Appropriate nutrition is fundamental for newborns, especially preterm infants, where their materno-fetal nutrients supply have been prematurely terminated(Reference De Rooy, Hamdallah and Dyall1). Breast milk provides nutrients, hormones, enzymes and immunological factors that are essential for infant development. Maternal breast milk is the first choice for feeding neonates; however, if mothers are unable to provide sufficient, donor human milk is the recommended alternative(Reference Weaver, Bertino and Gebauer2). The nutritional composition of human milk varies widely, not only over lactation, but also between individuals and populations(Reference Brenna, Varamini and Jensen3–Reference Floris, Stahl and Abrahamse-Berkeveld5). Factors that have been shown to affect the nutritional composition include maternal lifestyle and dietary habits(Reference Bravi, Wiens and Decarli6). It is therefore essential to identify which nutrients in breast milk are responsive to maternal diet in order to inform and update nutritional guidance for lactating mothers, milk donors and milk banks.
The impact of maternal diet on breast milk composition has been widely investigated; however, the results have been equivocal, with some studies showing positive effects whereas others have not. Systematic reviews in 2016(Reference Bravi, Wiens and Decarli6) and 2017(Reference Keikha, Bahreynian and Saleki7) report a positive association between oily fish consumption and higher levels of the n-3 PUFA, DHA (22:6n-3) and EPA (20:5n-3), and other fatty acids, such as the n-6 PUFA, linoleic acid (LA, 18:3n-6), and oleic acid (18:1n-9) in breast milk(Reference Bravi, Wiens and Decarli6). There was also evidence demonstrating a positive association between dietary vitamin C, B1 and vitamin A, D, E and K, with breast milk levels. The effects of dietary vitamin and/or mineral supplementation were reported in two systematic reviews(Reference Karcz and Krolak-Olejnik8,Reference Keikha, Shayan-Moghadam and Bahreynian9) , where the results were mixed, although there was some indication that vitamin supplementation had a greater effect on breast milk levels than mineral supplementation, with the strongest evidence seen for vitamin A, D, B1, B2, B12 and C.
Restricted diets, such vegan or vegetarian, can provide lower levels of nutrients, such as DHA, which may consequently affect the nutritional content of breast milk. In a 2020 systematic review by Karzc and Krόlak-Olejnik, the effects of vegan or vegetarian diets on breast milk composition were explored(Reference Karcz and Krolak-Olejnik8). Thirteen studies were summarised, and the authors identified that although milk from mothers following vegan, vegetarian and non-vegetarian diets was generally comparable in nutritional content, there were lower levels of some nutrients, particularly of DHA and vitamin B12, in the milk from vegan mothers and, therefore, recommended supplementation with these nutrients to enhance the nutritional content of the milk.
DHA, the long-chain n-6 PUFA and arachidonic acid (ARA, 20:4n-6) are essential for the development of optimal brain, visual and immune system functions(Reference Dyall10). In addition to those following vegan diets, lactating women may limit their consumption of fish, where DHA and EPA are highly enriched, due to concerns over the presence of heavy metal contaminants, such as methylmercury(Reference Grandjean, Jørgensen and Weihe11) and their effects on breast milk composition. This review also investigated the effects of dietary exposure to contaminants including heavy metals (As, B, Pb and Hg) and polychlorinated biphenyls (PCB) on breast milk levels.
The aim of this review is to extend the scope of previous systematic reviews and provide an up-to-date summary of the effects of short-term and long-term changes in maternal nutrient intake, including restrictive diets, and on breast milk nutritional composition. It is hoped that the results may be used to guide future research and inform nutritional guidance for lactating mothers, milk donors and milk banks.
Methodology
This review was designed and undertaken following the protocols for Preferred Reporting Items for Systematic Review and Meta-Analysis (PRISMA)(Reference Page, Moher and Bossuyt12). Study selection, assessment of eligibility, extraction of data and statistical analysis were performed according to a predefined protocol registered with the PROSPERO International prospective register of systematic reviews (ID: CRD42020221577).
Search strategy
The search was performed on four different databases: PubMed, CENTRAL, Web of Science and CINALH following the PRISMA(Reference Page, Moher and Bossuyt12) statement for systematic reviews. Additionally, three systematic reviews(Reference Bravi, Wiens and Decarli6,Reference Karcz and Krolak-Olejnik8,Reference Keikha, Shayan-Moghadam and Bahreynian9) were screened and articles meeting the selection criteria were also included. The review was designed upon the participants/population, intervention, comparison and outcome (PICO) model, with population being ‘lactating mothers’ or ‘human milk donors’; intervention, ‘experimental’ or ‘observation’ studies; comparison, ‘maternal dietary intake’ and outcome, ‘micronutrients’, ‘macronutrients’ and ‘contaminants’ breast milk content. The search was conducted on human studies and exclusively on lactating women. Publication types included were randomised controlled trials (RCT), experimental studies and observational studies. Limitations were applied to exclude conference papers, editorials, letters, commentary, and short survey, and grey literature was not searched. The search was run in English language up to 4 June 2023, with no time limitation. Online Supplementary Table S1 shows the search strategy.
Selection criteria
The selection criteria were based on the participants/population, intervention, comparison and outcome framework(Reference Cochrane, Watson and Timpson13). The participants/population were healthy, non-micronutrient-deficient, lactating women, as defined by investigators. The participants/population were breast-feeding or expressing breast milk within the first 12 months postpartum, and the exclusion criteria were participants/populations with predisposition to malnutrition, micronutrient deficiency as defined by investigators, any severe medical conditions or disorders including, HIV 1 or 2, hepatitis B or C, human T-lymphotropic virus type I or II, or syphilis, recreational drug users, smokers, or users of nicotine replacement therapy. The intervention was dietary supplementation for RCT and experimental studies, and assessment of dietary intake for observational studies. The comparison was to the control group or differences in relative levels of intake. The outcome was the differences in breast milk nutrient/contaminant level by maternal intake.
Data extraction
Two reviewers independently screened all titles and abstracts (CF and MS), according to the inclusion and exclusion criteria. Disagreements were resolved by discussion and where necessary involving a third reviewer (SCD). If the title or abstract appeared to meet the eligibility criteria or they could not determine its eligibility, the full texts of the articles were obtained. Full-text screenings and quality assessments for each of the included papers were also conducted by the two reviewers independently (CF and MS), and any discrepancies were discussed with a third author (SCD) until a decision on whether or not to include the paper in the review was reached. Rayyan software was used for handling and managing extracted studies that were found in the databases, and duplicates were removed(Reference Ouzzani, Hammady and Fedorowicz14).
Quality assessment and risk of bias
The quality assessment of the studies was performed by using the Cochrane Risk of Bias tool (ROB2_IRPG_beta_v7)(Reference Cumpston, Li and Page15,Reference Sterne, Savovic and Page16) for RCT and the Newcastle–Ottawa scale for the non-randomised cohort and case–control studies(Reference Lo, Mertz and Loeb17). The ROB2_IRPG_beta_v7 assessment tool contains five domains: randomisation process, deviations from intended interventions, missing outcome data, measurement of the outcome and selection of the reported result. An algorithm calculates the risk of bias for each domain as well as the overall risk, classifying it within three categories, high risk, low risk or some concerns. The Newcastle–Ottawa scale is comprised of eight items covering three domains: selection (including representativeness and source of sample), comparability (including study design and considerations in analysis) and exposure (for cohort studies, the exposure domain is instead the ‘outcome’ domain). Each paper can be assigned a score of 9 stars and was rated as either ‘good’, ‘fair’ or ‘poor’. The quality of each study was rated using the following scoring algorithms: ≥ 7 points were considered as ‘good’, 3–6 points were considered as ‘fair’ and ≤ 2 points was considered as ‘poor’ quality.
Statistical analysis
Information was extracted on author, type of study, geographical area, characteristics and number of participants, evaluated nutrients, type of supplement when intervention, breast milk extraction method, aim and outcome of the study, and most relevant findings. Among the examined nutrients in breast milk, results are reported for heavy metals, iodine, Fe, Cu, n-3 and n-6 PUFA, ovalbumin, persistent organic pollutants, protein, retinol, Se, vitamin A, B vitamins, vitamin B, vitamin C, vitamin D, vitamin E, vitamin K and Zn. A random effect meta-analysis was conducted with RevMan 5.4, Cochrane’s online review-writing platform on fatty acids (DHA, EPA and ARA), vitamins A, D and E, iodine and Se; however, due to high heterogeneity, it was decided not to publish the meta-analysis results. In the final summary, the overall certainty of the evidence was rated by the authors as either (1) very low, (2) low, (3) moderate or (4) high, following the Grading of Recommendations Assessment, Development and Evaluation system(Reference Guyatt, Oxman and Vist18).
Results
Description of the identified studies
The initial search identified 10 780 articles across four databases: PUBMED, CENTRAL, CINAHL and Web of Science; Fig. 1 shows the article selection procedure (PRISMA flow chart): 10 702 articles were excluded, 935 because they were duplicates, and 9816 after abstract and title screening. Fifty-nine additional articles were identified from reference lists.

Fig. 1. PRISMA 2009 flow diagram. From: Moher D, Liberati A, Tetzlaff J, Altman DG, The PRISMA Group (2009). Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLoS Med 6(7): e1000097. doi:10.1371/journal.pmed1000097. For more information, visit www.prisma-statement.org.
In total, eighty-eight articles were included in the final review, fifty-four experimental studies and thirty-four observational, comprising a total of 6577 participants. Twenty-nine articles examined fatty acids, thirty-one examined vitamins, twenty-three examined minerals, amino acids and proteins, and five examined contaminants (heavy metals: As, B, Pb and Hg) and PCB. For the rest of the nutrients, there were less than five studies each. The breakdown per nutrient is shown in online Supplementary Table S2.
Breast milk extraction
The breast milk collection details are summarised in Tables 1–4. The other studies either analysed samples taken over the day or did not specify the time of collection.
Table 1. Responsivity of breast milk fatty acid content to maternal diet

RCT, randomised control trial; BM, breast milk; PP, postpartum; AM, ante meridiem ; PM, post meridiem; M, median; GA, gestational age; Obs, observational study; ALA, α-linolenic acid; ARA, arachidonic acid; ALA, α-linolenic; LA, linoleic acid; DGLA, dihomo-γ-linolenic acid; DTA, docosatetraenoic acid; GLA, γ-linolenic acid.
Table 2. Responsivity of breast milk vitamin content to maternal diet

PP, postpartum; RCT, randomised control trial; BM, breast milk; AM, ante meridiem; PM, post meridiem; M, median; Obs, observational study.
Table 3. Responsivity of breast milk mineral, amino acid and protein content to maternal diet

RCT, randomised control trial; PP, postpartum; BM, breast milk, obs, observational study; IQR, interquartile range; GPC; glycerol-phosphocholine; PC, phosphocholine.
Table 4. Responsivity of breast milk contaminant levels in response to maternal diet

Obs, observational study; PP, postpartum; BM, breast milk; PCB, polychlorinated biphenyls.
Ethnicity
The articles included in this review involved African, Arabic, Asian, Australian, European and Hispanic participants, as summarised in Tables 1–4.
Main results
Fatty acids
Twenty-nine publications on fatty acids were included, fifteen experimental studies(Reference Argaw, Bouckaert and Wondafrash19–Reference Yang, Li and Li33), and fourteen observational studies(Reference Aitchison, Dunkley and Canolty34–Reference Scopesi, Ciangherotti and Lantieri47) and are summarised in Table 1.
Of the experimental studies, three were rated low risk of bias(Reference Fougere, Bilodeau and Lavoie22,Reference Mazurier, Rigourd and Perez25,Reference Smithers, Markrides and Gibson29) and twelve were identified having some concerns(Reference Argaw, Bouckaert and Wondafrash19–Reference Craig-Schmidt, Weete and Faircloth21,Reference Hawkes, Bryan and Makrides23,Reference Lauritzen, Jorgensen and Hansen24,Reference Mellies, Ishikawa and Gartside26–Reference Smithers, Markrides and Gibson29,Reference Valentine, Morrow and Pennell31–Reference Yang, Li and Li33) . For the observational studies, three were of good quality(Reference Liu, Ding and Li41,Reference Makela, Linderborg and Niinikoski42,Reference Perrin, Pawlak and Dean44) , nine were fair quality(Reference Aitchison, Dunkley and Canolty34–Reference Bzikowska-Jura, Czerwonogrodzka-Senczyna and Jasińska-Melon36,Reference Freitas, Macedo and Lessa39,Reference Juber, Jackson and Johnson40,Reference Olafsdottir, Thorsdottir and Wagner43,Reference Sanders, Ellis and Dickerson45–Reference Scopesi, Ciangherotti and Lantieri47) and two were poor quality(Reference de la Presa-Owens, Lopez-Sabater and Rivero-Urgell38,Reference Freitas, Macedo and Lessa39) .
PUFA
Nine experimental studies were identified with DHA and EPA, and participants were supplemented with DHA in the range of 200 to 1200 mg/d, and EPA between 70 and 300 mg/d(Reference Argaw, Bouckaert and Wondafrash19,Reference Boris, Jensen and Salvig20,Reference Fougere, Bilodeau and Lavoie22–Reference Mazurier, Rigourd and Perez25,Reference Smithers, Markrides and Gibson29,Reference Valentine, Morrow and Pennell31,Reference Yang, Li and Li33) . DHA and EPA supplementation was consistently shown to increase DHA and EPA breast milk levels, and this was in a dose-dependent manner. Two RCT investigated the effects of maternal α-linolenic acid (ALA) and LA maternal supplementation on breast milk, and ALA maternal intake was similarly show to increase breast milk ALA levels(Reference Mazurier, Rigourd and Perez25,Reference Valenzuela, Bascuñán and Chamorro32) . The observational studies also reported a significant positive correlation between maternal consumption of fatty fish intake and breast milk DHA, EPA and ALA(Reference Bzikowska-Jura, Czerwonogrodzka-Senczyna and Jasińska-Melon36,Reference de la Presa-Owens, Lopez-Sabater and Rivero-Urgell38,Reference Juber, Jackson and Johnson40,Reference Olafsdottir, Thorsdottir and Wagner43,Reference Perrin, Pawlak and Dean44) . The effect of vegan, vegetarian or omnivore diet patterns on breast milk fatty acids fat composition was investigated in three observational studies(Reference Perrin, Pawlak and Dean44–Reference Sanders and Reddy46). DHA levels were either significantly lower in vegans than omnivores or vegetarians(Reference Sanders and Reddy46) or low across all groups(Reference Perrin, Pawlak and Dean44). The LA to ALA ratio was significantly lower in breast milk from vegan participants compared with vegetarians and omnivores(Reference Perrin, Pawlak and Dean44). In comparison to omnivores, breast milk from vegans contains a higher proportion of SCFA (C10–C14) and lower proportion of medium-chain fatty acid (C16–C18). For ARA, only one study was identified, and in this experimental study participants were supplemented with 54 mg ARA per d for 2 weeks and no relationship was identified between maternal intake and breast milk levels(Reference Smithers, Markrides and Gibson29).
Others fatty acids
Trans-fatty acids, SFA and hydrogenated fats consumption and their content in breast milk were investigated in four studies(Reference Craig-Schmidt, Weete and Faircloth21,Reference Aitchison, Dunkley and Canolty34,Reference Daud, Mohd-Esa and Azlan37,Reference Makela, Linderborg and Niinikoski42) . The consumption of hydrogenated vegetable oils with high content of trans-fatty acid increased the trans-fatty acids concentration in breast milk after a 12–36-h lag period.
Lipid-soluble vitamins (A, D, E and K)
Vitamin A
Eight publications were included for vitamin A, which were all experimental studies(Reference Bahl, Bhandari and Wahed48–Reference Johnson, Qin and Krinsky55). One was low risk of bias(Reference Ding, Hu and Yang52), four presented some concerns(Reference Bahl, Bhandari and Wahed48,Reference Canfield, Kaminsky and Taren51,Reference Gossage, Deyhim and Yamini53,Reference Grilo, Medeiros and Silva54) and three were high risk of bias(Reference Basu, Sengupta and Paladhi49,Reference Canfield, Giuliano and Neilson50,Reference Johnson, Qin and Krinsky55) , with the results summarised in Table 2.
Maternal β-carotene supplementation increased β-carotene concentration in breast milk without impacting retinol, α-tocopherol or other carotenoid breast milk content. A similar effect is observed with retinol, lactating mothers supplemented with retinol produce a higher retinol concentration breast milk without affecting other carotenoids. The supplements in the experimental studies varied from 30 mg of β-carotene daily to 60 mg of retinyl palmitate or β-carotene single dose and 90 mg β-carotene as red palm oil in six doses over 10 d.
Vitamin D
Five publications were included for vitamin D, all experimental studies(Reference Ala-Houhala, Koskinen and Parviainen56–Reference Oberhelman, Meekins and Fischer60) Three were low risk of bias(Reference Basile, Taylor and Wagner57–Reference Niramitmahapanya, Kaoiean and Sangtawesin59), one with concerns(Reference Oberhelman, Meekins and Fischer60) and one rated high risk of bias(Reference Ala-Houhala, Koskinen and Parviainen56), with the results summarised in Table 2. One study reported that daily maternal supplementation had no significant effect on vitamin D breast milk concentration(Reference Ala-Houhala, Koskinen and Parviainen56). The other four studies reported that a single large dose supplementation was more effective in raising breast milk vitamin D concentration than a smaller daily supplementation(Reference Basile, Taylor and Wagner57–Reference Oberhelman, Meekins and Fischer60). The supplements in the experimental studies varied from 50 µg per d to 3750 µg single dose.
Vitamin E
Six studies were included for vitamin E, with five experimental(Reference Clemente, Ramalho and Lima61–Reference Pires Medeiros, Ribeiro and Lima65) and one observational(Reference Antonakou, Chiou and Andrikopoulos66), and are summarised in Table 2. Among the experimental studies, two were low risk(Reference Clemente, Ramalho and Lima61,Reference Gaur, Kuchan and Lai63) , one was rated with some concerns(Reference de Sousa Rebouças, Costa Lemos da Silva and Freitas de Oliveira62) and two were high risk of bias(Reference Kanno, Kobayashi and Yamauchi64,Reference Pires Medeiros, Ribeiro and Lima65) , whereas the observational study was considered good quality(Reference Antonakou, Chiou and Andrikopoulos66).
Maternal intake of vitamin E (α-tocopherol) was shown to influence breast milk vitamin E concentration. The supplements in the experimental studies ranged from 40 mg/d to 536 mg in a single dose.
Vitamin K
Three experimental studies measuring vitamin K were included(Reference Bolisetty, Gupta and Graham67–Reference von Kries, Shearer and McCarthy69), as summarised in Table 2. One was low risk of bias(Reference Greer, Marshall and Foley68), and two were considered high risk of bias(Reference Bolisetty, Gupta and Graham67,Reference von Kries, Shearer and McCarthy69) . The three studies reported that supplementing lactating mothers with vitamin K produced an increase in hind milk, foremilk and total breast milk vitamin K concentration, with a peak 12–24 h after supplementation. Vitamin K supplements varied from 0·5 to 5 mg per d for a period of 1 d up to 12 weeks.
Water-Soluble vitamins
B vitamins
Five experimental studies were included(Reference Chang and Kirksey70–Reference Thomas, Kawamoto and Sneed74), one was rated as low risk of bias(Reference Hampel, Shahab-Ferdows and Islam71), one presented some concerns(Reference Nail, Thomas and Eakin72) and three were high risk of bias(Reference Chang and Kirksey70,Reference Styslinger and Kirksey73,Reference Thomas, Kawamoto and Sneed74) , and are summarised in Table 2. Two studies investigated vitamins B1 and B2 (Reference Hampel, Shahab-Ferdows and Islam71,Reference Nail, Thomas and Eakin72) , four investigated vitamin B6 (Reference Chang and Kirksey70,Reference Hampel, Shahab-Ferdows and Islam71,Reference Styslinger and Kirksey73,Reference Thomas, Kawamoto and Sneed74) and one investigated vitamin B12 (Reference Thomas, Kawamoto and Sneed74). The effects of maternal vitamin B1 intake on breast milk levels showed mixed results(Reference Hampel, Shahab-Ferdows and Islam71,Reference Nail, Thomas and Eakin72) . When the maternal supplementation was 1·7 mg/d for 6 weeks from parturition, there was no significant impact on vitamin B1 breast milk concentration(Reference Nail, Thomas and Eakin72), whereas a supplement of 5 mg and then 10 mg over 2 d increased vitamin B1 content of breast milk.
Maternal vitamin B2 and B6 supplementation increased the breast milk vitamin B2 and B6 concentrations, respectively, in the first few postpartum weeks(Reference Chang and Kirksey70–Reference Nail, Thomas and Eakin72). Maternal supplementations were 2 mg per d for vitamin B2 and ranged from 4 mg to 20 mg per d for vitamin B6 and lasted between 3 d and 6 weeks. Although, vitamin B6 maternal intake positively impacts breast milk concentration in the first few weeks postpartum, the effects of vitamin B6 supplementation were shown to decrease after 40 d(Reference Thomas, Kawamoto and Sneed74).
Vitamin B12 maternal supplementation did not show a significant effect on breast milk content at 1 week postpartum; however, a daily 8 μg intake was shown to prevent its decline in breast milk over lactation(Reference Thomas, Kawamoto and Sneed74).
Vitamin C
Three experimental studies were included on vitamin C(Reference Thomas, Kawamoto and Sneed74–Reference Daneel-Otterbech, Davidsson and Hurrell76), all were rated high risk of bias, as summarised in Table 2. Overall, vitamin C in breast milk was only shown to be responsive to maternal intake following high-dose supplementation, that is, 1000 mg per d for 4 months(Reference Daneel-Otterbech, Davidsson and Hurrell76). Supplementation at lower doses, for example, 90 mg/d of vitamin C given to lactating mothers for 6 weeks, showed no difference in breast milk composition(Reference Thomas, Kawamoto and Sneed74,Reference Byerley and Kirksey75)
Minerals (iodine, iron, copper, zinc and selenium)
Iodine
Four studies(Reference Leung, Braverman and He77–Reference Ureta-Velasco, Keller and Escuder-Vieco80)were identified investigating iodine content, three were experimental(Reference Leung, Braverman and He77–Reference Nazeri, Mirmiran and Tahmasebinejad79) and one observational(Reference Ureta-Velasco, Keller and Escuder-Vieco80), as summarised in Table 3. Of the experimental studies, two were rated as low risk of bias(Reference Mulrine, Skeaff and Ferguson78,Reference Nazeri, Mirmiran and Tahmasebinejad79) , and one high risk of bias(Reference Leung, Braverman and He77), whereas the observational study was rated of good quality(Reference Ureta-Velasco, Keller and Escuder-Vieco80).
Maternal iodine supplementation increased breast milk iodine content and prevented a decline over lactation. Supplementation varied between 75 μg and 150 μg/d or a single 450 μg dose. The observational study found a positive correlation between breast milk iodine content and the consumption of at least three dairy products per d(Reference Ureta-Velasco, Keller and Escuder-Vieco80).
Selenium
Six publications(Reference Bianchi, Cruz and Zanetti81–Reference Valent, Horvat and Mazej86) were included on Se, four RCT(Reference Dodge, Wander and Butler82–Reference Trafikowska, Zachara and Wiacek85) and two observation studies(Reference Bianchi, Cruz and Zanetti81,Reference Valent, Horvat and Mazej86) , as summarised in Table 3. Among the experimental studies, one presented some concerns(Reference Dodge, Wander and Butler82), and three were rated high risk of bias(Reference Dylewski and Picciano83–Reference Trafikowska, Zachara and Wiacek85). The two observational studies were good quality(Reference Valent, Horvat and Mazej86) and fair quality(Reference Bianchi, Cruz and Zanetti81). Maternal Se supplementation increased breast milk Se concentration. The experimental studies supplements varied from 20 μg/d, 50 μg/d and 200 μg/d for 3 months. One study(Reference Dodge, Wander and Butler82)reported that maternal Se supplementation (50 μg per d) increased breast milk PUFA levels by 41 % (including LA and ARA) and decreased the levels of SFA by 11 %.
Iron, copper and zinc
One experimental study was included for Fe, which was rated low risk of bias(Reference Yalçin, Baykan and Yurdakök87). Three observational studies were included, which measured Cu, Fe and Zn(Reference Choi, Kim and Lee88–Reference Vuori, Makinen and Kara90). One was rated good quality(Reference Choi, Kim and Lee88), and two were fair quality(Reference Leotsinidis, Alexopoulos and Kostopoulou-Farri89,Reference Vuori, Makinen and Kara90) , summarised in Table 3.
Cu, Fe and Zn maternal intake in healthy non-deficient lactating women was not shown to impact breast milk composition in the observational studies. Furthermore, in the experimental study, Fe supplementation at 80 mg/d for 4 months did not increase breast milk Fe levels(Reference Yalçin, Baykan and Yurdakök87).
Protein, ovalbumin, choline and tyrosine
Protein (amino acids)
Two publications were included(Reference Forsum and Lonnerdal91,Reference Rana and Sanders92) , one experimental study, which was rated as presenting concerns(Reference Forsum and Lonnerdal91), and one observational study(Reference Rana and Sanders92), which was rated as poor quality. Results are summarised in Table 3. There was no significant difference in the breast milk true protein, lacto-ferrin, α-lacto-albumin and lactose content between lactating women consuming a low or a high protein diet for 4 d(Reference Forsum and Lonnerdal91). However, breast milk from vegans was shown to have a lower taurine concentration than that from omnivores(Reference Rana and Sanders92)
Ovalbumin
Two experimental studies(Reference Metcalfe, Marsh and D’Vaz93,Reference Palmer, Gold and Makrides94) were included, one was rated as some concerns(Reference Palmer, Gold and Makrides94) and the other high risk(Reference Metcalfe, Marsh and D’Vaz93), summarised in Table 3. A direct dose response between the number of cooked eggs ingested and the ovalbumin concentration in breast milk was identified.
Choline
One observational study rated fair quality(Reference Perrin, Pawlak and Allen95) was included for choline, and the results are summarised in Table 3. The study reported differences in breast milk choline forms for vegans, as they had a greater mean concentration and distribution of choline derived from glycerophosphocholine than vegetarian and omnivores. Also, there was a lower mean percentage of choline from phosphocholine in vegan breast milk compared with vegetarian and omnivores.
Tyrosine
One experimental study, rated with some concerns, was included on tyrosine(Reference Dowlati, Ravindran and Maheux96). Results are summarised in Table 3. The study reported that lactating women supplemented with tyrosine had a higher breast milk total tyrosine concentration. The supplementation was a single dose of 10 g of tyrosine.
Contaminants
Five observational studies were included(Reference Leotsinidis, Alexopoulos and Kostopoulou-Farri89,Reference Castro, Harari and Llanos97–Reference Ursinyova, Masanova and Uhnakova100) . One on Hg, which was rated good quality(Reference Ursinyova, Masanova and Uhnakova100), one on Hg and Pb, which was rated good quality(Reference Gundacker, Pietschnig and Wittmann99),one on As, B and Li, rated as fair quality(Reference Castro, Harari and Llanos97), one on Pb and Cd, rated as fair quality(Reference Leotsinidis, Alexopoulos and Kostopoulou-Farri89), and one on PCB, rated as poor quality(Reference Dewailly, Ryan and Laliberté98). The results are summarised in Table 4.
Heavy metals (arsenic, boron, cadmium, lead, lithium and mercury)
Maternal intake of freshwater fish was shown to be negatively associated with breast milk Hg levels, whereas maternal consumption of cereals was associated with higher breast milk Hg levels(Reference Ursinyova, Masanova and Uhnakova100).
One study identified a significant association between fish consumption and breast milk Pb levels(Reference Gundacker, Pietschnig and Wittmann99). The other studies show that environment can have a bigger impact on the presence of contaminants in breast milk than dietary habits(Reference Leotsinidis, Alexopoulos and Kostopoulou-Farri89,Reference Castro, Harari and Llanos97) .
Polychlorinated biphenyls
The reviewed study reported Inuit breast milk samples (300 g/d seafood intake) had a content in total 2,3,7-tetrachlorodibenzo-p-dioxinequivalents (TEQ) for PCB 3·5 times higher than Caucasian breast milk samples (12 g/d seafood intake)(Reference Dewailly, Ryan and Laliberté98).
Overall summary
Table 5 provides an overall summary of the results of this systematic review and provides ratings of the overall quality of the evidence by the authors using the GRADE system for each nutrient and contaminant(Reference Guyatt, Oxman and Vist18). The table also summarises the doses of supplementation provided in the experimental studies, and where relevant European Food Safety Authority recommended intake levels are provided for comparison, as well as toxicity information on contaminant levels(101).
Table 5. Synthesis of the nutrients in breast milk responsive to maternal diet
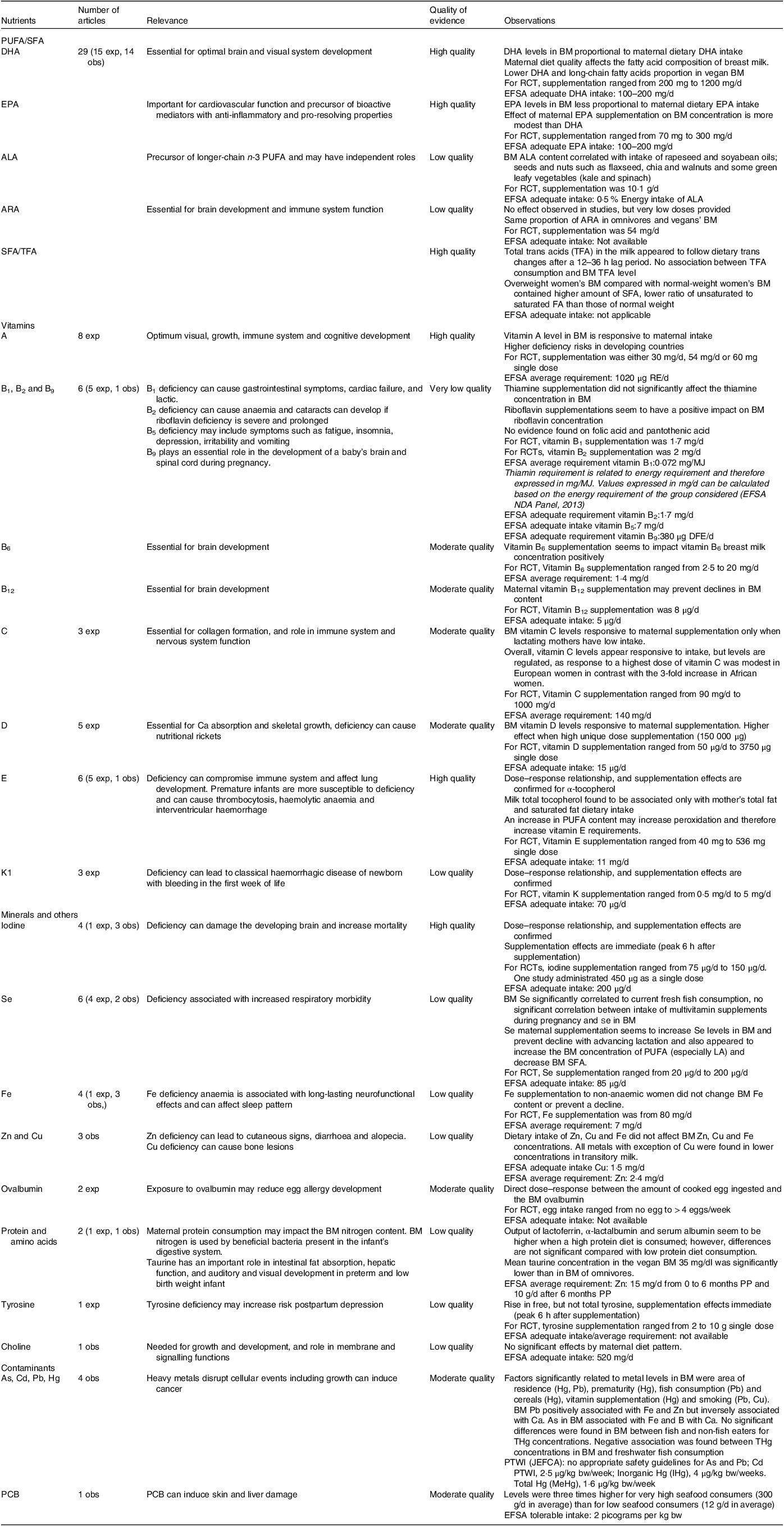
Based on GRADE rating system.
Obs, observational study; exp, experimental study (randomised control trial); ALA, α-linolenic acid; ARA, arachidonic acid; BM, breast milk, FA, fatty acid; PP, postpartum; DFE, dietary folate equivalent; EFSA, European Food Safety Authority; THg, total Hg; PCB, polychlorinated biphenyls; PTWI, provisional tolerable monthly intake; bw, body weight; JEFCA, joint FAO/WHO Expert Committee on Food Additives; RE, retinol equivalent.
Discussion
This study systematically reviewed the literature investigating the relationship between maternal intake and the levels of macronutrients, micronutrients and contaminants (heavy metals and PCB) in breast milk, for women without nutrient deficiencies. Due to the high heterogeneity between studies, it was not possible to undertake a meta-analysis, and so the results have been summarised with a narrative synthesis. The main findings were that there was strong evidence of response to maternal intakes of DHA, EPA, vitamins A, E and K, iodine and Se in breast milk composition, some evidence of response for ALA, B vitamins, vitamin C and D, ovalbumin, tyrosine and some contaminants, and insufficient evidence to determine the effects of ARA, Cu, Fe, Zn and choline. However, it should be noted that only a high dose of vitamin C was shown to produce an increase in breast milk vitamin C content, and although ARA intake was not found to affect breast milk ARA content, the supplemental dose used in this study was too low to allow definitive conclusions. Although maternal intake of Fe, Cu, Zn and total choline levels was not shown to affect their levels in breast milk, these findings are based on a limited number of studies, and so there remains uncertainty for these nutrients.
Maternal intake of DHA, EPA, ALA, B vitamins, and vitamins A, E, and K, iodine, Se, ovalbumin, and tyrosine was shown to affect their breast milk levels; however, the strength of evidence and quality of studies underpinning this evidence were highly variable. The relationship between DHA and EPA intake and breast milk levels has been extensively investigated, and the results across the experimental and observational studies show clear and consistent results that maternal intake influences their levels in breast milk(Reference Argaw, Bouckaert and Wondafrash19,Reference Boris, Jensen and Salvig20,Reference Fougere, Bilodeau and Lavoie22–Reference Mazurier, Rigourd and Perez25,Reference Smithers, Markrides and Gibson29–Reference Yang, Li and Li33,Reference Antonakou, Skenderi and Chiou35,Reference Bzikowska-Jura, Czerwonogrodzka-Senczyna and Jasińska-Melon36,Reference de la Presa-Owens, Lopez-Sabater and Rivero-Urgell38,Reference Juber, Jackson and Johnson40,Reference Olafsdottir, Thorsdottir and Wagner43–Reference Scopesi, Ciangherotti and Lantieri47) . With vitamin A, there was a much greater heterogeneity in experimental study design, particularly around the type of intervention and the dose and duration of supplementation; however, of the nine RCT, eight identified a positive relationship between intake and breast milk levels(Reference Bahl, Bhandari and Wahed48–Reference Ding, Hu and Yang52,Reference Grilo, Medeiros and Silva54,Reference Johnson, Qin and Krinsky55) . There have been fewer studies with B vitamins, and these are highly heterogeneous in study design, but overall, the results support the importance of maternal intake in influencing their levels in breast milk. Since the completion of this review, a relevant study has been published exploring the relationship between diet and nutritional status and the nutritional composition of donor milk(Reference Ureta-Velasco, Montealegre-Pomar and Keller102). Their results are consistent with our findings of a dose–response relationship between DHA intake and milk DHA content, and associations between maternal intake and milk levels of vitamins B1, B2, B6, C, and D. However, further high-quality studies are still needed, particularly for B12, to explore the effects of dose and also interactions between B vitamins(Reference Chang and Kirksey70–Reference Thomas, Kawamoto and Sneed74).
Studies of the effects of vitamin D intake are potentially confounded by seasonal effects, as breast milk concentrations of vitamin D and 25-hydroxyvitamin D have been shown to have significant seasonal variations(Reference Moller, Streym and Jensen103). Five experimental studies were identified that investigated the effects of maternal vitamin D supplementation on breast milk levels(Reference Ala-Houhala, Koskinen and Parviainen56–Reference Oberhelman, Meekins and Fischer60). Overall, the strongest effects were identified with the higher doses of supplementation, and importantly supplementation was shown to alleviate seasonal declines. It may therefore be advisable to recommend vitamin D supplementation in circumstances where endogenous synthesis is limited.
In all five experimental studies, maternal vitamin E supplementation increased breast milk α-tocopherol levels; however, two studies directly compared natural (RRR α-tocopherol) and synthetic (all-racemic α-tocopherol) sources(Reference Clemente, Ramalho and Lima61,Reference Gaur, Kuchan and Lai63) . Synthetic α-tocopherol is an equimolar mix of its eight stereoisomers, as the three chiral carbons of α-tocopherol can be in either an R or an S orientation, whereas in nature only one of these isomers (RRR) is found(Reference Ranard and Erdman104). Clemente and co-workers found that supplementation with both forms increased vitamin E concentrations in breast milk (colostrum); however, the RRR form was more efficient in increasing the levels(Reference Clemente, Ramalho and Lima61). Gaur and co-workers reported that supplementation with RRR increased the percentage of RRR α-tocopherol isomers in breast milk, whereas supplementation with all-racemic α-tocopherol decreased the percentage of RRR stereoisomers and increased the non-RRR-α-tocopherol stereoisomers, such as 2S-α-tocopherol(Reference Gaur, Kuchan and Lai63). Since the relative effects and potencies of these different forms of α-tocopherol on health outcomes are not well understood(Reference Ranard and Erdman104), it may be prudent at this time to recommend the RRR form of α-tocopherol, where supplementation is advised.
Three experimental studies were identified for vitamin K, and although a wide range of supplementation protocols were employed, a consistent dose–response relationship was identified between maternal vitamin K intake and breast milk levels(Reference Bolisetty, Gupta and Graham67–Reference von Kries, Shearer and McCarthy69). Three experimental and one observational studies were identified that explored the relationship between maternal intake of iodine and breast milk levels, and a consistent relationship was identified across all studies(Reference Leung, Braverman and He77–Reference Ureta-Velasco, Keller and Escuder-Vieco80). With Se four experimental studies were identified, and they all showed that maternal supplementation increases breast milk levels(Reference Dodge, Wander and Butler82–Reference Trafikowska, Sobkowiak and Butler84); however, the observational studies were discordant, with one identifying a significant association between fish intake and breast milk Se levels(Reference Valent, Horvat and Mazej86), whereas the other did not identify any significant associations(Reference Bianchi, Cruz and Zanetti81).
No clear relationship between nutrient intake and breast milk levels was identified for Cu, Fe and Zn; however, this is based on a limited number of studies(Reference Yalçin, Baykan and Yurdakök87–Reference Vuori, Makinen and Kara90). The only experimental study reported that Fe supplementation of non-anaemic women did not increase breast milk Fe levels(Reference Yalçin, Baykan and Yurdakök87), whereas although one observational study found that breast milk Fe concentration was significantly higher from those reporting taking daily Fe supplements(Reference Choi, Kim and Lee88), the other did not identify an association between Cu, Fe and Zn intake and breast milk levels(Reference Leotsinidis, Alexopoulos and Kostopoulou-Farri89). Albeit with this paucity of evidence, these results are consistent with previous observations that breast milk Cu, Fe and Zn concentrations are not associated with maternal mineral status(Reference Domellof, Lonnerdal and Dewey105).
Overall, the observations from the present review are consistent with the results of previous systematic reviews in this area(Reference Bravi, Wiens and Decarli6–Reference Keikha, Shayan-Moghadam and Bahreynian9), with the exception of vitamin C, where we did not identify a clear relationship between maternal intake and breast milk levels. This dissonance between may be based on differences in the study selection criteria between systematic reviews, as the present review only included studies on non-deficient populations. In the present review, three experimental studies were identified for vitamin C(Reference Thomas, Kawamoto and Sneed74–Reference Daneel-Otterbech, Davidsson and Hurrell76). All three studies were based on small numbers of participants, with a range of different supplemental dosing regimens administered. A relationship between maternal intake and breast milk content was only identified in one trail, which provided vitamin C at high doses, that is, 1000 mg per d(Reference Daneel-Otterbech, Davidsson and Hurrell76). It may therefore be hypothesised that in non-deficient populations, breast milk vitamin C levels are only responsive to higher levels of maternal supplementation; however, this needs further investigation.
It is important to highlight that in most of the reviewed experimental publications, the nutrient doses were higher than the EFSA adequate intake or average requirements(101). For instance, in all nine reviewed experimental studies, DHA and EPA supplementation was shown to increase breast milk DHA and EPA levels, and this was in a dose-dependent manner(Reference Argaw, Bouckaert and Wondafrash19,Reference Boris, Jensen and Salvig20,Reference Fougere, Bilodeau and Lavoie22–Reference Mazurier, Rigourd and Perez25,Reference Smithers, Markrides and Gibson29,Reference Valentine, Morrow and Pennell31,Reference Yang, Li and Li33) . The highest dose of DHA provided was 1·2 g for 14 d. The levels of supplementation provided were also higher than EFSA recommendations for vitamins A, B6, B12, E and K(101).
With regard to specific restrictive diets, breast milk from vegan mothers contained low DHA levels(Reference Perrin, Pawlak and Dean44), and in one study this was significantly lower than breast milk from vegetarian and omnivore mothers(Reference Sanders and Reddy46). The choline composition profile was also reported to be lower in breast milk from vegan mothers(Reference Perrin, Pawlak and Allen95), and taurine was also found to be lower in breast milk from vegan mothers(Reference Rana and Sanders92). Although the present review did not identify any studies that compared vitamin B12 content between mothers following vegan, vegetarian and non-vegetarian diets, vitamin B12 content was shown responsive to maternal intake. Therefore, our results support the previous recommendations in the systematic review by Karzc and Krόlak-Olejnik, that mothers following a vegan diet should consider supplementation with preformed DHA and vitamin B12, as maternal levels of intake of these nutrients may be low(Reference Karcz and Krolak-Olejnik8). Furthermore, as vegan and vegetarian diets become more popular, there is an urgent need to conduct further high-quality studies in this area, so lactating mothers and milk bank donors can be provided with specific nutritional recommendations.
With regard to the effects of intake of contaminants such as heavy metals and PCB, and breast milk levels, all studies reviewed were observational in nature, and it should be noted that any observed effects may therefore be confounded by environmental factors in addition to dietary intake(Reference Leotsinidis, Alexopoulos and Kostopoulou-Farri89,Reference Castro, Harari and Llanos97) . However, two studies reported a negative relationship between freshwater fish consumption and breast milk Hg content, and a positive relationship was found with cereals and vitamin supplements consumption(Reference Gundacker, Pietschnig and Wittmann99,Reference Ursinyova, Masanova and Uhnakova100) . A positive relationship was reported between maternal fish consumption (especially large fish species) and PCB content in breast milk, although the fish intake was extremely high (300 g/d)(Reference Dewailly, Ryan and Laliberté98). Overall, the number of studies published in this area is limited, with very few publications focusing on dietary sources; however, the overall presence of contaminants in breast milk appears below toxicity levels. Furthermore, based on these observations, fish intake was not identified as a potential source of elevated Hg in breast milk.
A strength of this systematic review is the extensive scope of nutrients and contaminants that have been considered, and recommendations in some areas are possible. Furthermore, our results have identified areas where there is a lack of high-quality evidence, particularly around the effects of ARA, Cu, Fe and Zn supplementation on breast milk content. The relationship between contaminant intake and breast milk levels also requires more comprehensive analysis, to delineate the effects of dietary intake from wider environmental exposures. A further strength is that this review considered studies undertaken in populations from a wide variety of countries and ethnicities, where healthy non-micronutrient-deficient lactating women were considered. Publications from developing countries were not included, unless it was clearly specified that the participants did not have a nutrient deficiency. However, as many publications on B vitamins were excluded during the selection process, as they were reporting on either deficient or low-income populations, we could not include any studies on vitamin B5 or folic acid.
Breast milk is a highly dynamic fluid, and the nutritional content has been shown to be affected by physiological factors in addition to nutritional intake, such as stage of lactation(Reference Ballard and Morrow106) and circadian rhythm(Reference Italianer, Naninck and Roelants107). Due to the variability in study reporting and high degree of design heterogeneity, it was not possible to draw specific conclusions about the effects of these factors in our analysis. Furthermore, other uncontrolled covariates that have been shown to influence the target compounds in breast milk and that were not analysed in the review, such as environment, obesity or genotype. For example, biosynthesis of DHA is influenced by genotype, as variations in SNP, particularly the fatty acid desaturase (FADS) gene cluster, affect PUFA levels. SNP within this cluster impact on expression of FAD genes across a wide range of tissues are associated with variations of n-6 and n-3 PUFA levels, such that those with the minor FADS allele have higher levels of LA and lower levels of ARA and DHA in serum phospholipids, plasma phospholipids and breast milk compared with carriers of the major allele(Reference Reynolds, Howard and Ruczinski108–Reference Xie and Innis110). Importantly, it has been shown that only women with the major allele appear to increase breast milk DHA by consuming fish or fish oil(Reference Xie and Innis110).
It should also be highlighted that the observational studies employed a wide variety of tools to assess dietary intake, and these have varying levels of precision, and there was also very little information across the publications as to how the supplements were ingested, and therefore absorbed, as it has been demonstrated that lipid-soluble nutrients are better absorbed when ingested with a fat meal(Reference Jeanes, Hall and Ellard111). These aspects should be considered in the design of future studies and also when providing nutritional advice to lactating mothers and breast milk donors. Our review limited the contaminants list to heavy metals and PCB; however, other ingredients should be further investigated. This is the case of non-nutritive sweeteners that were recently found in breast milk from lactating women in the USA(Reference Sylvetsky, Gardner and Bauman112).
In conclusion, the present systematic review assessed the available literature on quantitative associations between maternal diet and breast milk composition. Maternal intake, particularly DHA, EPA, ALA, and vitamins A, D, E, B6, B12 and K, ovalbumin and tyrosine were found to be responsive to maternal diet, whereas there is insufficient evidence to ascertain the effects of intake on breast milk ARA, vitamin C, Fe, Zn, Cu and choline. Although these results provide information that can be used to help inform nutritional guidelines for lactating mothers and breast milk donors, further high-quality research is needed to inform nutrient intake recommendations. At present, the recommendation for lactating women and milk donors should adopt a healthy and diversified diet, such as the Mediterranean diet, and consider supplementation with nutrients such as DHA and vitamins B12 and D when their diets are restricted or limited by external factors.
Acknowledgements
No funding was received for this work.
S. C. D. formulated the research question, C. F., Y. M. J. and S. C. D. designed the study protocol, C. F. and M. S. carried out data collection and quality assessment, and C. F. carried out interpretation of results and lead on the formulation the manuscript. C. F., Y. M. J. and S. C. D. critically reviewed the manuscript content, S. C. D. had primary responsibility for the final content of the manuscript, and all authors read and approved the final manuscript.
There are no conflicts of interest.
This research does not include studies on human subjects, human data or tissue, or animals.
All data are available upon request from the corresponding author.
Supplementary material
For supplementary material/s referred to in this article, please visit https://doi.org/10.1017/S0007114523002775











