Breast cancer (BC) is the most frequently diagnosed cancer in women worldwide(Reference Siegel, Miller and Jemal1) and the secondary leading cause of death. In the twenty-first century, malignancy is expected to be the primary barrier to increasing life expectancy and decreasing the per capita death rate in each country and region(Reference Bray, Ferlay and Soerjomataram2). Although the 5-year recurrence rate is not high in patients with BC(Reference Tallet, Racadot and Boher3), these patients require long-term medication and regular examinations, the sensitivity of chemotherapy and radiotherapy is poor when the tumour recurs and recurrence is accompanied by a high mortality rate(Reference Dando, Pozza and Ambrosini4–Reference Puig, Tenbaum and Chicote5). Therefore, we anticipate being able to prevent the incidence of BC through lifestyle changes.
Coffee is generally divided into regular coffee and decaffeinated coffee, and tea is mainly divided into three types, green tea, black tea and oolong tea, which are the most popular drinks worldwide. Recently, some studies have been reported a relationship between coffee and/or tea intake with tumourigenesis, such as BC, stomach cancer, colorectal cancer and glioma(Reference Creed, Smith-Warner and Gerke6–Reference Poorolajal, Moradi and Mohammadi8).
Coffee is the major dietary source of caffeine, which also includes diterpene and polyphenols; tea also contains caffeine, as well as tea polyphenols and epigallocatechin 3-gallate. All of the above may have anticancer effect, while caffeine may influence BC mechanisms a lot, caffeine interacts with the PI3K/AKT inhibitory kinase signalling pathway, indicating that caffeine may play important roles in tumour pathogenesis, metastasis and prognosis(Reference Tenbaum, Ordóñez-Morán and Puig9). However, the roles of coffee and/or tea consumption in reducing the risk of incident BC remain controversial. Although some published meta-analyses have shown that coffee and tea potentially reduce the risk of BC(Reference Wang, Zhao and Chong10–Reference Lafranconi, Micek and De Paoli11), other studies reached the opposite conclusion(Reference Yaghjyan, Colditz and Rosner12) and the recommended dosage was not conclusive.
The objective of our current research was to determine the most suitable population and recommended daily dosage intake for coffee and tea that would effectively prevent BC, which could also assist in clinical prevention. No previous systematic review has provided a comprehensive overview by performing a meta-regression and Bayesian network meta-analysis of this current topic.
Materials and methods
This systematic review and Bayesian network meta-analysis followed the guidelines of the Preferred Reporting Items for Systematic reviews and Meta-analyses extension statement(Reference Hutton, Salanti and Caldwell13). We registered the pre-established protocol for this trial with the International Prospective Register of Systematic Reviews (PROSPERO) with prospective registry number CRD42020177945(14).
Search strategy and study selection
The publication search strategy, selection of eligible studies, data extraction, risk of bias assessment and Grading of Recommendations Assessment, Development and Evaluation (GRADE) were conducted by two independent researchers (WS and LX), and any controversies were resolved by discussion with an experienced reviewer (ZYS or ZQC). We searched PubMed, Embase and the Cochrane Library to identify potentially eligible studies published in the last 30 years up to April 2020 with original search terms of coffee or tea and the risk of BC and breast carcinoma but no language restrictions (see the details in online supplementary material, Supplemental Table 1). Eligible studies evaluated coffee and/or tea consumption for the risk of incident BC with their MeSH terms. Manual searches using reference lists in similar publications of each potential studies were also conducted. Potential publications analysed the association of coffee or tea consumption with the risk of incident BC, compared dose–response correlations or non-coffee and tea groups and were either case–control studies or prospective cohort studies.
Meaningful baseline characteristics were extracted using preset tables. For studies comparing dose–response correlations, the first author, publication year, project name, country and region, research type, tested consumption, subject type, sample size, age, BMI, alcohol intake, height, smoking, family history of BC and hormone therapy have been extracted. In addition, for studies which compared with non-coffee and tea group, first author, publication year, country and region, research type, tested consumption, subject type, sample size, age, BMI, alcohol intake, smoking, family history of BC, previous history of benign breast diseases and hormone therapy have been extracted.
Data extraction, risk of bias assessment and Grading of Recommendations, Assessment, Development and Evaluation
The data were extracted using preset charts, and the baseline data and risk of incident BC were both extracted. For studies comparing dose–response correlations, the baseline data recorded the highest dose and the lowest dose of coffee or tea consumption, while the baseline data were extracted from studies comparing the regular coffee/tea group with a non-coffee and tea group. Additionally, the non-adjusted and adjusted values for effect size were extracted for the risk of incident BC, and the main adjusted values for effect size of were marked. Moreover, dichotomous outcomes of incident BC were also been extracted from all available studies. Therefore, we used the Newcastle–Ottawa Scale(Reference Wells, Shea and O’Connell15) to assess the risk of bias of both prospective cohort studies and case-controlled studies, and a Newcastle–Ottawa Scale score > 4 was considered acceptable quality and a score > 7 was considered high quality. We also used GRADE scales(Reference Guyatt, Oxman and Vist16) to evaluate the quality of the outcomes as high, moderate, low and very low.
Data synthesis and statistical analysis
In our systematic review and Bayesian network meta-analysis, we mainly considered the dose–response relationship between coffee/tea consumption and the risk of recurrent or new primary BC. We preset 0–2 cups/d coffee or tea intake as low consumption, 3–4 cups/d coffee or tea intake as moderate consumption, ≥5 cups/d coffee or tea intake as high consumption and a dose that was not mentioned as regular consumption. We also examined the differences in coffee/tea types, menopause status, hormone receptor status and BMI in subgroup analyses and meta-regression analyses to determine the most suitable level of coffee or tea consumption.
For both hazard ratios (HR) with their 95 % CI from effect size data and OR with their 95 % CI from dichotomous data, we are using ln(HR) for the accuracy of the data. Pairwise meta-analyses of heterogeneity were conducted when the I 2 statistic was > 25 % or the P value was < 0·10, and regardless of the results of the heterogeneity test, random effects models were applied to assess accuracy. Additionally, the P value for the source of heterogeneity was <0·05 from a meta-regression analysis(Reference Yang, Gong and Jin17). Moreover, publication bias was assessed with Begg’s test and Egger’s test, and a value < 0·05 indicates the existence of publication bias. Correlation coefficients were obtained from the average of available correlation coefficients per result.
Additionally, we performed a Bayesian random-effects network meta-analysis composed of four chains with 100 000 iterations after an initial burn-in of 10 000 and a thinning of 2·5 to determine the best recommended dose of caffeine intake (from coffee or tea). We calculated the HR and OR and corresponding 95 % credible intervals, and mean rank and surface under the cumulative ranking curve (SUCRA) values(Reference Feng, Jiang and Jia18) were produced from network meta-analysis estimates with a consistency model. All the aforementioned analyses were performed with StataMP version 14.0 and WinBUGS version 1.4.3.
Results
Description of included studies
Figure 1 shows the flow chart of identified potential publications and details of the study selection process. We initially identified 928 records, and after subsequently removing duplicates and screening titles and abstracts, we assessed 113 full-text articles. After removing sixty-eight articles that were not suitable for inclusion, forty-five original articles (3 323 288 participants)(Reference Sánchez-Quesada, Romanos-Nanclares and Navarro19–Reference Inoue, Tajima and Mizutani63) were eligible for the systematic review and Bayesian network meta-analysis (Table 1). Among those studies, thirty-eight evaluated coffee consumption and the risk of BC in 3 058 893 participants, while seventeen evaluated tea consumption and the risk of BC in 264 395 subjects. Additionally, twenty-eight articles analysed the dose–response correlations and seventeen studies compared the preventative efficacy with non-coffee/tea group; details of each publication are shown in online supplementary material, Supplemental Tables 2–3. A baseline analysis of the highest dose compared with lowest dose of coffee/tea intake in articles examining the dose–response correlations revealed that the highest dose of coffee/tea consumption may be accompanied by a lower BMI, higher alcohol intake and smoking rate. The comparison of the regular coffee/tea consumption group with non-coffee/tea group revealed that the regular consumption group may also exhibit a lower BMI in the pairwise baseline meta-analysis. Moreover, twenty studies were case–control studies, while twenty-five were prospective cohort studies, and only one of those was defined as a low-quality study based on the Newcastle–Ottawa Scale score (see online supplementary material, Supplemental Tables 4–5).

Fig. 1 Procedure used to select studies examining coffee and tea consumption and the breast cancer risk
Table 1 Summary of the baseline main characteristics of coffee and tea consumption and the breast cancer risk

* Significant differences.
Dose–response relationship between coffee/tea consumption
The relationship between coffee consumption and BC risk was evaluated in a dose–response manner (Table 2). Low, moderate and high coffee consumption reduce the risk of incident BC, based on the effect size of HR data and significant differences, either from non-adjusted and adjusted values for effect size (the first column) or from most adjusted values for effect size (the second column), accompanied by low heterogeneity. Therefore, we postulate that HR data from most adjusted values for effect size (the second column) were more accurate, and starting with low-dose coffee consumption might prevent BC.
Table 2 Ln(HR) and their 95 % CI of breast cancer risks according to low, moderate and high coffee consumption obtained from the subgroup analysis and meta-regression analysis of the coffee type, menopause status, hormone receptor status and BMI
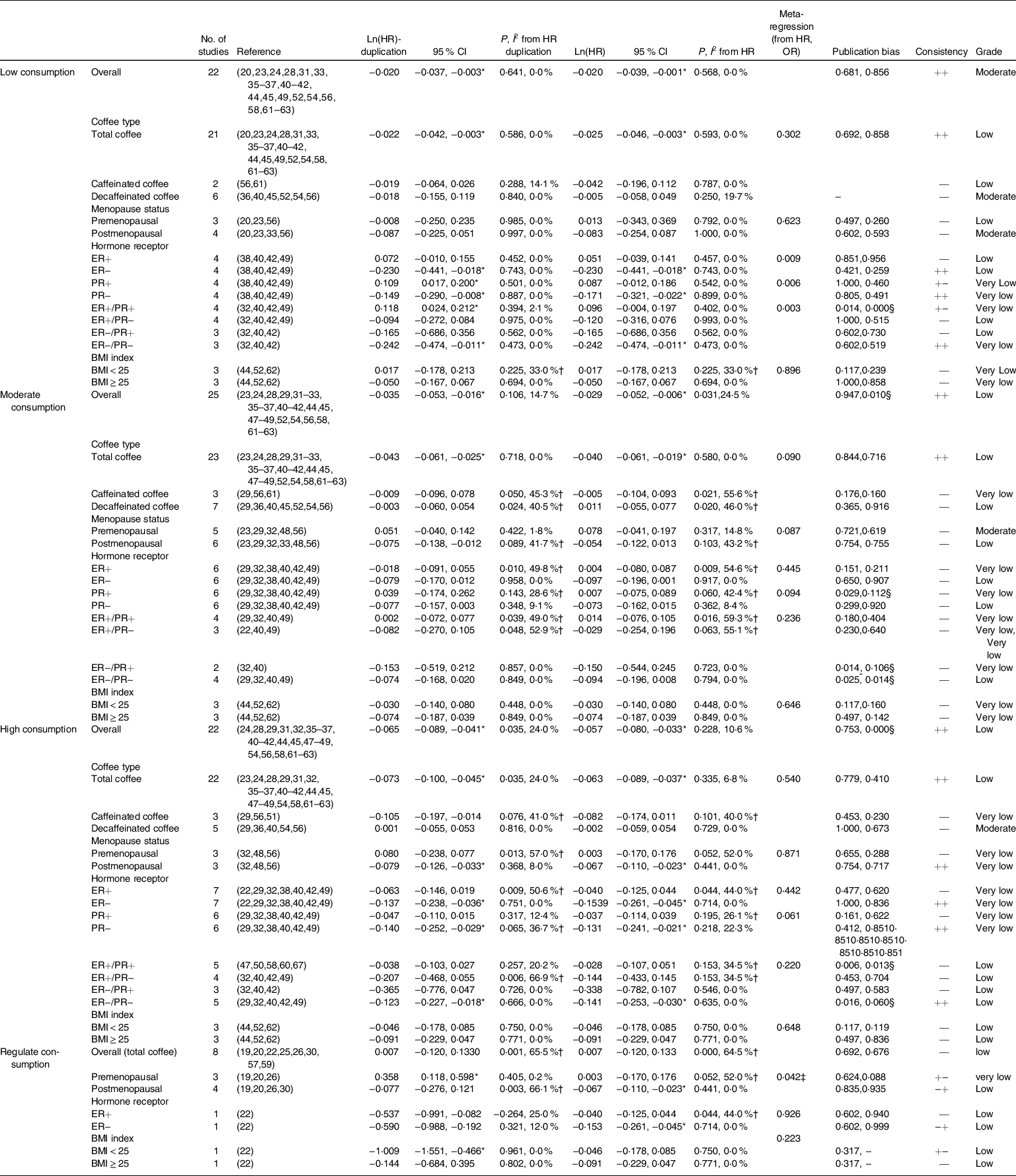
ER, estrogen receptor; PR, progesterone receptor.
* Significant differences.
† Existence heterogeneity.
‡ Source of heterogeneity from meta-regression.
§ Publication bias.
According to the subgroup analysis and meta-regression analysis of the coffee type, menopause status, hormone receptor status and BMI, significant differences often appeared in the subgroups of total coffee in coffee type, postmenopausal status in menopause status and the ER(estrogen receptor)−/PR(progesterone receptor)− status in the hormone receptor status. Sensitivity analysis did not find any single study that had a significant impact on the overall results (online supplementary material, Supplemental Fig. 1). Additionally, the meta-regression analysis produced similar results, because the P value was frequently < 0·05. In these pairwise meta-analyses, publication bias is generally not high and the GRADE quality of outcomes was acceptable (Table 2).
The relationship between the BC risk and tea consumption was also evaluated in a dose–response manner (Table 3) by conducting a subgroup analysis and meta-regression analysis of the type of tea, menopause status and hormone receptor status. We also considered the second column of HR data to determine the accuracy of the data, although no opposite outcomes and few inconsistent outcomes occurred. Notably, significant differences in high-dose tea consumption often appeared in the subgroups of postmenopausal status in the menopause status and ER−/PR− status in the hormone receptor status, and these inconsistencies were also confirmed by the meta-regression analysis. Sensitivity analysis did not find any single study that had a significant impact on the overall results (online supplementary material, Supplemental Fig. 2). Publication bias was detected in some of these outcomes, with an acceptable GRADE quality (Table 3).
Table 3 Ln(HR) and their 95 % CI of breast cancer risks according to the low, moderate and high dose of tea consumption obtained from the subgroup analysis and meta-regression analysis of the tea type, menopause status and hormone receptor status

ER, estrogen receptor; PR, progesterone receptor.
* Significant differences.
† Existence heterogeneity.
‡ Source of heterogeneity from meta-regression.
§ Publication bias.
In summary, the intake of coffee and tea produced similar results, but the difference was that a low dose of coffee has a role in reducing the incidence of BC but tea does not exert a protective effect until a high dose is consumed, and subgroups of postmenopausal women in the menopause status and ER−/PR− status in the hormone receptor status often revealed a protective effect with significant differences, which were confirmed by the meta-regression analysis (Tables 2–3). Therefore, we conducted a factor correlation analysis and indeed observed a relationship between the protective efficacy of coffee and tea intake with the menopause status and the status of the hormone receptors ER and PR in BC, particularly ER. No correlation was observed between these factors (Fig. 2). Although the protective effect of coffee was observed after the consumption of a low-dose, decaffeinated coffee is not as effective as regular coffee. Moreover, tea was only effective at high doses, particularly green tea. However, we have not determined the highest recommended daily intake doses of both coffee and tea, and thus we need to continue our research.

Fig. 2 Summary of the results of the correlation analysis of influencing factors and the incidence of breast cancer. *Significant influence.  , influence factors;
, influence factors;  , P values from coffee consumption;
, P values from coffee consumption;  , P values from tea consumption
, P values from tea consumption
Daily recommended doses of coffee/tea consumption
Low doses of coffee/tea consumption were preset to 0–2 cups/d and high doses of coffee/tea consumption were preset to ≥5 cups/d; however, some of the original studies considered 1–3 cups/d as low-dose consumption and ≥4 cups/d as high-dose consumption. Meanwhile, some studies did not describe the doses, which were only defined as low dose, moderate dose, high dose, etc. Therefore, low-dose coffee consumption included 0–1, 1, 1–2, 2–3, 1+ and 2+ cups/d, high-dose tea consumption included 3+, 4+, 5+ and 3–5 cups/d (Table 4).
Table 4 Relationships between the recommended daily doses of coffee, tea and caffeine consumption with breast cancer risks based on the Ln(HR) and their 95 % CI calculated using a subgroup analysis and meta-regression analysis of dose–response relationships and menopause status

* Significant differences.
† Existence heterogeneity.
‡ Source of heterogeneity from meta-regression.
§ Publication bias.
When we consider the recommended dose of coffee consumption, significant differences were observed in the subgroup of 2–3 cups/d (−0·034, −0·068 to −0·000), with low heterogeneity (P = 0·448, I 2 = 0·1 %) from the most adjusted effect size data, while the meta-regression analysis revealed a large difference among doses with P < 0·001. These results may support the hypothesis that 2–3 cups/d may be the daily recommended dose of coffee. When we studied high-dose tea consumption, a significant difference was only observed in the group that consumed 5+ cups/d (−0·153, −0·277 to −0·030), with low heterogeneity (P = 0·999, I 2 = 0 %). As a result, the recommended daily dose of tea was more than 5 cups/d.
These results may be due to the much higher caffeine content in coffee than in tea; therefore, we attribute the difference to the caffeine content and conducted another pairwise meta-analysis. The caffeine content was divided into four quartiles (low, moderate, moderate–high and high), and we noticed a significant result for the high caffeine content group (−0·068, −0·128 to 0·009), with some heterogeneity (P = 0·232, I 2 = 30 %), which was also obtained from the most adjusted effect size (Table 4). We performed a Bayesian network meta-analysis using effect size data and dichotomous data to obtain the highest recommended caffeine intake. Figure 3a presents the network plot including all direct contrasts among quartiles and the highest quartiles of caffeine content ranked first in two types of original data with significant differences (Fig. 3b).
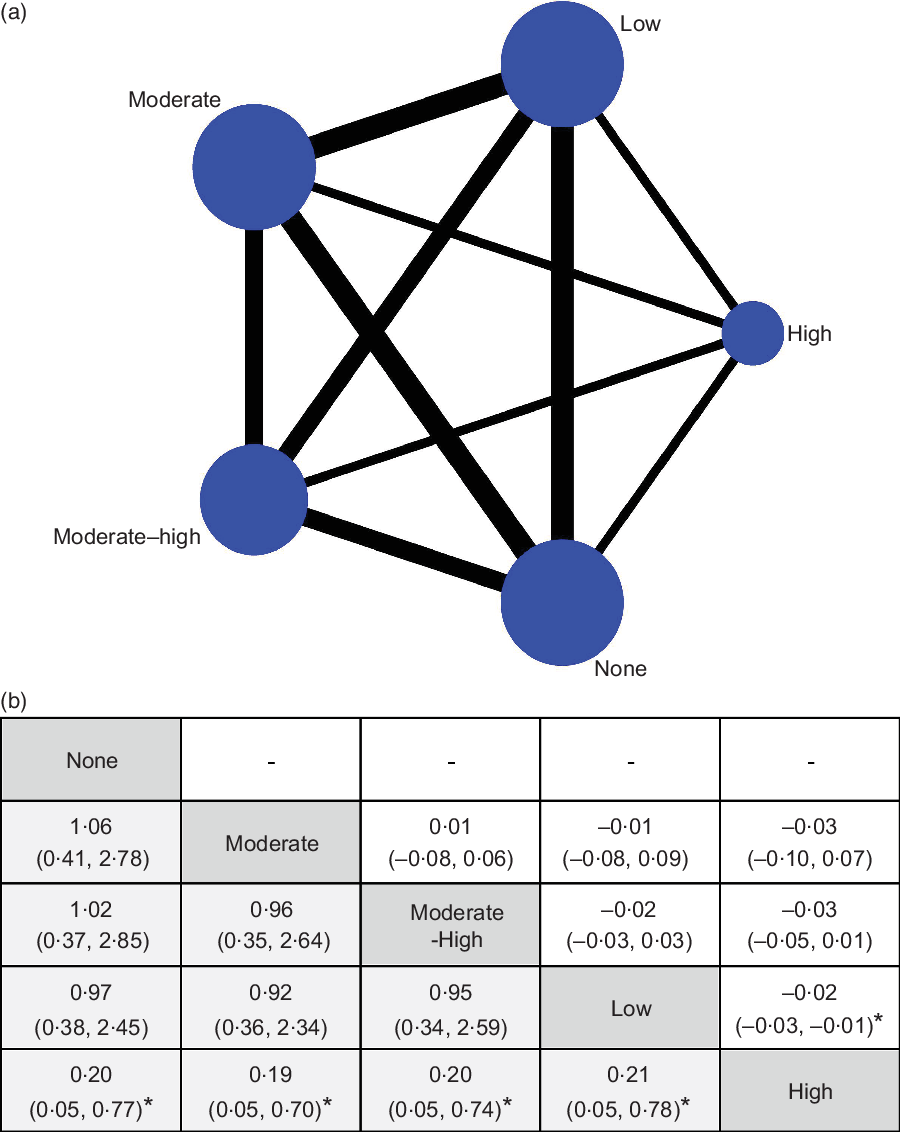
Fig. 3 Network meta-analysis plots relating to the eligible comparisons of caffeine intake (a) and dose–response effects obtained from the Bayesian network meta-analysis used to determine the recommend dose (b). Comparisons should be read from left to right and were ordered relative to overall prevention potential. The dose in the leftmost position is ranked as recommended from the hazard ratio (HR) and OR data. *Significant difference.  , dose–response of caffeine intake;
, dose–response of caffeine intake;  , OR (95 % CI);
, OR (95 % CI);  , HR (95 % CI)
, HR (95 % CI)
In addition, we compared the relationship between coffee intake, caffeine content and menopausal status. A significant difference was observed in postmenopausal women (Table 4), suggesting that the consumption of a high dose of caffeine may prevent the incidence of BC, particularly in postmenopausal women.
Discussion
The findings of our systematic review and Bayesian network meta-analysis have revealed the likely benefits of coffee or tea consumption in preventing BC from forty-five studies including 3 323 288 participants(Reference Sánchez-Quesada, Romanos-Nanclares and Navarro19–Reference Inoue, Tajima and Mizutani63). First, from the pairwise analysis of the dose-dependent response, the consumption of a low dose of coffee (0–2 cups/d) and high dose of tea (≥5 cups/d) may achieve the goal of preventing the incidence of BC (Tables 2–3). Additionally, coffee/tea consumption may be more beneficial for postmenopausal patients and preventing the occurrence of ER−/PR− BC, particularly ER− BC, according to the subgroup meta-analysis, meta-analysis and correlation analysis (Tables 2–3; Fig. 2). Moreover, for low-dose coffee and high-dose tea intake, we determined the recommended daily intake of coffee, tea and caffeine using a pairwise meta-analysis and Bayesian network meta-analysis. We confirmed that the consumption of 2–3 cups of coffee, more than 5 cups tea intake and a high level of caffeine exerts a preventive effect on BC and may be more effective in preventing BC in postmenopausal women (Table 4; Fig. 3).
Our research follows the Preferred Reporting Items for Systematic reviews and Meta-analyses guideline and the protocol has been registered. Regarding the caffeine content, a total caffeine consumption of ≥414·1 mg or ≥693 mg/d may exert a protective effect on BC. In addition to BC, caffeine may protect against the occurrence and development of many types of malignancy, such as ovarian cancer and skin cancer(Reference Shafiei, Salari-Moghaddam and Milajerdi64–Reference Caini, Cattaruzza and Bendinelli65). The results have also been verified in vitro and in vivo (Reference Bułdak, Hejmo and Osowski66–Reference Kolberg, Pedersen and Mitake67). As an antioxidant, a high concentration of caffeine might induce the formation of oxygen-centred radical, resulting in a decrease in reactive oxygen species (ROS) production and protection from cell damage, DNA mutation and inflammation, which are the causes of tumourigenesis. Additionally, caffeine not only affects immune cells, such as T and B lymphocytes, NK cells and macrophages, but also affects cytokines, such as TNF-α and IL-2, which have a variety of functions, to improve immunity in the body(Reference Cui, Wang and Pan68). Moreover, caffeine may represent a therapeutic agent for BC by activating apoptosis-inducing mechanisms(Reference Shashni, Sharma and Singh69). Therefore, the intake of decaffeinated coffee is not as effective as total coffee consumption, and similar results have been reported in published studies(Reference Micek, Godos and Lafranconi70–Reference Kennedy, Roderick and Buchanan71).
The effect of caffeine intake on postmenopausal women was more effective in our study, probably because of the positive association of coffee and caffeine intake with sex hormone binding to globulin in postmenopausal women, suggesting another potential mechanism by which coffee may reduce the levels of circulating oestrogens and subsequently the risk of malignancies. This mechanism may explain why coffee consumption may reduce the incidence of ER− BC(Reference Kotsopoulos, Eliassen and Missmer72). Numerous studies have discussed the adverse cardiovascular reactions associated with caffeine consumption; however, moderate caffeine intake is not associated with increased risks of total CVD, arrhythmia, heart failure and blood pressure changes;(Reference Turnbull, Rodricks and Mariano73–Reference Grant, Magruder and Friedman74) the evidence described above confirms that caffeine intake is safe. According to our research, the consumption of coffee and tea, particularly a low dose of coffee (2–3 cups/d) and high dose of tea (≥5 cups/d), exerted effects on preventing BC. According to the baseline meta-analysis, caffeine intake may reverse the cancer risk associated with obesity, smoking and alcohol intake(Reference Picon-Ruiz, Morata-Tarifa and Valle-Goffin75–Reference Shield, Soerjomataram and Rehm77); and also tea consumption seems more beneficial than harmful(Reference Shirai, Kuriki and Otsuka78), this discovery is very new and must be confirmed in other studies.
Our network meta-analysis has some limitations. First, heterogeneity often occurs in dichotomous data for OR, and the results of HR data and OR data are likely to be inconsistent, probably because the dichotomous data have not been adjusted. Meanwhile, the heterogeneity in HR data was much lower (Tables 2 and 3) and the heterogeneity of the original data analysis was substantial at baseline (Table 1); therefore, we used the most adjusted HR data to determine the accuracy of the data in subsequent analyses (Table 4). Second, the GRADE score for the outcome is not high, likely because all the publications we included are not randomised controlled trials and because dietary factors are unable to be randomised; publication bias also occasionally appeared in the tea group, due to the difficulty in publishing negative results. Third, the quality and the preparation of coffee were difficult to standardise, such as filtered or unfiltered, coffee bean roasting level and species of coffee beans; additionally, numerous types of tea were consumed, such as green tea (non-fermented), black tea (fermented) and oolong tea (semifermented), and thus the quality of tea was also difficult to standardise.
Compared with the previously published meta-analysis, our study includes the largest sample size. Our study is the first to determine the recommended daily intake of coffee and tea, which have value in guiding epidemiology and clinical practice. However, as mentioned above, the differences between different types of coffee and tea are substantial, and few studies mention the concentration of each cup of coffee or tea, and how many ml is consumed per cup. In the future, we hope to regulate the daily intake of caffeine and anticipate high-quality, preferably prospective studies, as well as ethnic and regional subgroup meta-analyses.
In conclusion, our systematic review and Bayesian network meta-analysis provide compelling evidence for the association between coffee/tea consumption and a decreased risk of BC, especially postmenopausal women, and particularly ER− BC. The recommended daily dose for preventing BC is 2–3 cups of coffee/d and more than 5 cups of tea/d, which are also safe doses. Future studies are expected to regulate the caffeine dosage in coffee and tea to determine the recommended daily caffeine dosage.
Acknowledgements
Acknowledgements: None. Financial support: This research received no specific grant from any funding agency, commercial or not-for-profit sectors. Conflict of interest: There are no conflicts of interest. Authorship: S.W, X.L., Y.S.Z. and M.Y.L. designed the research; S.W., X.L., X.C.Q. and J.P.X. conducted the research; S.W., Y.Y., M.Y.L., Y.Z. and Y.Z. analysed the data; S.W., Y.S.Z. and Q.Z. wrote the paper; Q.C.Z. had primary responsibility for final content. All authors are involved in writing the article. Ethics of human subject participation: Not applicable.
Supplementary material
For supplementary material accompanying this paper visit https://doi.org/10.1017/S1368980021000720












