The micronutrients that we have come to know as vitamins had their road of discovery pathed by a multitude of deficiency diseases. A clear intervention, then still in the form of foods, relieved symptoms and cured diseases such as limes and scurvy, unpolished rice and beriberi and cod liver oil and rickets. Diseases nowadays are not marked by deficiency, rather overconsumption of foods tends to be the major cause of chronic diseases such as CVD, diabetes and cancer(Reference Lim, Vos and Flaxman1–Reference Tapsell, Neale and Satija3). These lifestyle diseases are multifactorial, where diet/nutrients play a role in disease development; however, more than a narrow focus on micronutrients is necessary to treat or prevent them.
Yet, dietary supplements remain popular in the general population where supplement users (SU) have been labelled as the ‘worried well’. Positive beliefs about supplements, such as ‘Help me to be healthy’, ‘Stop me getting ill’, ‘Not do me any harm’ and ‘Be the best I can do for myself’ have been observed among SU in the UK(Reference Conner, Kirk and Cade4). A Dutch survey found that 61 % thought that supplements were ‘sufficiently proven’ and 48 % believed that supplements were ‘an easy way to stay healthy’(Reference de Jong, Ocké and Branderhorst5). Also in the USA, the National Health and Nutrition Examination Survey reasons for supplement use relate to disease prevention/treatment and supplementing the diet(Reference Bailey, Gahche and Miller6). These opinions are in contrast with public-health guidelines in these countries, where there is, in general, no role for supplement use for adults, apart from illness/special conditions, and more recently, for vitamin D supplementation in at risk groups in the UK(7, 8).
So, is there a role for dietary supplements? Should we have to make up a balance of food v. supplements even if health guidelines are not encouraging the use of dietary supplements? The fact that supplements continue to be used means that the general population derives nutrients from both foods and supplements and the supplement contribution may be substantial. Supplement use is therefore an exposure that cannot be ignored in relation to (i) nutrient deficiency, sufficiency and toxicity, (ii) biomarker associations and sometimes (iii) disease, in case of suboptimal nutrient status or food intake (e.g. fish v. fish oil and the association with CVD). Alternatively, in observational research it is not always about establishing whether there is a benefit from supplement use itself, but also, how can we control for this health-seeking behaviour when we are interested in this (or another) exposure and health(Reference Satia-Abouta, Kristal and Patterson9). The typical SU does not exist, there is heterogeneity in the characteristics of SU, depending on the type of supplement consumed(Reference Denison, Jameson and Syddall10–Reference Patterson, Neuhouser and White13). Therefore, adjusting the supplement–disease analyses for yes/no supplement use might not take away the suspected confounding, but could potentially create (more) noise/attenuation in the associations.
The present paper aims to describe dietary supplement assessment methodology in the context of observational research and characterise the heterogeneity among SU. A secondary aim is to focus on the role of supplements in the nutrient distribution, circulating biomarkers and disease, using a variety of examples illustrating their (in)effectiveness in public health.
Dietary supplement assessment: definition, instruments and prevalence of use
Within Europe since 2002, dietary supplements have been regulated by the directive 2002/46/EC which defines supplements as(14): ‘Food stuffs the purpose of which is to supplement the normal diet and which are concentrated sources of nutrients or other substances with a nutritional or physiological effect, alone or in combination, marketed in dose form, namely forms such as capsules, pastilles, tablets, pills and other similar forms, sachets of powder, ampoules of liquids, drop dispensing bottles, and other similar forms of liquids and powders designed to be taken in measured small unit quantities’. Definitions of what are considered to be dietary supplements, or indeed specific types of supplements, have been reported to vary across American surveys(Reference Yetley15). Also in UK studies, definitions are lacking although the answer categories or the examples given to participants in the questionnaires give an indication of what was studied(Reference Denison, Jameson and Syddall10, Reference Kirk, Cade and Barrett16, Reference Harrison, Holt and Pattison17). Depending on the aim of the study, prescribed medication (as sources of folate, calcium and iron) can be included in order to calculate what is known as total nutrient intake (TNI), i.e. the sum of nutrient intake from foods and supplements(Reference Bates, Prentice and van der Pols18). Moreover, separating medication-derived nutrients from dietary supplements (or indeed food intake from dietary supplement intake) might provide additional information regarding reverse causality or confounding by indication, which might obscure the association with biomarkers or illness, e.g. the use of prescribed ferrous sulphate for anaemia, which itself might be caused by an underlying illness/treatment, will be differently associated with health than ferrous sulphate part of a multivitamin/multimineral (MVMM) supplement consumed out of choice.
The following issues arise when wanting to assess the nutrient contribution from supplements: (i) the potential for short-term use by participants, (ii) constant change in the supplement supply and (iii) constant change in supplement composition. The choice of the dietary supplement assessment instrument will have consequences for how well these issues can be dealt with. Dietary supplement use is assessed in similar ways to diet. There is self-reported data, using a variety of questionnaires, as well as objective measures, in the form of biochemical markers each with advantages and disadvantages (Table 1). The gold standard in supplement assessment is considered to be a face-to-face supplement inventory, which enables label transcription and/or collection of supplement bottles to retrieve nutrient composition as well as tablet count and hence provides very detailed information. This method has been applied in sub-cohorts or pilot studies, mainly to validate questionnaires(Reference Patterson, Kristal and Levy19, Reference Murphy, Wilkens and Monroe20). Label transcription has also been applied in the UK National Diet and Nutrition Surveys (NDNS) and the North/South Ireland Food Consumption Survey. General questionnaires can include question(s) regarding supplement use. Answer categories will enable categorisation into non-SU (NSU) and SU and might ask more detailed (possibly in free text) information on the type of supplement used, frequency or dose. The recall time and words such as ‘regular’, ‘usual’ or ‘seasonal’ will reflect the prevalence of supplement use obtained(Reference Dickinson, Blatman and El-Dash21, Reference Lentjes, Welch and Luben22). In a supplement frequency questionnaire, supplements are grouped, for example ‘fish oils’, ‘vitamin C’, ‘once a day multivitamins’ and frequency and/or amount of use are asked for each supplement group, sometimes specifying a minimal frequency of use required(Reference White, Patterson and Kristal23). The nutrient intake is calculated by assuming a nutrient formulation for each of these supplement groups. The recall period varies between studies and can be up to 10 years(Reference White, Patterson and Kristal23). A recall covers a period of 24 h, whereby supplement nutrient intake can be calculated using default nutrient profiles or manufacturers’ data matched with the exact supplement used, multiplied by the frequency of consumption. The number of days collected will influence the findings regarding prevalence of supplement use(Reference Murphy, Wilkens and Hankin24). In records, supplements can be recorded as they are consumed, which could minimise omissions due to forgetfulness (and thereby the potential for recall bias) and capture full label content. Participants are asked to fully describe the supplement, the dose (or enclose the label), the quantity and potentially also the clock time. The number of days collected will influence the results regarding prevalence of supplement use. Biomarkers, such as blood or urine samples, tend to be used to measure concentrations of the compound of interest or its metabolite. Biomarkers cannot differentiate between sources of the nutrient (i.e. whether the vitamin C was derived from foods or supplements), they vary in reference time (they may reflect recent or long-term exposure) and some nutrients are homoeostatic or may be affected by illness. Laboratory measures are independent of errors made during self-report, but sample collection can be burdensome for the participant as well as expensive.
Table 1. Overview of dietary supplement assessment instruments and characteristics of collected data
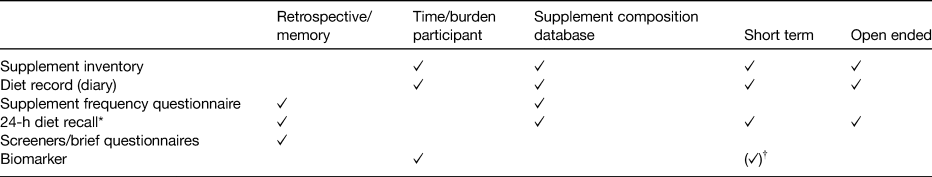
A summary based on Dwyer et al.(Reference Dwyer, Costello, Coulston, Boushey and Ferruzzi110).
* When repeated measures are taken, the time/burden approaches that of the diet record method.
† The measure is not uniform in its characteristic/use; see examples in the text.
In summary, all these instruments have limitations and the quality of the data obtained will influence how the obtained data may be used in analysis. Supplement–disease analysis may be fraught with confounding when simply comparing SU against NSU; supplement nutrient intake may require researchers to maintain time-consuming, detailed supplement composition data; while biomarkers will leave the researcher with a sample concentration, but without an idea of what was actually consumed. Indeed, a combination of instruments might be a better way forward(Reference Bates, Prentice and van der Pols18, Reference Nicastro, Bailey and Dodd25).
The choice of instrument is reflected in the prevalence of dietary supplement use observed. By using a similar instrument, secular trends can be monitored. Using a 1-year recall, the NDNS in 2012/13–2013/14 estimated the use of any type of dietary supplement in the UK among adults aged 19–64 years to be 15 % in men and 24 % in women and for those ≥65 years, 30 % and 41 %, respectively(Reference NatCen26). In years 5 and 6 of the rolling programme, the percentage using dietary supplements has not changed greatly for the oldest age category (38 and 41 %, respectively); for the younger age groups, up to a 3-fold increase was observed. Compared with earlier adult survey data collections in 1986/87, the change has been substantial since it was estimated to be approximately 9 and 17 %, respectively(Reference Henderson, Irving and Gregory27). Secular trends have also been observed in the USA, where the use of any type of supplement might have stabilised, but, for example, vitamin D supplementation increased between 1999 and 2012 from 5 to 19 % and n-3 containing supplements increased 7-fold up to 13 %(Reference Kantor, Rehm and Du28). A trend analysis of supplement use in the health professionals follow-up study and the nurses’ health study indicated continued increase of supplement use up to 2006, but a marked decrease of β-carotene after 1994, partly because trials suggested potential harm(Reference Kim, Giovannucci and Rosner29). The changes in trends may be a consequence of health policies (e.g. Healthy Start) and/or media coverage of trials. Supplement use varies greatly across Europe(Reference Flynn, Hirvonen and Mensink30), both in prevalence and in the type of supplement consumed. Comparisons across countries are hampered by the variety in recall time and choice of instrument. In European Prospective Investigation into Cancer (EPIC)-Europe, the choice of a single 24 h recall between 1995 and 2000 might have underestimated the usual supplement exposure; however, a clear North–South gradient was observed (Fig. 1), as well as positive trends with age(Reference Skeie, Braaten and Hjartåker31). The stark differences in the prevalence of supplement use between countries and continents needs to be considered when comparing results regarding supplement-sourced nutrient intake between studies.
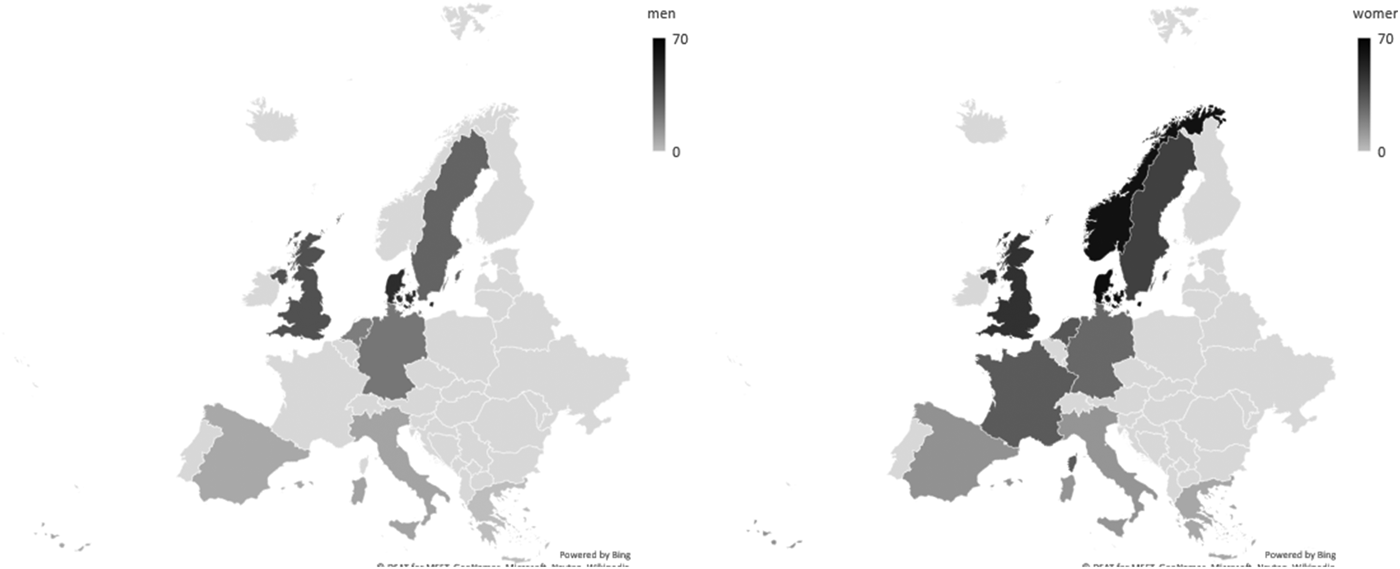
Fig. 1. Prevalence of any type of dietary supplement in European Prospective Investigation into Cancer-Europe as assessed by 24-h recall(Reference Skeie, Braaten and Hjartåker31). Data collection of the calibration study between 1995 and 2000.
Supplement nutrient intake: extremes of the distribution
All of the earlier listed assessment instruments, except the biomarkers, require the researcher to make assumptions regarding the supplement nutrient composition. The pre-structured questionnaires will assume a default nutrient composition. Open-ended questionnaires, such as used in the NDNS(Reference Bates, Lennox and Prentice32, Reference Bates, Cox and Nicholson33) and in the Norfolk arm of the EPIC (EPIC-Norfolk) study(Reference Lentjes, Bhaniani and Mulligan34), can be more specific, but will equally rely on the labels printed on dietary supplement packaging, and therefore the potential for label-transcription errors(Reference Dwyer, Saldanha and Bailen35). The packaging may contain errors, the supplement may have been kept in poor storage conditions or the supplement may contain ‘overages’, the latter mainly for vitamins, and taking into account safety limits, in the range of 5–100 % of the label value(36, 37). All these factors make what is ‘on the label’ not an accurate reflection of what is ‘in the dietary supplement’ and therefore a less accurate, or possibly even biased, measure of supplement nutrient intake (at least attenuating any association between nutrient intake and the biomarker or disease). A long-term process of developing a composition table based on analytical data has for these reasons been proposed and developed(Reference Dwyer, Picciano and Betz38, Reference Dwyer, Picciano and Raiten39).
Once the nutrient intake from supplements is assessed, it can be added to the food-sourced intake, to obtain TNI. This widens the range of the studied nutrient, and therefore enables risk assessment at either side of the nutrient intake distribution (Fig. 2). The at risk population is situated in the tails of the nutrient intake distribution (either because the intake remains low or becomes too high after inclusion of supplement sources), the intakes of which are less accurately measured. For this reason, researchers may take the upper/lower 5th percentile of the nutrient intake distribution as a more stable assessment rather than the proportion in the distribution above or below the exact cut-off set by the dietary reference values (DRV)(40, 41). When a limited number of dietary intake days are collected, researchers prefer application of statistical techniques such as ‘Shrink & add’ or ‘Add & shrink’ (see the measurement error webinar series for information about these methods(42)). The TNI distributions are used to establish the contribution that supplements make in meeting or exceeding DRV. The estimated average requirement (EAR) is used for comparing populations against a standard. It is the average nutrient requirement in a healthy group of people meant to maintain sufficient concentrations of a particular biomarker (blood/tissue concentration; enzyme saturation) in order to prevent nutrient deficiencies. The exact requirement is often unknown and assumed to be symmetrical(40), but reasonable estimates of the proportion at risk can be obtained using the EAR cut-point method(Reference Carriquiry43), which assumes that the proportion below the average nutrient intake is, under certain conditions, approximately the same as the proportion of people with an intake below their average nutrient requirement. The reference nutrient intake (RNI) is the EAR value plus two standard deviations, and covers the need of 98% in a population(40, Reference Carriquiry43). EAR value minus two standard deviations, termed the lower RNI, is likely to cover the need of only 2% of the population. The RNI might provide a good estimate for comparison against an individual's requirement; however, at the population level, this measure is (too) cautious(Reference Carriquiry43). The safe upper level (SUL) is defined by the expert group on vitamins and minerals to represent an intake that can be consumed daily over a lifetime without significant risk to health on the basis of available evidence(36) and refers to the supplement-sourced intake only. The guidance level is defined by the expert group on vitamins and minerals as an approximate indication of levels that would not be expected to cause adverse effect, but have been derived from limited data and are less secure than SUL(36).
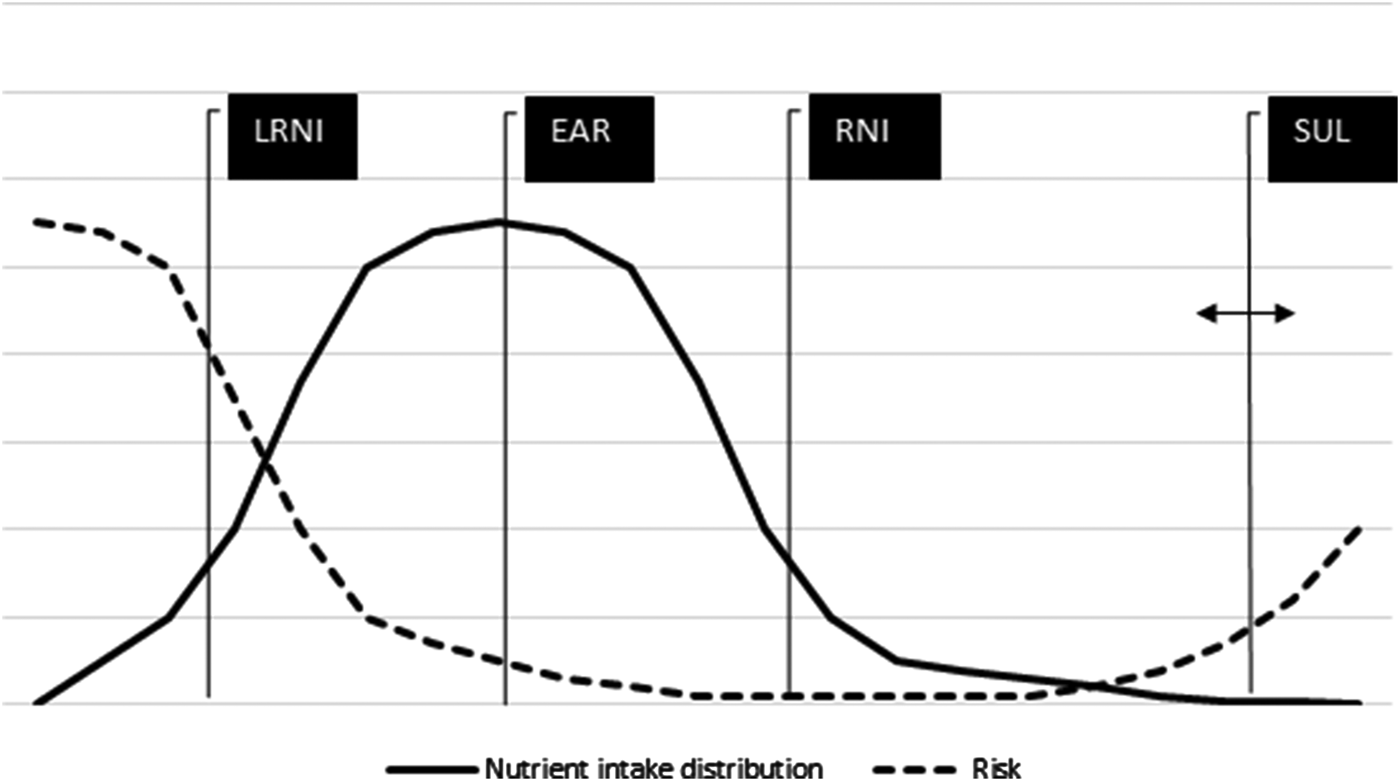
Fig. 2. Schematic of the various dietary reference values. Adapted and combined from Refs.(40,Reference Mulholland and Benford83,Reference Mason109) . LRNI, lower reference nutrient intake; EAR, estimated average requirement; RNI, reference nutrient intake; SUL, safe upper level.
Considering the variation in supplement use across Europe(Reference Flynn, Hirvonen and Mensink30, Reference Skeie, Braaten and Hjartåker31), supplements vary in the contribution that they make to food-sourced intake and the proportion of the populations at risk of not meeting the sufficiency DRV. There are however various complications when wanting to assess this across countries, not in the least because of different dietary assessment methodologies applied in surveys, but also what is considered sufficient across countries varies due to(Reference Doets, de Wit and Dhonukshe-Rutten44, Reference Roman Viñas, Ribas Barba and Ngo45): different expert panels, the currency of the evidence assessed, use of different DRV, different cut-off points for age groups, criteria for adequacy (i.e. the condition that the nutrient needs to prevent) and the extrapolation of data. Mensink et al.(Reference Mensink, Fletcher and Gurinovic46) streamlined participant-level data with regard to DRV and age cut-offs from dietary surveys in eight countries in the EU, with data collections between 1997 and 2010. Using vitamin C from this publication as an example, mean food-sourced intake in adults aged 18–60 years varied from 81 (Poland) to 152 (Germany) mg/d in women and from 81 (France, The Netherlands) to 152 (Germany) mg/d in men. After the contribution of supplements, TNI ranged from 96 (France) to 175 (Germany) mg/d in women and from 87 (France) to 173 (Germany) mg/d in men. There was a very small decrease (0–1 % women; 0–0·7 % men) in the percentage of the populations meeting the EAR after inclusion of supplements; only among the 65+ age group were reductions of 0–4 % obtained. Particularly for the vitamins A, D and E, and the minerals iron (among women) and selenium, a lower prevalence of intakes below the EAR (up to 34 % decrease for vitamin D) were observed after inclusion of supplement sources of these nutrients in adults. When it comes to exceeding upper limits due to supplements, Flynn et al.(Reference Flynn, Hirvonen and Mensink30) studied dietary survey data of seven vitamins and eight mineral nutrient distributions gathered in a selection of European countries between 1994 and 2006. Food-sourced intake (with fortified foods making a small contribution) was responsible for the majority of the populations’ intakes. The nutrient intake associated with the 95th percentile of retinol, zinc, iodine, copper and magnesium increased considerably after inclusion of supplement sources; however, it only exceeded the upper limits in a small percentage of the studied populations.
When supplement use is compared between countries or continents, its use and contribution do not only vary because of participant-associated variation (i.e. the choice of supplement), but also due to the choices in data handling and analysis by researchers. When comparing publications, large differences between studies may be explained due to SU all being grouped together v. nutrient-by-nutrient distinction among SU. This is the case when interpreting publications using National Health and Nutrition Examination Survey data for example(Reference Bailey, Fulgoni and Keast47–Reference Bailey, Fulgoni and Keast49). Here, far greater effects on meeting the EAR and exceeding the tolerable upper intake level are obtained because of different supplement nutrient groupings of participants (in addition to different DRV cut-offs and the majority of the supplements being MVMM-type supplements). Applying this nutrient-by-nutrient grouping strategy and UK DRV to the vitamin C intake as assessed in the NDNS data of years 1–4 of the rolling programme(Reference Bates, Lennox and Prentice32), Supplementary Table 2 is obtained. When the food-sourced vitamin C intake of all the men or all the women within the same age group are compared against the TNI, the median intake increased with 3–9 mg/d and the percentage of participants in this population not meeting the EAR was maximally 0·1–1·1 % lower once supplements were included, as was observed EU-wide(Reference Mensink, Fletcher and Gurinovic46). When we additionally ask the question ‘Who is at risk?’ and stratify the strata further by supplement status, we can allocate the supplement exposure to those who were truly exposed and not dilute the exposure with non-vitamin C containing supplements. When the vitamin C SU (SU + C) are identified, the contribution of the supplement was approximately 2-fold that of the food-sourced intake (Supplementary Table 2). The SU + C group had a lower risk of not meeting the sufficiency DRV (not just because of the supplement, but also because of higher food-sourced vitamin C intake among the SU − C compared to SU + C); moreover, only the SU + C group, and only when studying TNI, were exceeding quantities >1000 mg/d, intakes which have been associated with gastrointestinal problems(36). A visual representation of this TNI distribution and DRV is provided in Fig. 3.
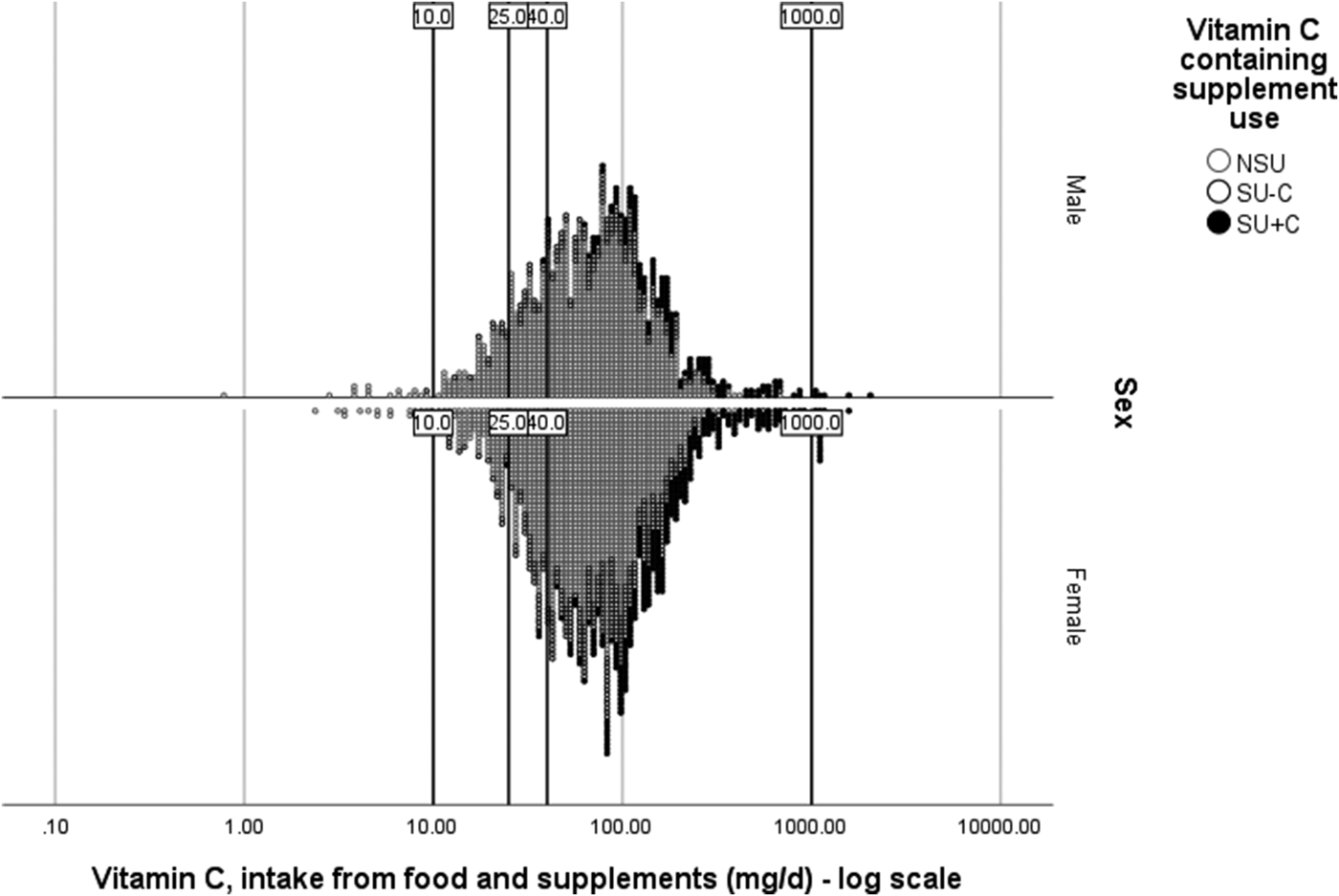
Fig. 3. Vitamin C total nutrient intake (food + supplements) distribution by vitamin C supplement user group status among men and women aged >18 years. Data from National Diet and Nutrition Surveys from years 1–4 of the rolling programme(Reference NatCen26). NSU, non-supplement users; SU, supplement users; SU + C, SU consumes a vitamin C containing supplement; SU − C, SU consumes a supplement without vitamin C; Lower reference nutrient intake (10 mg/d); Estimated average requirement (25 mg/d); Reference nutrient intake (40 mg/d); 1000 mg/d being the intake at which gastrointestinal problems have been reported.
Conclusion: intake
Supplement intakes shift the nutrient exposure distribution to the right; however, nutrient sufficiency, in most cases, may be obtained from food sources only. The (small) reduction in the proportion at risk after including supplements depends on the nutrient, but also on the grouping of the supplements. There is a modest higher risk of exceeding the upper limits when supplement intake is included (among those using that nutrient in supplement form).
Association between supplement intake and biomarkers
Objectively measured nutrient biomarkers may serve to validate the self-reported nutrient intake, by providing an indication of the ‘internal dose’, the absorption. Biomarkers may be influenced by a variety of factors described in detail elsewhere(Reference Jenab, Slimani and Bictash50, Reference Giovannucci51); however, with regard to dietary supplements as a source of nutrient intake, a few points stand out. First, the range of nutrient intake is made wider and different dose–response associations may be detected with TNI v. food-sourced intake alone. Secondly, the statistical parameters chosen in observational research are mostly there to establish correlations and quantify reclassification of participants, but a dose–response association is different and some of these results may be counterintuitive with regard to the ‘internal dose’. Thirdly, just as foods contain multiple nutrients which may interact (e.g. fat-soluble vitamins as antioxidants in high-fat foods), colinearity in supplement nutrient ingestion exists (e.g. use of MVMM-type supplements). Therefore, biomarkers other than the nutrients studied may be affected (e.g. vitamin C supplement use and tocopherol concentrations). These points are illustrated here.
In (large) cohort studies, circulating biomarkers are commonly used as an indicator of absorption/bio-availability. The nutrient exposure may be classified into N-tiles (e.g. tertiles, quintiles) and the means of both intakes and biomarkers may be presented for each N-tile, this to establish any type of dose–response association. Researchers may be interested in the (improvement of the) agreement in classification between the objectively and subjectively collected data, i.e. establish whether participants ranked and placed into a specific N-tile according to the biomarker are the same participants as those placed in this N-tile according to the questionnaire (comparing this agreement using the intake without and with supplements). Alternatively, researchers may wish to summarise the association between intake and biomarker in a single number, using either (i) a correlation or (ii) a β-coefficient. A correlation is a standardised measure (disregarding the unit) indicating the strength between two variables. If the correlation is high, then a standardised higher intake is associated with a standardised higher or lower biomarker concentration; however, it does not reflect a dose–response association (even when the value approaches 1 or −1), since the standardisation process has removed this aspect from the results. Using linear regression, which obtains the (adjusted) β-coefficient, the unit in which the variables are measured remains (although the input variables might be transformed), and the results may be interpreted as a dose–response since the intake of x amount of mg/d can be associated with a higher/lower y concentration of the biomarker. For example, correlations between TNI or supplement-sourced vitamin E intake and α-tocopherol concentration biomarkers have been reported to range from 0·3–0·7 using a variety of parameters on transformed or non-transformed data(Reference Satia-Abouta, Patterson and King52–Reference Bodner, Soutar and New55). In the Vitamin and Lifestyle cohort(Reference Satia-Abouta, Patterson and King52), adjusted correlations between supplement intake and biomarker were 0·69 with a significant linear trend across N-tiles (P < 0·0001); however, when plotting the means of the supplement intake groups (NSU: 0; quartiles: 18, 180, 194 and 360 mg/d) against the blood biomarker (NSU: 28, quartiles: 34, 44, 50 and 60 µmol/l), three issues become apparent: (i) supplement-sourced intake exceeds food-sourced intake 30–40-fold; (ii) due to the non-normal distribution of supplement-sourced intake, a wide range of supplement-sourced intake is grouped together, creating then small, then large differences between the N-tile means of intake; and consequently (iii) the dose–response of supplement intake is not the same at every amount of supplement-sourced vitamin E intake. Such observations were also observed by Zhao et al. in the Irish National Adult Nutrition Survey data(Reference Zhao, Monahan and McNulty56). α-Tocopherol concentrations are positively associated with vitamin E intake, γ-tocopherol is negatively associated with vitamin E intake due to preference of hepatic α-tocopherol transfer proteinase; furthermore, potential differences in the associations of plasma tocopherol and natural v. synthetic forms of vitamin E may exist(Reference Zhao, Monahan and McNulty57).
When assessing the association between nutrient intake (from both food and supplement sources) and a biomarker, Block et al. draw an analogy with smoking(Reference Block, Sinha and Gridley58). When the association between smoking and a nicotine biomarker is assessed, we could analyse the amount smoked at home separately from the amount smoked at work, or analyse the amount smoked at work adjusted for the amount smoked at home however the total amount smoked is the exposure of interest in aetiology(Reference Block, Sinha and Gridley58). Moreover, when applied to nutrient–biomarker associations, the biomarker has no ability to detect a difference between food or supplement sources. One more analogy may be added to the ones listed by Block et al. and that is that we would not average the number of cigarettes smoked whilst including the non-smokers. However, this is what happens by grouping all SU into a single group, the supplement contribution of a nutrient is diluted by SU who consume different types of supplements. A nutrient-by-nutrient supplement group distinction can provide insights not only in potentially differential food-sourced intakes (as described earlier in the intake distribution section), but also in potentially differential dose–response associations. Particularly so, since supplement-sourced intake could surpass food-sourced intake and has therefore been associated with biomarker saturation. In the EPIC-Norfolk study, dose–response associations have been observed to vary across subgroups of SU. A sex-adjusted analysis of published results(Reference Lentjes, Mulligan and Welch59), obtains the following associations between food-sourced vitamin E intake (per 10 mg/d) and back-transformed log-biomarkers of α-tocopherol concentrations (and therefore representing a percentage change (95 % CI)) among NSU, SU − E and SU + E respectively of: 10 % (9, 12 %), 9 % (6, 12 %) and 5 % (2, 9 %). When replacing food-sourced intake with TNI, the associations in the SU + E group weakened to 1 % (1, 2 %); although the adjusted correlation strengthened from 0·09 (food only) to 0·43 (TNI) among the SU + E (since supplement-sourced vitamin E intake may be over 10-fold higher than food-sourced intake in the UK). This linear model indicates saturation, which has been reported with intakes varying between 9 and 17 mg/d(Reference Lebold, Ang and Traber54, 60); and indeed, when only participants with TNI <17 mg/d were included, the coefficient among the SU + E was 9 %, although with wide CI (1,16 %). The urinary excretion products of vitamin E have for this reason been studied as a substitute to indicate sufficiency, or very high ingested doses(Reference Lebold, Ang and Traber54). Saturation thresholds also exist for vitamin C since kidneys excrete vitamin C at intakes higher than 120 mg/d(40); whereas retinol concentrations are largely homoeostatic, even after a state of toxicity has been reached(Reference Penniston and Tanumihardjo61) and therefore dose–response associations are not observed in replete individuals.
The n-3 fatty acids EPA and DHA are mostly obtained from oily fish, for which the most recent dietary guideline recommendations (one portion of oily fish per week, approximately 0·45 g/d or 3·15 g/week EPA + DHA)(62) have not been met in the UK population(Reference Bates, Lennox and Prentice32, Reference Bates, Cox and Nicholson33). A source of EPA and DHA may also be obtained from cod liver oil and fish oil type supplements (referred to as EPA/DHA-containing supplements), which could approximately double the exposure among those using EPA/DHA-containing supplements (SU + EPA/DHA). In EPIC-Norfolk, a general population-based cohort, aged between 39 and 79 years, the median TNI was 0·39 g/d in men and 0·29 g/d in women among SU + EPA/DHA between 1993 and 1998(Reference Lentjes, Mulligan and Welch59). For EPA or DHA supplements, when these nutrients are ingested separately or combined, in doses up to 7 g/d (i.e. over fifteen times the Scientific Advisory Committee Nutrition recommendation), dose–response associations in trials have resulted in increased plasma concentrations with the most efficient dose–response when the respective fatty acids is supplemented(Reference Arterburn, Hall and Oken63). Dose–response associations between the sum of EPA and DHA intake (3:2 ratio) and plasma EPA and DHA, have been found to be linear up to 3 g/d in a trial of healthy young men who consumed fish <1 time/week at baseline(Reference Blonk, Bilo and Nauta64). A trial among healthy men and women aged 20–80 years, who did not consume fish or supplements thereof, showed linear dose–response associations up to four portions of oily fish per week (where six capsules totalling 3·27 g EPA + DHA reflected a single portion)(Reference Browning, Walker and Mander65). However, in a cohort study where SU + EPA/DHA were excluded and fish consumption was 0·5–1 serving per week, a linear association was observed up to 0·5 g/d EPA + DHA intake(Reference Mozaffarian, Lemaitre and King66, Reference Mozaffarian, Bryson and Lemaitre67). The differences in dose–response between cohorts and trials may be explained by differences in bio-availability of food-sourced and supplement-sourced EPA + DHA due to varying fat content of meals and biochemical form of the supplemented fatty acids(Reference Schuchardt and Hahn68, Reference Ghasemifard, Turchini and Sinclair69) or the frequency of EPA + DHA consumption. Supplements in trials are advised to be taken daily, whereas fish is an episodically consumed food. Browning et al. observed that similar weekly doses of EPA and DHA (6·54 g/week, i.e. two times the Scientific Advisory Committee Nutrition recommendation), but taken either daily or dispersed over only 2 d per week, resulted in faster and sustained incorporation into plasma, platelets and erythrocytes when supplements were taken daily, although after 12 months no difference was observed in plasma concentration when comparing the weekly v. the daily exercise(Reference Browning, Walker and Mander70).
Not just pharmaceutical supplement doses, but also supplement doses not exceeding the RNI are associated with circulating biomarker concentrations. A recent publication from the Lung Cohort Cancer Consortium combined cohorts across four continents and analysed biomarkers in a single laboratory(Reference Midttun, Theofylaktopoulou and McCann71). It illustrated a wide range in vitamin status across the continents, with higher concentration among MVMM-type SU. In the 1994/95 NDNS 65+ sample, vitamin but not mineral intake from supplements, was associated with higher status indices, regardless of the supplement assessment tool used(Reference Bates, Prentice and van der Pols18). In the UK, vitamin D is mostly contained in cod liver/fish oil supplements as well as multivitamin and MVMM supplements. Here, the doses do not tend to exceed 5 μg/d and still 10 nmol/l higher 25(OH)D concentrations were observed among participants in the 1958 Birth Cohort who took such supplements(Reference Hyppönen and Power72), lowering their risk of a 25(OH)D concentration being <40 nmol/l by 64 (95 % CI 56,70) %.
Conclusion: biomarker
The supplemented nutrients are capable of raising plasma concentrations of the respective nutrients, particularly vitamins and fatty acids. Supplements at pharmaceutical doses might obtain high correlations between intakes and biomarker; however, the dose–response associations indicate saturation. A biomarker may be influenced by many other factors; moreover, it does not automatically mean that higher circulating concentrations indicate better health or functionality, since circulating biomarkers might not reflect storage or the effectiveness of the nutrient in an organ.
Health outcomes
In this last section, the balance between food and supplements is discussed in light of positive and negative health outcomes. Evidence for causality of a putative beneficial nutrient is generally taken from double-blinded, placebo-controlled trials; however, evidence with regard to side effects, contamination or toxicity are mostly gathered from extensive risk assessment using animal models, observational studies and case reports or sensitivity analysis from trial data. I will first contrast these study designs, followed by a summary of systematic reviews evaluating the role of dietary supplements and emphasising the differences between foods v. supplements.
Trials and observational studies have advantages and disadvantages when studying associations between supplement use and health/disease (Table 2). Trials are limited in the number of exposures that can be tested in a single experiment(Reference White, Patterson and Kristal23, Reference Patterson, White and Kristal73, Reference Byers74). The conclusion of dietary supplement efficacy in relation to the outcome is hence limited to the number of compounds tested, the dose tested (potentially higher than a commonly available dose) and the outcome tested. Moreover, particularly when the outcome is cancer, the follow-up in trials tends to be too short since the disease might take 10–20 years to develop(Reference Huang, Caballero and Chang75–Reference Taylor and Greenwald77). Trial findings can be obscured by the use of supplements beside the trial dose, particularly when these are unrecorded. Similarly, past use of supplements by trial participants (treatment or control) could obscure findings as well as pre-cancerous stages which may modify the risk to the intervention arm(Reference Patterson, Neuhouser and White13, Reference Taylor and Greenwald77, Reference Greenwald, Anderson and Nelson78). Regarding observational studies and supplements, such studies can be more inclusive in their eligibility criteria and the follow-up time tends to be longer than in trials. They can assess a wide range of commonly used dietary supplements and doses(Reference White, Patterson and Kristal23). Depending on the frequency of assessment, cohorts can take into account the variability of supplement use over time, since a single measure cannot be considered to reflect habitual supplement use(Reference Patterson, Neuhouser and White79, Reference Bailey, Fakhouri and Park80). Conversely, observational studies suffer from confounding and, if retrospective measures are used, potentially recall bias(Reference Huang, Caballero and Chang75, Reference Manson, Gaziano and Spelsberg81). The distribution of socio-demographic characteristics, behavioural factors, and prevalent illnesses are not uniformly distributed between SU and NSU(Reference White, Patterson and Kristal23, Reference Patterson, White and Kristal73, Reference Radimer, Bindewald and Hughes82). Additionally, the role of specific nutrients is difficult to assess due to colinearity, i.e. nutrients are commonly consumed as part of a MVMM-type supplement for which factorial trial designs are better equipped(Reference White, Patterson and Kristal23, Reference Patterson, White and Kristal73, Reference Taylor and Greenwald77).
Table 2. The advantages and disadvantages of using observational or trial data to ascertain efficacy of dietary supplements in disease prevention

Since supplements contain (isolated) nutrients in concentrated forms, TNI may lead to chronic intakes exceeding SUL(Reference Mulholland and Benford83) (Fig. 2). In the Iowa Women's Health Study, supplement use has (potentially for this reason, but also due to confounding by indication) observed harmful associations between supplemental iron and mortality(Reference Mursu, Robien and Harnack84). High retinol TNI (about 2500 µg/d) in combination with low vitamin D TNI (<11 µg/d) has been associated with fractures in post-menopausal women(Reference Caire-Juvera, Ritenbaugh and Wactawski-Wende85). For vitamin C the difference between the RNI and (reversible) harm in the form of gastrointestinal problems ranges between 40 and 1000 mg/d; whereas for retinol this is 600 v. 1500 µg/d (the difference being just over a common vitamin A dose in a supplement). The European Food Safety Authority(86) and the Expert Group on Vitamins and Minerals in the UK have extensively reviewed trials and safety reports for a wide range of nutrients(36). A selection of the SUL set by the expert group on vitamins and minerals are provided in Table 3. When compared against the 95th percentile of supplement-sourced intake among the adult population in the NDNS, it is observed that the intake of zinc and vitamin B6 could exceed the SUL. Although such intakes would need to be sustained over a long period of time to affect health and the collection of a single 4-d diary might not be sufficient to reflect a person's usual intake or capture the varying behaviour of supplement use.
Table 3. Safe upper limits as set by the Expert Group on Vitamins and Minerals (EVM)(36), applied to the National Diet and Nutrition Survey rolling programme years 1–4 where participants were 18 years or older(Reference NatCen26)
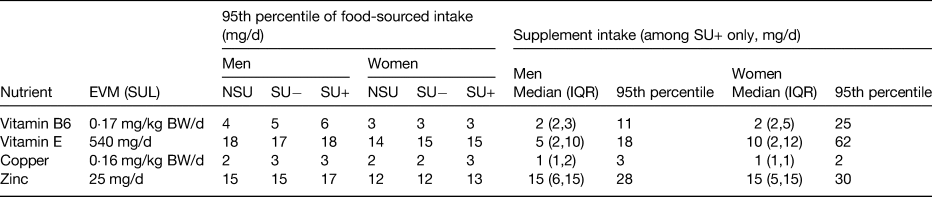
IQR, interquartile range; BW, body weight; NSU, non-supplement users; SU, supplement users; SU+, SU consuming the nutrient of interest in supplement form; SU−, SU not consuming the nutrient of interest in supplement form; SUL, safe upper level.
Systematic reviews with meta-analyses of trials randomising participants to placebo or single/combinations of anti-oxidant supplements (vitamin A, C, E, β-carotene and selenium), observed significant associations with harm in unbiased trials (relative ratio 1·04; 95 % CI 1·01, 1·07), but significant beneficial associations (relative ratio 0·91; 95 % CI 0·85, 0·98) for biased trials(Reference Bjelakovic, Nikolova and Gluud87). Significantly higher all-cause mortality risks were observed for β-carotene (relative ratio 1·05; 95 % CI 1·01, 1·09), and potentially for vitamins A and E, but not for vitamin C or selenium. Also the US Preventive services Task Force recommendation statement concluded that overall no benefit could be observed for primary prevention of cancer or CVD when using single nutrient supplements(Reference Moyer88, Reference Fortmann, Burda and Senger89). A meta-analysis of MVMM-type supplement trials concluded no benefit with regard to total, cardiovascular or cancer mortality(Reference Macpherson, Pipingas and Pase90).
The Linxian Nutrition Intervention Trials in the general population, studied the effects of the use of any of the four supplement combinations: retinol and zinc, riboflavin and niacin, vitamin C and molybdenum, or β-carotene, vitamin E and selenium in the prevention of all-cause mortality, cancer mortality and cancer incidence(Reference Blot, Li and Taylor91). It observed significant reductions in mortality (9 %), cancer mortality (13 %), but particularly for stomach cancer (21 %) when β-carotene, vitamin E and selenium were supplemented. Potential explanations for the observed effects were marginal micronutrient intake at baseline due to low consumption of fruit and vegetables. Indeed, plasma vitamin C concentrations were low at the start of the trial and a daily supplement dose of 120 mg/d raised these concentrations comparable to or just below the UK mean. Suboptimal circulating vitamin concentrations have also been proposed as an explanation for the decrease in cancer incidence in the supplementation v. placebo arm in men of the Supplementation en Vitamines et Mineraux Antioxydants trial, since the baseline antioxidant concentrations were lower in men. In post hoc analysis, an interaction (P = 0·04) between baseline concentrations and trial arm could only be observed for vitamin C and only among men(Reference Galan, Briançon and Favier92).
Since nutrients may be derived from a variety of (potentially fortified) foods, and not necessarily from foods which are recommended for public health, one can argue that food intake might be a better marker of optimal intake rather than nutrient intake. For example, median vitamin C TNI expressed as a percentage of the RNI was 185 and 197 % in men aged 19–64 years and 65+ years, respectively, and 192 and 209 % in women(Reference Bates, Lennox and Prentice32). Contrasting this to fruit and vegetable consumption, the UK diet meets 30 and 40 % of the 5-a-d guidelines in both men and women aged 19–64 years and 65+ years, respectively(Reference Bates, Lennox and Prentice32). The role of multivitamins in the past was partly seen as a means to compensate poor dietary choices(Reference Patterson, White and Kristal73); or, where after various considerations, the likely benefits outweighed harm of supplement use(Reference Willett and Stampfer93). However, as observed in earlier described meta-analyses, such use has not been successful in the prevention of disease or early death in populations. Potentially, since foods contain more than vitamins and minerals alone and dietary patterns as a whole play an important role in health(Reference Tapsell, Neale and Satija3).
An example of a sub-optimally consumed food group in the UK is fish, of which the recommendation is to consume two portions/week (about 280 g/week). In men, intake reached 161 g/week and 252 g/week for the age groups 19–64 years and 65+ years, respectively; in women 154 g/week and 189 g/week(Reference Bates, Lennox and Prentice32). Data on the contribution of EPA + DHA from the most commonly consumed supplement, cod liver oils and fish oils, are lacking in the national surveys. These results are available from the baseline EPIC-Norfolk cohort (Supplementary Table 5). The low dose EPA + DHA from mainly cod liver oil resulted in 15–20 % more participants meeting the EAR of 0·45 g/d.
Higher fish consumption has been associated with lower CHD/CVD mortality in cohort studies, despite differences across the globe due to differences in dietary assessment methods, absolute amounts of fish consumed, fish preparation and water contamination(Reference Jayedi, Shab-Bidar and Eimeri94, Reference Zheng, Huang and Yu95). Various biological mechanisms relating to long chain n-3 fatty acids and CHD have recently been reviewed in these Proceedings, including the prevention of arrhythmia and anti-inflammatory properties(Reference Calder96, Reference Hall97). Fish may also exert its benefit as a source of protein, vitamin D, iodine, calcium (bones), or due to the substitution effect when consumed as part of a meal(Reference Kiefte-de Jong, Chowdhury and Franco98, Reference Bowen, Harris and Kris-Etherton99). Although, trials using EPA + DHA supplements in secondary/tertiary prevention groups showed promising results initially, later trials observed no benefit(Reference James, Sullivan and Metcalf100). A recent review by the Omega-3 Treatment Trialists’ Collaboration confirmed no benefit in relation to fatal CHD or nonfatal myocardial infarction among those with existing CHD(Reference Aung, Halsey and Kromhout101). Supplementation with n-3 fatty acids for primary prevention of CVD has not been advised due to lack of trial results in primary prevention(Reference Siscovick, Barringer and Fretts102, Reference Nestel, Clifton and Colquhoun103) (the results from the first primary prevention trial on Vitamin D and EPA + DHA, the Vitamin D and Omega-3 Trial, are not yet available(Reference Manson, Bassuk and Lee104)), only the consumption of oily fish and seafood is currently advocated. Since cod liver oil is a low dose source of EPA + DHA and a commonly consumed supplement in the EPIC-Norfolk study (Supplementary Table 5), it was possible to assess the role of this supplement in primary prevention of CHD mortality. A low dose of 250 mg/d EPA/DHA is considered sufficient for prevention of arrhythmia(Reference Mozaffarian and Rimm105). Due to supplement use, an additional 19–24 % of the participants met this threshold. The confounding associated with SU + EPA/DHA and SU − EPA/DHA as well as the changes over time in supplement use was modelled using time-varying covariates analysis. It was observed that CHD mortality was 26 % lower (95 % CI 16, 34 %) among SU + EPA/DHA compared with NSU, but no significant association was observed when comparing SU − EPA/DHA v. NSU(Reference Lentjes, Keogh and Welch106). Due to the observational nature of the study, residual confounding and colinearity of nutrients could have occurred.
Conclusion: health
Whenever supplement use and health are being associated, the heterogeneity among SU cannot be ignored. The typical SU does not exist. The obvious distinction between SU lies in the variety of the supplements consumed, but also in the many other disease risk factors which might confound or bias the supplement–health association in observational research. Supplements may be considered natural; however, the concentrated form puts the user at risk of harm when overdosed. Meta-analyses of trials studying MVMM supplements thus far have indicated that if populations are optimally nourished, there is no role for supplement us: enough is enough(Reference Guallar, Stranges and Mulrow107).
Conclusions
How does the balance tip between foods and supplements? Supplements continue to be used by an increasing proportion of the population, so their contribution to diet, health and disease needs to be monitored. Traditionally, essential nutrients have been studied in relation to health, and although micronutrient deficiencies are still prevalent in the UK population, the relatively high nutrient intake may not be a marker of healthy food choices, as reflected in the low fruit, vegetable and fish consumption from national surveys. Resolving unhealthy dietary patterns with micronutrient supplements is a too narrow-minded solution. Nowadays, public-health nutrition guidelines take the role of the nutrient, its food source and its place in the diet into account to optimise diet. The current role of supplements herein seems restricted to certain age groups, life circumstances or diseases with impaired nutrient absorption(7, Reference Manson and Bassuk108). The challenge in observational research methodology is to assess and describe nutrient intake, as well as diet as a whole, in the general population and to clarify the role, if any, of nutrient supplements in primary disease prevention.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S0029665118002525
Acknowledgements
I would like to thank Prof Kay-Tee Khaw and Prof Ailsa Welch for their supervision during my PhD studies. I thank staff at the Elsie Widdowson Laboratories for answering questions on the use of the NDNS datasets and Angela Mulligan for reading the draft manuscript.
Financial Support
The author reports programme grants from Cancer Research UK (G0401527 and G1000143) and the Medical Research Council (C864/A8257 and C864/A14136).
Conflicts of Interest
None.










