What is gluten?
Gluten is the main storage protein used by some classes of flowering plants to nourish seeds during development and germination(Reference Shewry, Napier and Tatham1). It is a high molecular weight protein found in the endosperm of grass-related grains, including wheat, barley and rye. It is the composite of two classes of protein, a glutenin and a prolamin (gliadin in wheat), which can be fractionated to produce α, β and γ peptides. As plant seeds are the plant tissue most consumed by men, seed storage proteins have been long studied and characterised. Wheat gluten was first isolated in 1745(Reference Beccari2) and since then further advances in the knowledge of protein structure have established that the prolamin components of gluten are responsible for the ability to process wheat to form dough by means of creating a viscoelastic network(Reference Field, Shewry and Miflin3, Reference Shewry, Halford and Belton4).
History of gluten and mankind
Humankind has existed for about 2·5 million years with cereal crops being introduced to the human diet relatively recently, during the Neolithic Revolution about 10 000 years ago. This saw a transition from hunting and gathering of food to settled agriculture. The first signs of cultivation have been found in the Fertile Crescent in South West Asia and the subsequent farming expansion lasted until 4000 bc(Reference Harlan and Zohary5).
Cereal harvesting and consumption have gradually increased since then, until its major outbreak in the 20th century. Between the two World Wars, the need to develop a more efficient rationing system and increased agricultural production became evident. The improvement of wheat cultivation became one of the main objectives of the Nutrition Society which was founded in 1941 in Britain to advance the scientific study of nutrition and its application to the maintenance of health(Reference Copping6). This goal was achieved, with modern day global wheat production amounting to over 700 million tonnes per year (http://faostat.fao.org).
Moreover, the need to ensure an efficient agricultural production has led to the artificial breeding and selection of wheat variants with better adaption to extreme climate conditions, bread-making qualities and resistance to diseases(Reference van den Broeck, de Jong and Salentijn7). This has contributed to a dramatic change in the genetic variety and possibly immunogenic qualities of wheat over time(Reference van den Broeck, de Jong and Salentijn7). Currently, about 95 % of the wheat grown worldwide is bread wheat (Triticum aestivum), a hexaploid species which resulted from the spontaneous hybridisations between more ancient tetraploid (Emmer) and diploid species (Wild grass) and was then selected by farmers for its superior qualities and yields, such as higher number and bigger seeds(Reference Dubcovsky and Dvorak8). Furthermore, the awareness of the potential role of gluten in processing food has led to the industrial extraction of gluten from plant seeds and its use in the baking industry as an additive with various functions, such as increasing elasticity and stability of food products or as a protein supplement to low-protein food(Reference Kasarda9).
It is, therefore, believed that the rate of increase in gluten exposure, from the development of wheat cultivation to modern intensive farming, along with its genetic modification, has been too high to give our immune system the time to develop optimal adaptive mechanisms, although this ‘evolutionary theory’ has yet to be fully clarified(Reference Catassi10). Nevertheless, perhaps as a result of all these factors have come the changing epidemiology of coeliac disease (CD) and other gluten-related disorders.
Coeliac disease
CD is a chronic inflammatory enteropathy caused by dietary exposure to gluten(Reference Mooney, Hadjivassiliou and Sanders11). Although the manifestations of CD may have been described more than 100 years ago, it is only from the 1940s that the relationship between gluten and CD has been established(Reference van Berge-Henegouwen and Mulder12). However, more than 70 years later, the pathogenesis of CD has yet to be fully elucidated, but it is agreed that the ingestion of gluten in genetically predisposed individuals carrying the HLA-DQ2 and/or DQ8 alleles can arise in a T-cell mediated immune reaction, leading to small bowel villous atrophy and subsequent clinical manifestations(Reference Marsh13, Reference Sollid, Markussen and Ek14).
Historically, CD was rare with an incidence in the UK of 1 in 8000 being reported in the 1950s(Reference Davidson15). However, contemporary epidemiological studies estimate a worldwide prevalence of approximately 1 in 100 or 1 %(Reference Fasano, Berti and Gerarduzzi16, Reference Volta, Bellentani and Bianchi17). Nevertheless, a considerable proportion of patients still remain undiagnosed with estimates that for every patient diagnosed with CD approximately three cases are yet to be detected(Reference West, Fleming and Tata18). Furthermore, our understanding of the coeliac patient has drastically changed. Whereas previously most cases diagnosed were children, it has now been shown that in fact adult cases (characteristically presenting between the fourth to sixth decades) are more frequent occurring at a ratio of 9:1 compared with the paediatric cohort. We are also seeing new horizons of CD where previously rice-based cultures such as China and the Indian sub-continent are now ‘Westernising’ their diet with the introduction of bread, pasta and pizza, CD is being reported in epidemiological studies(Reference Wu, Xia and von Blomberg19–Reference Makharia, Verma and Amarchand22).
The clinical manifestations of CD are heterogeneous. The classical presentation of malabsorption characterised by chronic diarrhoea, weight loss and failure to thrive is relatively rare. Far more commonly, patients present with non-classical symptoms which include irritable bowel syndrome (IBS)(Reference Sanders, Carter and Hurlstone23), iron deficiency anaemia(Reference Corazza, Valentini and Andreani24), osteoporosis(Reference Kemppainen, Kröger and Janatuinen25), ataxia or peripheral neuropathy(Reference Hadjivassiliou, Gibson and Davies-Jones26). Indeed, given that IBS is extremely common affecting about 11 % of the population national guidelines now propose that all patients presenting with such symptoms should have CD excluded(Reference Kemppainen, Kröger and Janatuinen25). In fact, a meta-analysis has shown that CD accounts for 4 % of those cases presenting with IBS(Reference Richey, Howdle and Shaw27, Reference Ford, Chey and Talley28).
To date, the only therapy for CD is a lifelong gluten-free diet (GFD)(Reference Rubio-Tapia, Hill and Kelly29). Adherence to a restrictive GFD leads to gradual healing of the mucosa of the small bowel and to the resolution of malabsorptive symptoms(Reference Lee, Lo and Memeo30), although there is a consistent proportion of patients who continue to show a low grade of mucosal inflammation even on a GFD(Reference Lanzini, Lanzarotto and Villanacci31). The Codex standard (which is used in the UK and Europe), and similarly the Food and Drug Administration in the USA, suggest that foods containing 20 mg/kg or less of gluten or 20 parts per million of gluten can be labelled as ‘gluten-free’ and that foods containing between 21 and 100 parts per million of gluten can be labelled as ‘very low gluten’.
Non-coeliac gluten sensitivity
The definition of non-coeliac gluten sensitivity (NCGS) encompasses a spectrum of gastrointestinal and extra-intestinal symptoms which are triggered by the ingestion of gluten-containing food, yet in the absence of the serologic and histological hallmarks of CD or wheat allergy(Reference Catassi, Bai and Bonaz32, Reference Sapone, Bai and Ciacci33). This terminology was defined following double-blind placebo-controlled studies showing gluten per se to induce symptoms in the absence of CD(Reference Biesiekierski, Newnham and Irving34). The symptoms reported include abdominal pain, diarrhoea, constipation and bloating, as well as chronic fatigue, behavioural changes, bone or joint pain and muscle cramps(Reference Catassi, Bai and Bonaz32–Reference Biesiekierski, Newnham and Irving34). Symptoms typically occur shortly after the ingestion of gluten, resolve on a GFD and relapse after gluten challenge.
NCGS is part of a spectrum of gluten-related disorders, as outlined in Fig. 1. It is often self-reported or suspected by the patients themselves and then confirmed by physicians after other forms of gluten-related disorders have been excluded(Reference Catassi, Bai and Bonaz32). In fact, while the diagnosis of CD can be made in most patients on the basis of positive serology (presence of endomysial and/or tissue transglutaminase antibodies) and villous atrophy at duodenal biopsy(Reference Rubio-Tapia, Hill and Kelly29, Reference Ludvigsson, Bai and Biagi35), patients with NCGS present with negative serology and absence of villous atrophy(Reference Sapone, Bai and Ciacci33). However, the presence of antigliadin antibodies has been described in up to 50 % patients with NCGS(Reference Carroccio, Mansueto and Iacono36–Reference Volta, Bardella and Calabrò38), and an increase in duodenal intraepithelial lymphocytes, corresponding to the grade 1 of the Marsh–Oberhuber histologic classification, has been observed in a subset of patients with NCGS in the absence of other criteria for CD(Reference Carroccio, Mansueto and Iacono36). Moreover, the prevalence of NCGS seem to be higher in first-degree relatives of subjects with CD, and carriers of HLA-DQ2 and/or DQ8 seem to be at greater risk of experiencing symptoms related to NCGS than the general population although these data have not been confirmed in different epidemiological studies(Reference Carroccio, Mansueto and Iacono36, Reference Volta, Bardella and Calabrò38).
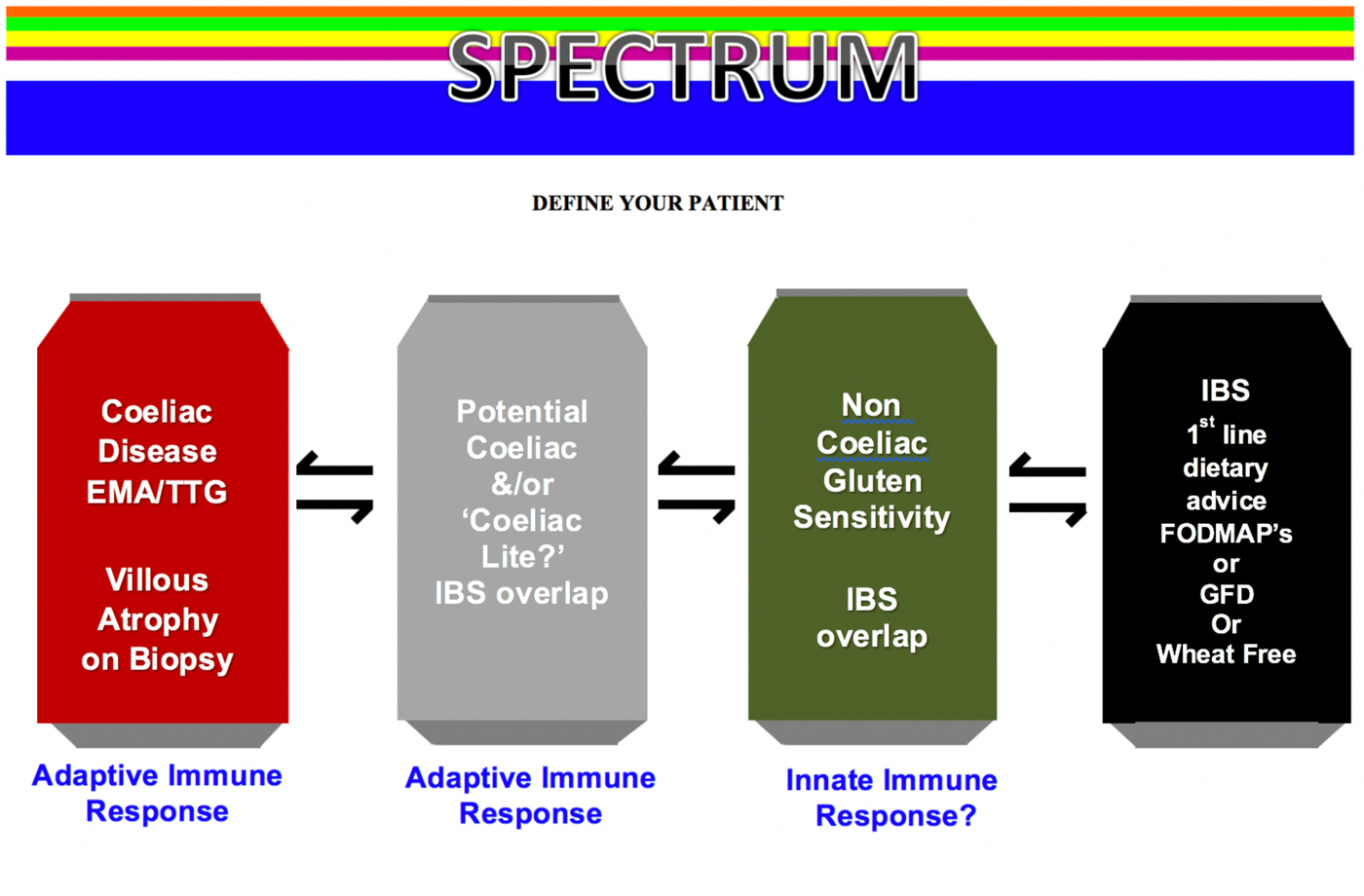
Fig. 1. (Colour online) Spectrum of gluten-related disorders. EMA, endomysial antibodies; TTG, tissue transglutaminase; IBS, irritable bowel syndrome; FODMAP, fermentable oligo-, di- and mono-saccharides and polyols; GFD, gluten-free diet.
The growing interest in this clinical entity has led to the advancing of several hypotheses about NCGS pathogenesis, yet all of them still remain to be fully elucidated. Altered intestinal permeability similar to that involved in the pathogenesis of CD and activation of the innate immune system following gluten exposure have been considered and are under investigation(Reference Sapone, Bai and Ciacci33, Reference Sapone, Lammers and Mazzarella39–Reference Brottveit, Beitnes and Tollefsen41).
In the absence of clear serologic or histopathologic criteria to orient toward a diagnosis, NCGS has often been perceived as being an IBS-like entity, mainly due to an evident overlap of clinical features between those two syndromes(Reference Verdu, Armstrong and Murray42). Furthermore, it has also been observed that IBS patients, previously naive to the effects of gluten, may benefit from a GFD(Reference Vazquez-Roque, Camilleri and Smyrk43). To date, the reference standard for the diagnosis of ‘true’ NCGS is an elimination diet followed by double-blind placebo-controlled gluten challenge, a method which could hardly be introduced into clinical practice(Reference Sapone, Bai and Ciacci33). Recently, a diagnostic algorithm based on the absence or presence of, clinical, serologic and histological criteria has been proposed to diagnose and differentiate NCGS from CD(Reference Kabbani, Vanga and Leffler44). This novel study provides a clinically pragmatic approach as it takes into consideration the difficulties that arise when evaluating patients who present with gluten-based sensitivity and are already taking a GFD, which in cases of CD can lead to negative coeliac serology and normal duodenal biopsies(Reference Kabbani, Vanga and Leffler44). It has been suggested that where available a negative HLA-DQ2 and DQ8 genotype is useful in that it can exclude CD with certainty given its 100 % negative predictive value; this will account for almost half of presenting cases(Reference Aziz, Lewis and Hadjivassiliou45). However, if HLA-DQ typing is not readily available, or is positive, then a gluten challenge followed by coeliac investigations is required(Reference Kabbani, Vanga and Leffler44). Traditionally, a gluten challenge has been suggested to be ≥10 g gluten (equivalent to about four slices of bread) daily for 6 weeks, prior to formalised testing. More recently this could be as little as ≥3 g gluten (equivalent to 1·5 slices of bread) daily for 2 weeks(Reference Leffler, Schuppan and Pallav46), which may be more suited to patients specifically presenting with gluten sensitivity. By adopting this approach in secondary-care gastrointestinal practice only a minority of adult patients will have a diagnosis of CD (7 %), with the remaining 93 % subsequently diagnosed as NCGS(Reference Aziz, Lewis and Hadjivassiliou45). Furthermore, individuals with NCGS do not appear to suffer the nutritional deficiencies (anaemia and haematinic deficiencies) and low mean BMI commonly associated with CD, which is a reflection of the state of normal villi as seen in NCGS as opposed to the villous atrophy in CD(Reference Aziz, Lewis and Hadjivassiliou45).
Gluten, fermentable oligo-, di- and mono-saccharides and polyols and irritable bowel syndrome
Diet appears to play a pivotal role in symptom generation in patients with IBS, with two-thirds of patients developing symptoms soon after food ingestion(Reference Simrén, Månsson and Langkilde47–Reference Böhn, Störsrud and Simrén49). A proportion of patients presenting with IBS may have a sensitivity to wheat. In a large retrospective study involving 920 patients fulfilling the Rome II criteria for IBS, 30 % (276/920) demonstrated wheat sensitivity or multiple food hypersensitivities, including wheat(Reference Carroccio, Mansueto and Iacono36). Participants from this study were subsequently followed up prospectively, demonstrating persistent wheat sensitivity over a median follow up of 99 months(Reference Carroccio, D'Alcamo and Iacono50).
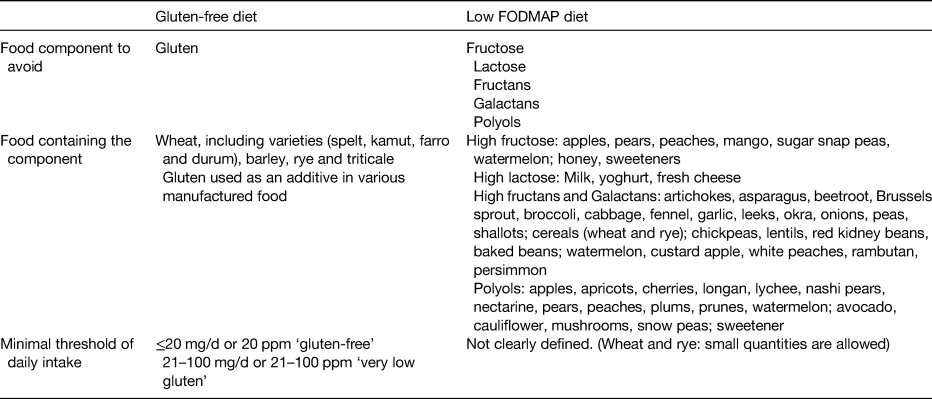
It is unclear which component of wheat is the causal agent for symptoms in IBS; gluten has been proposed as a causal factor, as well as fructans, which are part of the fermentable oligo-, di-, and mono-saccharides and polyols (FODMAP) family. Other agents such as α-amylase trypsin inhibitors and wheat germ agglutinins have also been suggested as causal agents(Reference Aziz, Hadjivassiliou and Sanders51). Research has recently focused on the role of a low FODMAP diet and GFD for symptom relief in patients with IBS. Table 1 outlines the main characteristics of these diets.
Table 1. Main characteristics of the gluten-free diet and the low fermentable oligo-, di-, and mono-saccharides and polyols (FODMAP) diet
There have been several trials assessing the role of a GFD in IBS. A randomised control trial in forty-five patients, who had been diagnosed with diarrhoea-predominant IBS, demonstrated increasing bowel movements daily on a gluten-containing diet v. a GFD, especially in those who were HLA-DQ2/8 positive(Reference Vazquez-Roque, Camilleri and Smyrk43). Also increased bowel permeability was noted in HLA-DQ2/8 positive patients, suggesting that gluten may alter intestinal barrier in patients with diarrhoea-predominant IBS (IBS-D)(Reference Vazquez-Roque, Camilleri and Smyrk43). A prospective study by our own group, in forty-one patients with IBS-D, demonstrated a statistically significant reduction in mean IBS symptom severity scores after 6 weeks of a GFD, following evaluation by a dietitian(Reference Aziz, Trott and Briggs52). There have been several double-blind placebo-controlled trials demonstrating the efficacy of a GFD in IBS, as seen in Table 2.
Table 2. Summary of double-blind placebo-controlled (DBPC) trials assessing the effect of gluten-free diet in irritable bowel syndrome

VAS, visual analogue scale; GI, gastrointestinal; FODMAP, fermentable oligo-, di-, and mono-saccharides and polyols.
The benefits of a low FODMAP diet was hypothesised at Monash University, Australia(Reference Gibson and Shepherd53), with the group focusing on the implementation of a low FODMAP diet in IBS(Reference Gibson and Shepherd54). A double-blind placebo-controlled trial by this group demonstrated that dietary reduction in fructose or fructans was likely to lead to symptom improvement in IBS, demonstrating the benefits of FODMAP restriction in general(Reference Shepherd, Parker and Muir55). There have been several randomised control trials demonstrating the efficacy of a low FODMAP diet, as seen in Table 3.
Table 3. Summary of randomised controlled trials (RCT) assessing low fermentable oligo-, di-, and mono-saccharides and polyols (FODMAP) diet in irritable bowel syndrome (IBS)
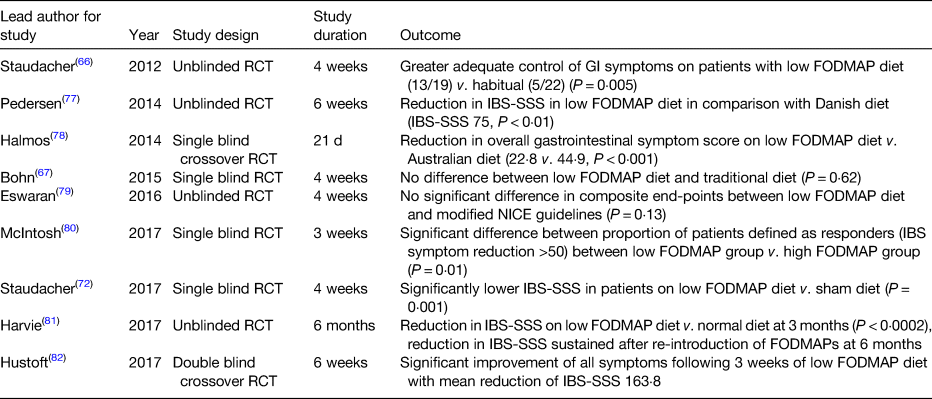
GI, gastrointestinal; IBS-SSS, IBS symptom severity score; NICE, National Institute for Health and care Excellence.
It is likely that there is significant overlap between both the GFD and low FODMAP diet, as it is unclear which component of wheat leads to induction of symptoms(Reference De Giorgio, Volta and Gibson56). Regardless of the mechanism, there appears to be compelling evidence to use both these dietary therapies in IBS. The implementation of these diets are best led by a dietitian, on the basis that most studies have been dietitian-led(Reference O'Keeffe and Lomer57), with a dietitian identifying the most appropriate diet for the patient based on a detailed history. This could be implemented through group education rather than one-to-one sessions, to help prevent a strain on existing resources(Reference Whigham, Joyce and Harper58).
Gluten-free diet as a ‘lifestyler’ choice
Historically, gluten-free products have been of limited availability with knowledge of CD amongst the general population shown to be lacking(Reference Karajeh, Hurlstone and Patel59). This inevitably contributed to the social phobia that individuals with CD experienced when dining out(Reference Sverker, Hensing and Hallert60). However, over the past decade, there has been a paradigm shift with a drastic rise in the availability of gluten-free products paralleled by an increase in awareness among the public(Reference Aziz, Karajeh and Zilkha61). Such findings are not only as a consequence of a rise in the incidence and recognition of CD. In fact, surveys conducted among the general population confirm that a greater number of consumers worldwide are following a GFD irrespective of the presence of CD(Reference Aziz, Lewis and Hadjivassiliou45, Reference Tanpowpong, Ingham and Lampshire62). Observational studies have reported that up to 13 % of the population may self-report sensitivity to gluten-based products and that up to 5 % of the population may be taking a GFD of their own volition(Reference Aziz, Lewis and Hadjivassiliou45, Reference Tanpowpong, Ingham and Lampshire62). Despite the prevalence of CD remaining stable in the US general population, the prevalence of people avoiding gluten has significantly increased(Reference Choung, Unalp-Arida and Ruhl63).
In some, the avoidance of gluten-containing food is viewed as a healthier lifestyle change rather than an actual treatment, whereas in others it is a consequence of reporting ill-effects to ingestion of gluten-based products. In fact, the relationship between the ingestion of gluten-containing products and the development of clinical symptoms even in the absence of CD has been described since the late 1970s(Reference Cooper, Holmes and Ferguson64, Reference Ellis and Linaker65). Healthy people have started to take a GFD as a lifestyle choice, leading to the rising interest of a GFD as a ‘lifestyler’, ‘free from’ or ‘clean eater’ choice.
Our own group has investigated the role of a GFD in healthy individuals, by performing a double-blind placebo-controlled trial, in which twenty-eight participants were recruited. Following a 2-week run-in period of a GFD, participants were randomised to receive either gluten-containing (14 g/d) or gluten-free products for 2 weeks. No significant difference in the primary endpoint of Gastrointestinal Symptom Rating Scores was noted between both groups. On the basis of this study, we suggest that gluten is unlikely to be the culprit agent in healthy individuals, and would not recommend commencement of a GFD in a healthy population.
Nutritional implications of restrictive diets
The use of restrictive diets, such as the low FODMAP diet and GFD, can lead to nutritional consequences. A reduction in calcium intake and short-chain fermentable carbohydrate intake has been demonstrated in those in a low FODMAP diet, in comparison with a habitual diet after 4 weeks(Reference Staudacher, Lomer and Anderson66). A statistically significant reduction in energy intake has also been demonstrated in patients following a low FODMAP diet (P < 0·001), in a randomised control trial comparing the low FODMAP diet with traditional dietary advice(Reference Böhn, Störsrud and Liljebo67). It must also be noted that there is emerging data that utilisation of an adapted FODMAP diet may be nutritionally adequate, with a long-term follow-up postal questionnaire study demonstrating no significant difference in carbohydrate and calcium intake between an adapted low FODMAP diet and habitual diet at long-term follow up, between 6 and 18 months(Reference O'Keeffe, Jansen and Martin68). Lower intakes of magnesium, iron, zinc, manganese and folate have been demonstrated in patients with CD following a GFD(Reference Wild, Robins and Burley69). A statistically significantly higher fat content on a GFD has also been demonstrated in children with CD(Reference Ferrara, Cicala and Tiberi70). It is, therefore, imperative that these diets are only implemented when necessary.
There are also concerns with restrictive diets with regard to the gut microbiota. A reduction in total bacterial abundance v. a normal diet has been demonstrated in patients with IBS taking a low FODMAP diet(Reference Halmos, Christophersen and Bird71), as well as a significant reduction in luminal bifidobacteria after 4 weeks of a low FODMAP diet(Reference Staudacher, Lomer and Anderson66). It is interesting to note that a recent placebo-controlled study, in 104 patients with IBS, demonstrated that patients had a lower abundance of Bifidobacterium species in faecal samples on a low FODMAP diet in comparison with a sham diet, but higher levels when given a probiotic(Reference Staudacher, Lomer and Farquharson72). Supplementation with probiotics could therefore potentially avoid this potentially deleterious effect of a low FODMAP diet, although long-term data are lacking. Similar changes in gut microbiota have also been demonstrated on a GFD, with a study in ten healthy subjects on a GFD demonstrated reductions in proportions of Bifidobacterium, Clostridium lituseburense and Faecalibacterium prausnitzii after 4 weeks(Reference De Palma, Nadal and Collado73). The effect on the gut microbiota of these restrictive diets requires further exploration, with long-term data lacking.
Conclusion
The rise in gluten production and consumption has led to the recognition of gluten-related disorders. CD affects 1 % of the population, which is important to diagnose in the first instance in patients presenting with symptoms induced by gluten. However, there is a growing body of evidence to show that individuals without CD are taking a GFD of their own volition. This clinical entity is defined as NCGS, although it is not without its controversy and uncertainty given the lack of diagnostic biomarkers and associated conflicting substrates which can provoke similar symptoms. Current evidence suggests there is a role for a GFD in the management of IBS, in addition to the role of a low FODMAP diet as a treatment in this group of patients. However, as demonstrated by our double-blind placebo-controlled study, there appears to be no role of a GFD in healthy individuals, which should be discouraged.
Financial Support
None.
Conflict of Interest
None.
Authorship
A. R., I. A. and D. S. S. wrote the initial manuscript and D. S. S. approved the final version.








