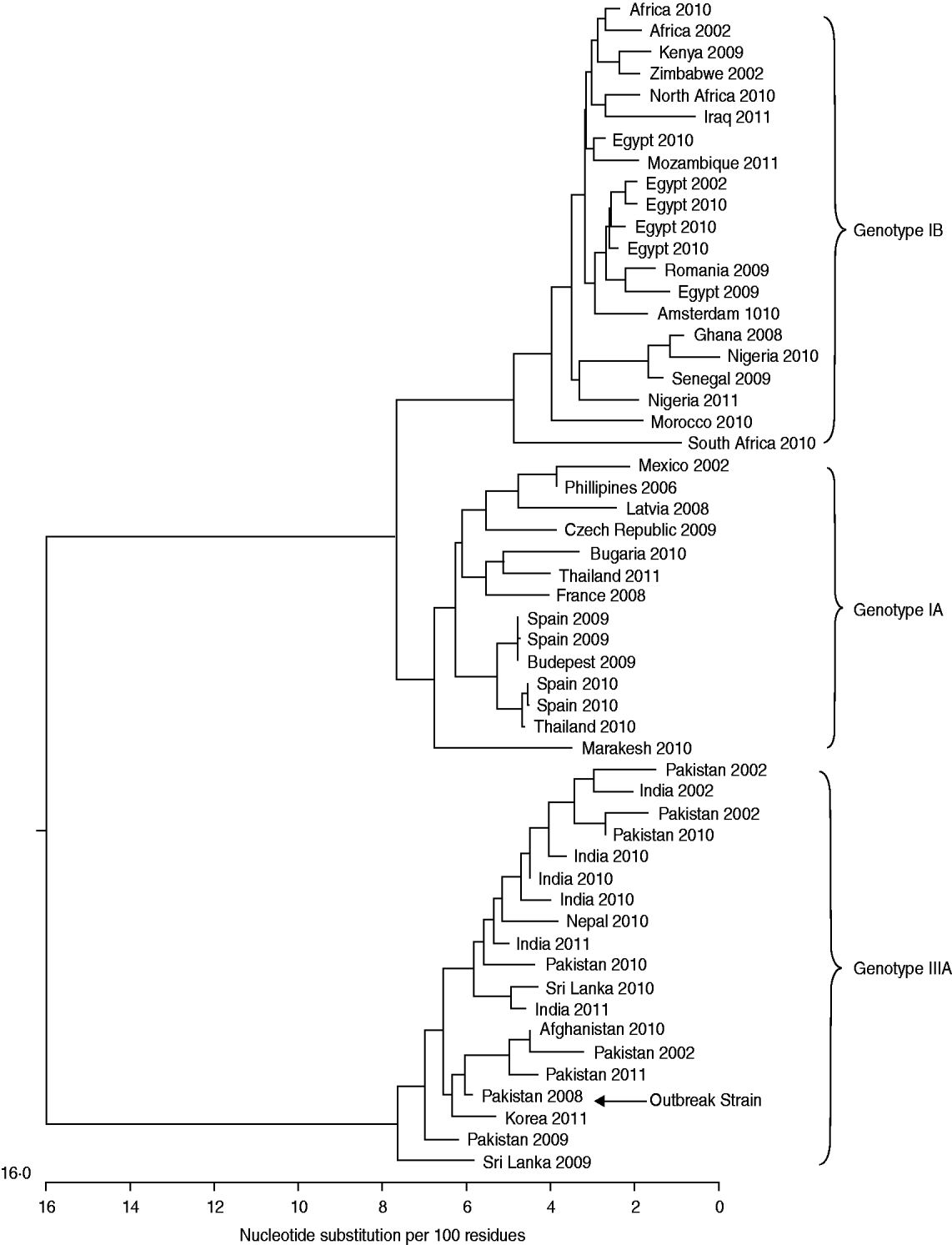
Fig. 1. Dendrogram featuring HAV sequences from patients with known travel history and year of infection.
Hepatitis A immunization was offered to 27 family contacts. The uptake of immunization was 100%. Another arm of the family (household G) was subsequently identified which had five children but as this was outside the 7 days after contact with a case they were given human normal immunoglobulin (HNIG) in accordance with current guidelines [4]. Individuals were not screened for immunity prior to immunization. No contacts were identified outside of the family and there were no other cases in the community. Figure 2 illustrates the chronology of the outbreak and immunization.
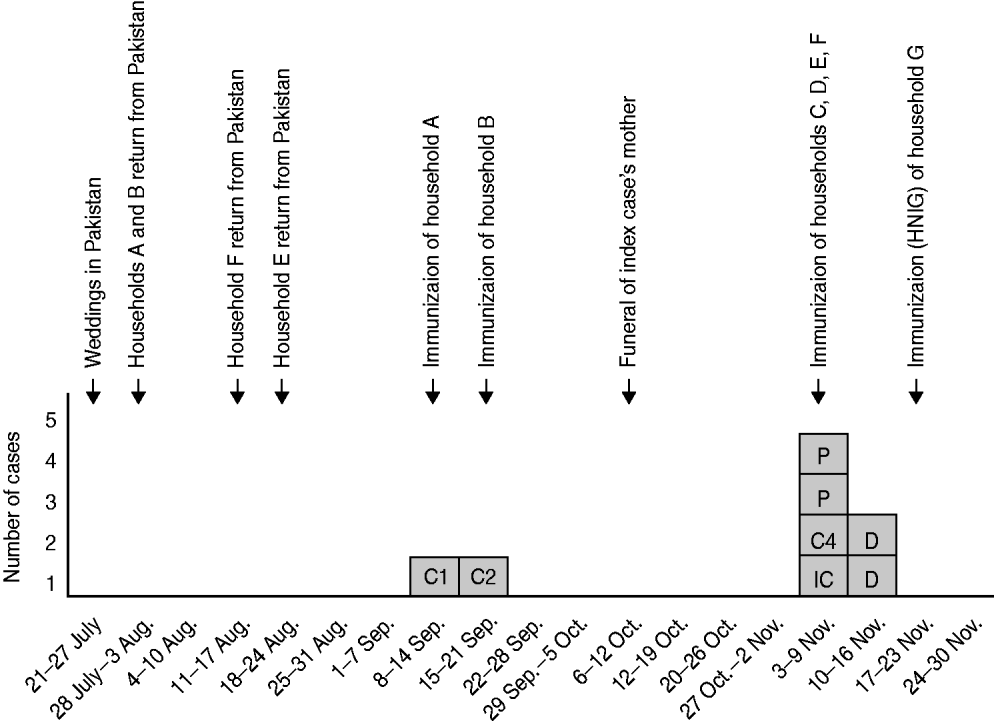
Fig. 2. Diagram illustrating the chronology of the outbreak and immunizations. IC, Index case; C, case; P, probable case; D, current/incubating infection identified by dried blood spot testing; HNIG, human normal immunoglobulin.
DISCUSSION
The investigation into this outbreak was initiated when a third adult, closely followed by a fourth adult, from the same extended family, presented with hepatitis A infection. These two cases presented more than 11 weeks after returning from Pakistan and 6 weeks after the first two cases which is outside the inferred incubation period for them to have become infected while abroad or from contact with the first two cases. The genotype of the virus that caused this outbreak was type IIIA which is consistent with reports of the virus being imported into Europe from Pakistan [Reference Tjon16, Reference van Steenbergen17] and from our own findings (Fig. 1). Genotype IIIA is common in South Asia but can be indigenous in parts of Europe [Reference Pérez-Sautu18]. Cases 1 and 2 were initially assumed to have acquired their infections while in Pakistan, as they became symptomatic 38 days and 46 days, respectively, after returning to the UK. These incubation periods are at the extreme end of the spectrum, the average incubation period is 28 days (range 15–50 days) and peak excretion of the virus occurs in the 2 weeks preceding jaundice [2], therefore it is plausible that the outbreak was caused by importation of the virus by an asymptomatic child and followed by asymptomatic spread within the children after their return to the UK making cases 1 and 2 secondary infections acquired in the UK. This is evidenced by the screening of some of the children within the family by DBS which uncovered three recent, one ongoing and one incubating HAV infection. The three recent and one current infection were all asymptomatic; however, the child who was incubating the infection at the time of testing developed symptoms 15 days after he was found to be HAV RNA positive by DBS, he was admitted to A&E and confirmed to have anti-HAV IgM and he had been immunized.
To cover the time that elapsed between the two reported pairs of adult cases the infection of the children would have had to have been sequential rather than simultaneous infections. The data suggest that the virus was passed by the young children from household A to the other households, as illustrated in Figure 3. Households C, D and E were most likely affected before household F (home of the index case) as these households had children who had already recently cleared their infections and/or probable cases prior to the index case becoming ill. It has been reported that in a normal course of infection viraemia is cleared within 3 weeks of onset [Reference Sagnelli19], if this is the case then one or all of the three children who had cleared their infection at the time of testing (week 46) could still have been infectious up to week 42 of 2008. This suggests how transmission between the children of the family maintained the virus within the family long enough for the index case and case 4 to become infected. Outbreaks are known to be associated with children returning from visiting family in endemic countries [Reference van Gorkom8, Reference Christenson9] and it is not uncommon for asymptomatic spread between children to be the cause of lengthy community outbreaks [Reference Staes20, Reference Smith21].

Fig. 3. Diagram illustrating the make up of the households within the extended family and how hepatitis A virus (HAV) may have been transmitted between them. * Child tested by dried blood spot. Underlining denotes an asymptomatic, recent past HAV infection; ‡, asymptomatic, current HAV infection; † incubating HAV infection, child symptomatic 15 days later; • probable case.
In many countries including the UK [4, Reference Ward22] immunization guidelines recommend hepatitis A immunization for persons travelling to endemic areas. However, this recommendation is often not followed [Reference Couturier23]. There could be a number of possible reasons for this. First, there is confusion about the need for payment for hepatitis A immunization. Although hepatitis A vaccine is free to those who travel to endemic countries, there is anecdotal evidence that there may be some degree of confusion among GP practices. The price of hepatitis A immunization can be as much as £55 per dose or £110 per course; a full course of vaccine for each member of this family could have been as much as £4180 collectively. Second, travellers might not perceive the risk especially those born in or who spent their childhood in endemic areas and then moved to UK might think that they are immune to hepatitis A. Travellers may not visit a travel health clinic or GP to get travel health advice because they are travelling ‘home’ and it may not occur to them that there may be susceptible people among those travelling. It has been reported that only one-third of travellers going abroad, especially to developing countries, obtain travel health information [Reference Heudorf, Tiarks-Jungk and Stark24]. Third, the vaccine is recommended preferably 2 weeks prior to travel [4]. It is possible that people may think after this time it is too late to be immunized even though immunization is still effective even if given as late as the day of travel [Reference Conner25]. Last, increasing numbers of travellers use the internet to plan and book their holidays [Reference Ward22]; thereby missing the opportunity to receive any travel health advice including the need for hepatitis A immunization either from a healthcare professional or travel health agent. With regard to this extended family, none of the households with cases perceived that there was a risk of contracting hepatitis A as they were visiting friends and relatives and had been doing so for many years. All the cases were born and raised in the UK and none of them sought pre-travel health advice before travelling to Pakistan.
CONCLUSION
This study is a good example of an outbreak of hepatitis A caused by importation from abroad followed by secondary spread on return to the UK, most of which could have been prevented by immunization before travelling abroad. Uptake of immunization was 100% in identified contacts which suggests that immunization (vaccine or HNIG) is acceptable, and can be rapidly arranged by liaising with GPs. DBS testing was a useful tool for investigating this outbreak and was found to be more acceptable to parents than taking blood samples from children by venesection.
It is clear that a mixture of common and innovative approaches at local, regional and national levels is needed to enhance hepatitis A immunization uptake in travellers to endemic countries, particularly children. There will always be challenges in promoting uptake in travellers, although the focus should not just be on travellers themselves but also on first-and second-generation migrants. GPs could easily identify such individuals and could proactively offer immunization in anticipation of them travelling ‘home’ at some point to visit friends and family rather than relying on them to seek travel health advice prior to a trip. In addition local Health Protection Units (HPUs) and the Primary Care Trusts (PCTs) should take proactive measures to better educate their populations about the risk of hepatitis A infection associated with travelling to endemic countries and the benefit of pre-travel hepatitis A vaccination.
DECLARATION OF INTEREST
None.








