145 results
The Crusades, the Latin East and Medieval History-Writing: An Introduction
-
-
- Book:
- Crusade, Settlement and Historical Writing in the Latin East and Latin West, c. 1100-c. 1300
- Published by:
- Boydell & Brewer
- Published online:
- 22 February 2024
- Print publication:
- 02 January 2024, pp 1-33
-
- Chapter
- Export citation
List of Illustrations
-
- Book:
- Crusade, Settlement and Historical Writing in the Latin East and Latin West, c. 1100-c. 1300
- Published by:
- Boydell & Brewer
- Published online:
- 22 February 2024
- Print publication:
- 02 January 2024, pp vii-vii
-
- Chapter
- Export citation
Notes on Contributors
-
- Book:
- Crusade, Settlement and Historical Writing in the Latin East and Latin West, c. 1100-c. 1300
- Published by:
- Boydell & Brewer
- Published online:
- 22 February 2024
- Print publication:
- 02 January 2024, pp ix-xii
-
- Chapter
- Export citation
Frontmatter
-
- Book:
- Crusade, Settlement and Historical Writing in the Latin East and Latin West, c. 1100-c. 1300
- Published by:
- Boydell & Brewer
- Published online:
- 22 February 2024
- Print publication:
- 02 January 2024, pp i-iv
-
- Chapter
- Export citation

Crusade, Settlement and Historical Writing in the Latin East and Latin West, c. 1100-c. 1300
-
- Published by:
- Boydell & Brewer
- Published online:
- 22 February 2024
- Print publication:
- 02 January 2024
List of Abbreviations
-
- Book:
- Crusade, Settlement and Historical Writing in the Latin East and Latin West, c. 1100-c. 1300
- Published by:
- Boydell & Brewer
- Published online:
- 22 February 2024
- Print publication:
- 02 January 2024, pp xiii-xiv
-
- Chapter
- Export citation
Acknowledgements
-
- Book:
- Crusade, Settlement and Historical Writing in the Latin East and Latin West, c. 1100-c. 1300
- Published by:
- Boydell & Brewer
- Published online:
- 22 February 2024
- Print publication:
- 02 January 2024, pp viii-viii
-
- Chapter
- Export citation
Index
-
- Book:
- Crusade, Settlement and Historical Writing in the Latin East and Latin West, c. 1100-c. 1300
- Published by:
- Boydell & Brewer
- Published online:
- 22 February 2024
- Print publication:
- 02 January 2024, pp 289-298
-
- Chapter
- Export citation
Contents
-
- Book:
- Crusade, Settlement and Historical Writing in the Latin East and Latin West, c. 1100-c. 1300
- Published by:
- Boydell & Brewer
- Published online:
- 22 February 2024
- Print publication:
- 02 January 2024, pp v-vi
-
- Chapter
- Export citation
Iodine intakes in school age girls aged 5–18 years in Ireland
-
- Journal:
- Proceedings of the Nutrition Society / Volume 82 / Issue OCE4 / 2023
- Published online by Cambridge University Press:
- 05 September 2023, E272
-
- Article
-
- You have access
- HTML
- Export citation
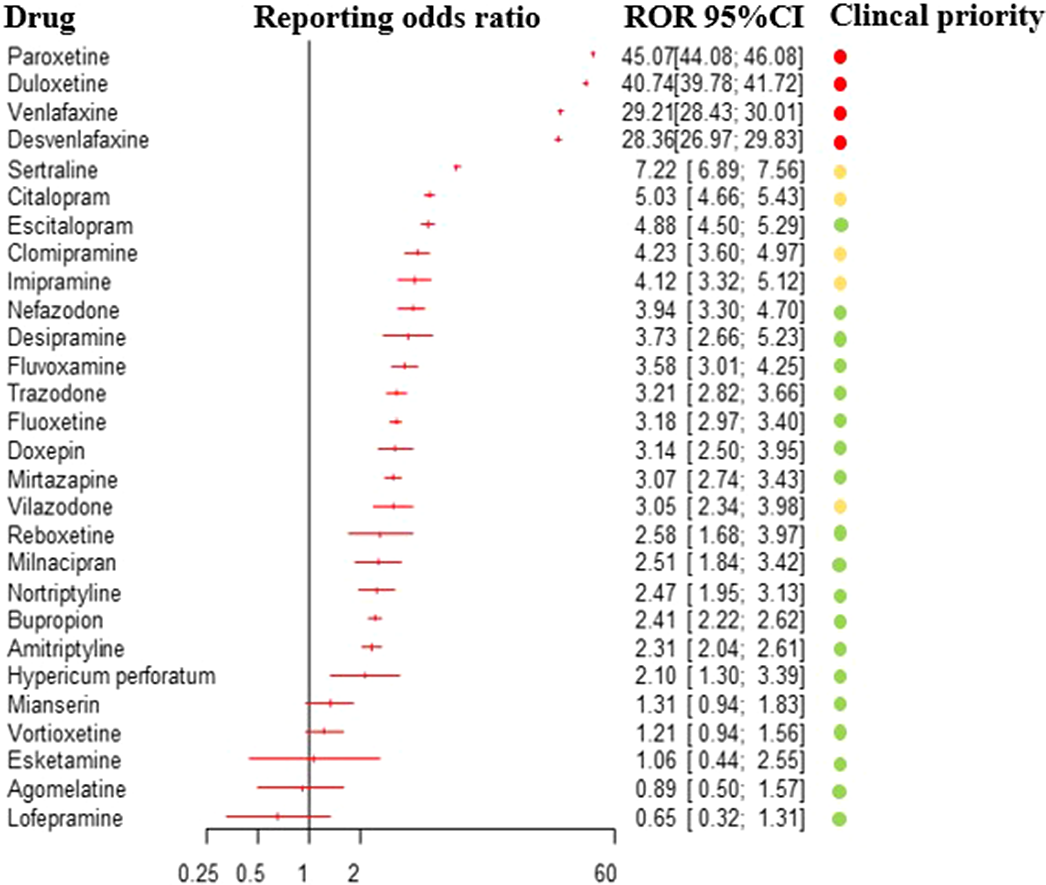
Pharmacovigilance analysis of the Vigibase on antidepressants-related withdrawal syndrome in adults and adolescents
-
- Journal:
- European Psychiatry / Volume 66 / Issue S1 / March 2023
- Published online by Cambridge University Press:
- 19 July 2023, pp. S95-S96
-
- Article
-
- You have access
- Open access
- Export citation
7 - Attention, Affect, and Creativity, from Mindfulness to Mind-Wandering
- from Part II - The Development of Creativity
-
-
- Book:
- The Cambridge Handbook of Creativity and Emotions
- Published online:
- 16 February 2023
- Print publication:
- 23 February 2023, pp 130-148
-
- Chapter
- Export citation
Do Clinicians Need to Rethink Antipsychotic Maintenance Treatment?: Pro
-
- Journal:
- European Psychiatry / Volume 65 / Issue S1 / June 2022
- Published online by Cambridge University Press:
- 01 September 2022, p. S6
-
- Article
-
- You have access
- Open access
- Export citation
Co-occurrence of clozapine-related DRESS syndrome core clinical manifestations: results of a systematic review
-
- Journal:
- European Psychiatry / Volume 65 / Issue S1 / June 2022
- Published online by Cambridge University Press:
- 01 September 2022, p. S719
-
- Article
-
- You have access
- Open access
- Export citation
Laboratory-Based 3D X-ray Imaging of Neutron-Irradiated Ceramic Particle Nuclear Fuel
-
- Journal:
- Microscopy and Microanalysis / Volume 28 / Issue S1 / August 2022
- Published online by Cambridge University Press:
- 22 July 2022, pp. 2038-2039
- Print publication:
- August 2022
-
- Article
-
- You have access
- Export citation
Understanding Fission Gas Bubble Distribution and Zirconium Redistribution in Neutron-irradiated U-Zr Metallic Fuel Using Machine Learning
-
- Journal:
- Microscopy and Microanalysis / Volume 28 / Issue S1 / August 2022
- Published online by Cambridge University Press:
- 22 July 2022, pp. 82-83
- Print publication:
- August 2022
-
- Article
-
- You have access
- Export citation
Advanced Crack Analytics on 3D X-ray Tomography of Irradiated Silicon Carbide Claddings
-
- Journal:
- Microscopy and Microanalysis / Volume 28 / Issue S1 / August 2022
- Published online by Cambridge University Press:
- 22 July 2022, pp. 208-210
- Print publication:
- August 2022
-
- Article
-
- You have access
- Export citation
Implementation of personalised medicine policies in mental healthcare: results from a stated preference study in the UK
-
- Journal:
- BJPsych Open / Volume 8 / Issue 2 / March 2022
- Published online by Cambridge University Press:
- 03 February 2022, e40
-
- Article
-
- You have access
- Open access
- HTML
- Export citation
Contraception Deserts: The Effects of Title X Rule Changes on Access to Reproductive Health Care Resources
-
- Journal:
- Politics & Gender / Volume 18 / Issue 3 / September 2022
- Published online by Cambridge University Press:
- 03 February 2022, pp. 672-707
-
- Article
-
- You have access
- Open access
- HTML
- Export citation
Iodine intakes in Irish children aged 5–12 years
-
- Journal:
- Proceedings of the Nutrition Society / Volume 80 / Issue OCE3 / 2021
- Published online by Cambridge University Press:
- 17 August 2021, E123
-
- Article
-
- You have access
- HTML
- Export citation