Insulin resistance (IR) is the state in which tissues have reduced sensitivity to the metabolic actions of insulin, with decreased glucose uptake and, consequently, reduced muscle and adipose tissue utilisation, impairing suppression of IR(Reference Muniyappa, Lee and Chen1–Reference Vieira5). These metabolic changes increase the risk of chronic diseases during adulthood, such as type 2 diabetes mellitus, the metabolic syndrome and CVD. That being the case, the identification of IR at the earliest age has great clinical and epidemiological relevance(Reference Vieira5,Reference Moon, Park and Ahn6) .
In this context, direct diagnostic methods of IR, such as the gold standard technique of hyperinsulinaemic–euglycaemic glucose clamp, are costly, invasive and complex, with limited use in younger age groups(Reference Kim, Park and Kim7). Because of the practicality and the large scale of studies demonstrating its validity, including in children, the most used indirect method is the homoeostasis model of assessment – insulin resistance (HOMA-IR), which uses insulin values and fasting glucose. However, the examination of insulin is still expensive in certain countries, making the method inaccessible(Reference Vasques, Novaes and de Oliveira4).
Thus, some alternative methods, derived from simple and universally available tests, have been proposed to detect early IR in children of developing countries, thus preventing the onset of other chronic diseases(Reference Simental-Mendía, Rodriguéz-Morán and Guerrero-Romero8). One of these emerging methods is the TAG–glucose (TyG) index, which is extensively researched in adults(Reference Vasques, Novaes and de Oliveira4,Reference Simental-Mendía, Rodriguéz-Morán and Guerrero-Romero8–Reference Zheng and Mao14) .
The TyG index has been useful in assessing the risk of IR in comparison with the gold standard (glucose clamp technique) and the HOMA-IR in adults(Reference Vasques, Novaes and de Oliveira4,Reference Guerrero-Romero, Simental-Mendía and González-Ortís9,Reference Kang, Yang and Lee13,Reference Irace, Carallo and Scavelli15,Reference Du, Yang and Zhang16) . In addition, it functions as a risk marker for the development of type 2 diabetes mellitus, the metabolic syndrome and CVD, such as atherosclerosis in adults(Reference Unger, Benozzi and Peruzza10,Reference Irace, Carallo and Scavelli15–Reference Nor, Lee and Bacha17) . In view of the above, the current study aimed to propose cut-off points for the TyG index in Brazilian children and to evaluate their association with cardiometabolic risk. Our hypothesis is that children with increased TyG index present higher prevalence of cardiometabolic risk factors, as well as greater accumulation of these.
Methodology
Population and study design
This is a cross-sectional study, with data from two surveys carried out with children aged 4–9 years, in the municipality of Viçosa, MG, Brazil in 2015 and 2016: The Lactation Support Program and the School Health Assessment Survey.
The first is a cross-sectional analysis of a retrospective cohort of the birth of children in the only maternity in the municipality of Viçosa, MG, who were monitored during their 1st year of life and evaluated between 4 and 7 years of age in the years 2015 and 2016. The information about the calculation of sample size, selection and data collection was previously published(Reference Ribeiro, Fonseca and Andreoli18). The sample consisted of 141 children.
The second survey is School Health Assessment Survey, which is a cross-sectional study with a representative sample of 8- and 9-year-old children enrolled in seventeen public and seven private schools in the urban area of Viçosa, MG, in 2015, whose objective was to evaluate the cardiovascular health of these children. The information on the calculation and sample size, children’s selection and data collection was further detailed in other studies(Reference Milagres, Rocha and Filgueiras19–Reference Filgueiras, Vieira and Fonseca21). The School Health Assessment Survey sample consisted of 376 children.
After the data collection, the power of the study was calculated in the OpenEpi online software (www.openepi.com), considering as an end point of the IR by TyG index between two groups: exposed (altered waist circumference (WC)) and not exposed (normal WC). Based on the average and sd of the TyG index group of children with a normal circumference (7·89 ± 0·38) and with an altered WC (8·13 ± 0·43), a sample size of 515 children with 100 % power was estimated at a significance level of 5 %.
Anthropometry and body composition
The anthropometric measures were performed by a member of the trained team (nutritionists), with the children without shoes, wearing light clothing and accompanied by their guardians. Height (with vertical stadiometer measured in centimetres and millimetres) and weight (in electronic digital scale with capacity for 150 kg and accuracy of 10 g) were measured for the calculation of BMI. The nutritional status was classified according to BMI for age in z-score, according to sex(Reference Friedewald, Levy and Fredrickson22,23) , and overweight and obesity were grouped as ‘overweight’ for purposes of analysis. The numbers for WC and waist:height ratio were classified according to cut-off points proposed by Filgueiras et al. (Reference Filgueiras, Vieira and Fonseca21).
Body composition was assessed by dual-energy X-ray absorptiometry. The children remained in supine position, and the rays were emitted and measured by an energy discriminating detector, while wearing light clothes, no earrings, a bracelet or any metal ornament. Body fat (%) and android fat (%), stratified by sex, higher than the 85th percentile of the sample, were considered excessive(Reference Filgueiras, Vieira and Fonseca21,24) . All evaluations were performed at the Federal University of Viçosa Health Division.
Biochemical and blood pressure data
Blood samples were collected in children fasting for 12 h at the Clinical Analysis Laboratory of Federal University of Viçosa’s Health Division. Fasting serum glucose was performed by the glucose oxidase enzymatic method using the Cobas Mira Plus automation equipment (Roche Corp.) or by the enzymatic colorimetric method without deproteinisation (Bioclin). Fasting insulin was dosed by the electrochemiluminescence method or analysed in serum using Access Immunoassay Systems. TAG, total cholesterol (TC) and lipid fractions were evaluated (LDL-cholesterol, HDL-cholesterol and TAG). TC, HDL-cholesterol and TAG were measured by the enzymatic colorimetric method, with automation by a Cobas Mira Plus equipment (Roche Corp.), and LDL-cholesterol concentration was calculated by Friedwald’s ‘formula(Reference McCarthy and Ashwell25) in the Lactation Support Program study. In the School Health Assessment Survey study, TC, HDL-cholesterol and LDL-cholesterol were measured by the enzymatic colorimetric test (Bioclin) and analysed by the BS-200 apparatus (Bioclin). The ratios TC:HDL-cholesterol, LDL-cholesterol:HDL-cholesterol and TAG:HDL-cholesterol were also calculated.
The following values were deemed altered: fasting serum glucose ≥ 100 mg/dl, TC ≥ 170 mg/dl, LDL-cholesterol ≥ 110 mg/dl, HDL-cholesterol < 45 mg/dl and TAG ≥ 75 mg/dl(Reference Faludi, Izar and Saraiva26,Reference Oliveira, Foss-Freitas and Junior27) . Due to the absence of cut-off points, values higher than the 85th percentile of the sample, according to sex and age, were considered increased for TC/HDL-cholesterol, LDL-cholesterol/HDL-cholesterol and TAG/HDL-cholesterol.
The children’s blood pressure (BP) was assessed and classified according to the protocol established by the VII Brazilian Guideline for Hypertension using an automatic BP monitor(28).
Insulin resistance
To assess the risk of IR, the TyG index was calculated using the formula: Ln(fasted TAG (mg/dl) × fasting serum glucose (mg/dl)/2) with values expressed in logarithmic scale(Reference Simental-Mendía, Rodriguéz-Morán and Guerrero-Romero8). IR was also evaluated by HOMA-IR according to the formula: Fasting insulin (μU/ml) × fasting serum glucose (mmol/l)/22·5(Reference Matthews, Hosker and Rudenski29). HOMA-IR was categorised according to the 75th percentile of the sample, by sex, and was adopted as a reference to define the TyG index cut-off point(Reference Shashaj, Luciano and Contoli30) for the children in the current study.
Cardiometabolic risk factors
The number of cardiometabolic risk factors was obtained by the sum of the following present changes: overweight (overweight and obesity), body fat (%) > 85th percentile, increased WC and waist:height ratio, BP ≥ 90th percentile, fasting serum glucose ≥ 100 mg/dl; TC ≥ 170 mg/dl, LDL-cholesterol ≥ 110 gm/dl; HDL-cholesterol < 45 mg/dl, TAG ≥ 75 mg/dl/l, as well as android fat and increased TC:HDL-cholesterol, LDL-cholesterol:HDL-cholesterol and TAG:HDL-cholesterol ratios (>85th percentile, according to sex).
Statistical analyses
Statistical analyses were performed on STATA 13.0 and SPSS version 23.0 (SPSS Inc.), adopting a significance level of 5 % in all analyses. The Shapiro–Wilk normality test, histograms, kurtosis and asymmetry were used to evaluate the distribution of quantitative variables. The data’s descriptive analysis was performed through measures of frequency distribution, central tendency (average or median) and dispersion (sd or interquartile range).
To determine the TyG index cut-off point in predicting the risk of IR in children, we analysed the receiver operating characteristic, considering the cut-off point with the best balance between sensitivity and specificity values, according to sex. HOMA-IR was used as a reference method for the identification of the TyG index cut-off point. The analysis of the receiver operating characteristic curve and the identification of cut-off points were performed by MedCalc software version 9.4.2.0.
The sample was stratified according to the TyG index (normal and increased), according to the cut-off point identified by the receiver operating characteristic curve. The χ 2 test was used to compare the distribution of anthropometric, body composition, BP and biochemical variables among the groups according to the risk of IR by the TyG index. Student’s t test was used to compare the average number of cardiometabolic risk factors, according to the TyG index (normal and increased).
To verify the association between the risk of IR, defined by the TyG index, and the cardiometabolic risk factors, we performed Poisson regression models. Cardiometabolic risk factors were considered as independent variables and the increased TyG index as a dependent variable. The variables that were not categorised according to cut-off points specific for sex and age were also adjusted by sex and age. Variables with a lower level of significance P ≥ 0·05 were excluded one by one from the model until the definition of the final model. The Hosmer–Lemeshow test was used to verify the fit of the final model. The association measure used was the prevalence ratio and their respective 95 % CI.
Results
The sample consisted of children with a mean age of 7·84 ± 1·28 years, of which 50·3 % were girls. It was observed that girls had a higher percentage of high LDL-cholesterol (61·7 %), hypertriglyceridaemia (55·9 %) and a high TC:HDL-cholesterol ratio (60·5 %) when compared with boys (Table 1). The mean of TyG index was 7·98 ± 0·42 in the total sample. There was no statistical difference in TyG values between the sexes (P = 0·057).
Table 1 Distribution of cardiometabolic risk factors in children aged 4–9 years, according to sex, Viçosa, MG, Brazil (2015/2016)

WC, waist circumference; WtHR, waist:height ratio; FSG, fasting serum glucose; TC, total cholesterol.
* Fisher’s exact test.
† χ 2 test (P < 0·05).
The cut-off points identified that depict the best balance between the values of sensitivity (S) and specificity (E) were 7·9 for boys and 8·1 for girls. These cut-offs presented satisfactory values of sensitivity and negative predictive values, with specificity values being moderate and the predictive values being low (Table 2). Considering these cut-off points, the prevalence of IR risk by the TyG index was 48·7 % (n 251). The prevalence of IR by HOMA-IR was 21·2 %.
Table 2 AUC, cut-off points, sensitivity, specificity and predictive values of the TAG–glucose index to identify the risk of insulin resistance in children aged 4–9 years, Viçosa, MG, Brazil (2015/2016)
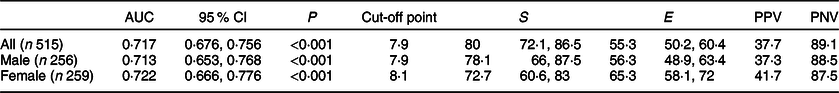
S, sensitivity; E, specificity; PPV, positive predictive value; PNV, negative predictive value.
Children with higher TYG index had an increased prevalence of total excess weight and central adiposity, hypertension and altered lipid profile (P ≤ 0·05) (Table 3).
Table 3 Distribution of cardiometabolic risk factors, according to the TAG–glucose (TyG) index, in children aged 4–9 years, Viçosa, MG, Brazil (2015/2016)

WC, waist circumference; WtHR, waist:height ratio; TC, total cholesterol.
* Cut-off point proposed by the current study (male ≥ 7·9; female ≥ 8·1).
† χ 2 test.
In the Poisson regression model, the prevalence of increased TyG was higher in overweight children, with high WC, increased waist:height ratio, excess body fat, android excess fat and hypertension, with increased values of TC, LDL-cholesterol and TC:HDL-cholesterol and LDL-cholesterol:HDL-cholesterol ratios, as well as in children with low HDL-cholesterol (Table 4).
Table 4 Association between cardiometabolic risk (independent variables) and increased TAG–glucose (TyG) index (dependent variable) in children aged 4–9 years, Viçosa, MG, Brazil, 2015/2016

WC, waist circumference; WtHR, waist:height ratio; TC, total cholesterol; PR, prevalence ratio.
Poisson regression.
* Variables classified according to sex and adjusted for age.
† Adjusted by sex and age.
Children with an increased TyG index had a higher rate of cardiometabolic risk factors than those with normal TyG index (Fig. 1).

Fig. 1 Number of cardiometabolic risk factors according to TAG–glucose (TyG) index in children, Viçosa, MG, Brazil, 2015/2016. *Student’s t test
Discussion
Our results demonstrated that the cut-off points of the TyG index to identify the risk of IR were 7·9 and 8·1 for boys and girls, respectively. Analysis of the receiver operating characteristic curve showed that the TyG index may be useful to identify the risk of IR, since it has AUC of 0·71 for boys and 0·72 for girls. It is known that the highest possible value for the AUC is 1·0, and the closer this value, the greater the accuracy of the test. Since these with AUC close to 0·75 are very useful clinically, it can be considered that the TyG index, in the current study, presented moderate accuracy to identify IR risk in Brazilian children from 4 to 9 years of age(Reference Faraggi and Reiser31,Reference Angoorani, Heshmet and Ejtahed32) .
Considering the best balance between S and E, the test had a moderate accuracy in the identification of the risk of IR, since for the cut-off points, the numbers were S = 78·1 % and E = 56·2 % for boys and S = 72·7 % E = 65·3 % for girls; therefore, it can be a useful method for metabolic screening of children, since it has an easy calculation and uses common biochemical tests(Reference Angoorani, Heshmet and Ejtahed32). Due to its higher sensitivity, the TyG index was more appropriate to identify children with IR and, consequently, had a reduced number of false negatives.
The low positive predictive value can be determined by the low specificity and the low prevalence of the outcome in the sample(Reference Akobeng33). We can observe in the current study that the positive predictive value was influenced by the moderate value of specificity. Due to the lack of cut-off points for HOMA-IR for the age group of the current study, we adopted the value of the 75th percentile of the sample, resulting in a proportion of 25 % of children with inadequate HOMA-IR. Hence, future studies that will adopt other criteria for the HOMA-IR may find different predictive values for the cut-off points proposed here.
In children and adolescents aged 9–13 years old from South Korea, a cut-off point of the TyG index for IR diagnosis of 8·2 was identified, with S and E similar to the current study: 77·3 and 68·3 %, respectively(Reference Kang, Yang and Lee13). Furthermore, it should be considered that children with a TyG index greater than these cut-off points should be submitted to more precise and specific methods to confirm the diagnosis(Reference Ribeiro, Fonseca and Andreoli18). In addition, it is necessary to validate the cut-off points proposed in the current study for children of other age groups and places of residence.
We observed that children with cardiometabolic risk factors, such as with excess weight and total and central body adiposity, hypertension and altered lipid profile, presented higher prevalence of increased TyG index. Similar results were found by other authors who observed a positive association of high TyG index with overweight, high BP and low HDL-cholesterol in children and teenagers(Reference Ribeiro, Fonseca and Andreoli18,Reference Silva34) .
It is worth noting that childhood obesity may lead to increased hepatic TAG production and decreased HDL-cholesterol, and these abnormalities may be observed mainly in children with abdominal obesity(Reference Kang, Yang and Lee13). The accumulation of visceral fat is a key factor in the development of IR. Visceral fat has higher lipolysis rates and increases the supply of NEFA to the liver through the portal vein, which is associated with changes in glucose and lipid metabolism. As a consequence of the expansion of adipose tissue, which also has an endocrinologic function, there is an increase in tissue hypoxia, infiltration of inflammatory cells and changes in the cytokine profile, which is also associated with IR(Reference Sinaiko and Caprio3).
The state of IR can also increase BP through several mechanisms(Reference Ferreira, Nóbrega and França35). Among them, by causing a failure in NO-mediated vasodilatory activity produced by insulin in the endothelial cells, leading to endothelial dysfunction and hypertension(Reference Ferreira, Nóbrega and França35,Reference Carvalho, Colaço and Fortes36) . On the other hand, as IR increases the risk of hypertension, factors related to this increase in BP influence the occurrence of IR, such as increased activation of the renin–angiotensin–aldosterone system and the sympathetic nervous system, oxidative stress, inflammation and functional mitochondrial abnormalities(Reference Whaley-Connell and Sowers37).
Another factor that associates IR to the occurrence of CVD is dyslipidaemia, characterised by hypertriglyceridaemia, low levels of HDL-cholesterol and/or increased LDL-cholesterol levels(Reference Faludi, Izar and Saraiva26). In IR, the decrease in insulin function increases lipolysis, with the formation of NEFA, and there is less activity of lipoprotein lipase, generating a remnant of TAG-rich chylomicrons, causing elevation of hepatic free fatty acids (FFA) and secretion of TAG-rich VLDL, processes that also affect the metabolism of HDL-cholesterol(Reference Tangvaarasittichai38).
In addition, in the current study, the accumulation of cardiometabolic risk factors in children with high TyG index was higher in relation to the others. Simental-Mendía et al. (Reference Simental-Mendía, Hernández-Ronquillo and Gómez-Diaz39), in a study with children and adolescents aged 6–15 years, observed that those distributed in the highest quintile of the TyG index had higher prevalence of cardiometabolic risk in relation to the others, suggesting a direct association between cardiovascular risk and the increased TyG index.
Some positive points of the current study should be considered. This is one of the few studies in developing countries that proposed cut-off points for the TyG index in childhood, being the first Brazilian study with children, according to our knowledge. Our sample is homogeneous in relation to the physiological characteristics, which contributes to the reduction of possible influences of the body composition. As IR increases the risk of CVD in the future, this is an important step in assessing the risk of this metabolic change, for greater monitoring and prevention throughout life. It is worth noting that the cut-off points established in the current study are specific to the population studied and should not be generalised to other ethnic groups. It is necessary to carry out multi-centred studies with children from other locations and age groups(Reference Er, Wu and Chou12).
However, some limitations must be mentioned. First, there was an imbalance between the sensitivity and specificity of the cut-off points found, which could be false positive, and the positive predictive value was low, indicating caution in its use in clinical practice. However, the method correlated well with other risk factors and cardiometabolic alterations and has a relatively high sensitivity, making its use interesting for screening services, with the need for more specific tests to confirm diagnosis. Secondly, we highlight the absence of a well-established cut-off point for classifying IR from HOMA-IR, which was the reference method used in the current study. Due to the sample size, we were unable to stratify the analyses by age. Nevertheless, the sample consists of prepubertal children, which is a slightly heterogeneous group without major biological changes that could affect results.
We concluded that the cut-off points of the TyG index for identifying the risk of IR were 7·9 for boys and 8·1 for girls aged 4–9 years. The children at risk of IR estimated by the TyG index, according to the cut-off points proposed by the current study, presented a higher cardiometabolic risk. The proposed cut-off points can be used as a routine to assess children at risk of developing IR, provided that they are validated for the age group in which they will be applied, in order to help in the early identification of IR and associated cardiometabolic risk.
Acknowledgements
Acknowlegements: The authors thank the children and their parents for participating in the study, the Coordination for the Improvement of Higher Education Personnel, the National Council for Scientific and Technological Development and Quibasa/BioClin laboratory. Financial support: The current work was supported by a scholarship granted by the Coordination for the Improvement of Higher Education Personnel (code 001), by the National Council for Scientific and Technological Development (number of cases: 478 910/2013–2014 and 407 547/2012–2016). Conflict of interest: There are no conflicts of interest. Authorship: A.D.M.d.B.: conception of the study, accomplishment, analysis of the data and writing of the article; M.D.S.F.: data analysis and critical manuscript review; H.H.M.H. and S.d.C.C.F.: critical manuscript review; S.A.V.R.: study design, data analysis and critical manuscript review; J.F.d.N.: study design, supervision and critical manuscript review. Ethics of human subject participation: The current study was conducted according to the guidelines laid down in the Declaration of Helsinki, and all the procedures involving human subjects were approved by the Ethics Committee on Human Research of the Federal University of Viçosa, cases no. 892476/2014 and 663.171/2014. Moreover, the current project was presented to the Municipal Department of Education, the Regional Superintendent of Education and principals of schools. All participants, as well as their responsible, were informed about the objectives of the research, and informed consent was obtained from all children’s parents.










