Article contents
A strategy for imaging-guided cocktail cancer therapy using dual-colored polymeric drug carriers with amplified aggregation-induced emission behavior by donor–acceptor/Förster resonance energy transfer adjustment
Published online by Cambridge University Press: 12 November 2020
Abstract
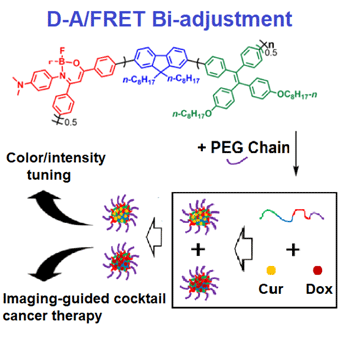
Nowadays, theranostics drug delivery systems (DDSs) with imaging and therapy bi-functions have been regarded as a future orientation for imaging-guided cancer therapy. To achieve high imaging quality, a donor–acceptor (D–A)/Förster resonance energy transfer (FRET) bi-adjustment strategy is carried out for designing dual-colored DDSs with amplified aggregation-induced emission (AIE) behavior for imaging-guided cocktail cancer therapy in this study. In detail, four AIE-active conjugated polymers P-1 to P-4 are synthesized via the Suzuki reaction. Noteworthily, the D–A-type structure is applied in tuning the fluorescence color from orange (P-1) to far-red/near-infrared (P-2), while the intramolecular FRET process further enhanced the fluorescence signal for six times (P-3). Afterwards, P-3-based amphipathic polymer P-4 further acts as a drug carrier in preparing doxorubicin (Dox)- and curcumin (Cur)-loaded polymer dots (Pdots) (Dox-loaded Pdots as PDox and Cur-loaded Pdots as PCur). PDox + PCur DDS is successfully applied in imaging-guided cocktail cancer therapy to give obviously higher in vivo anticancer efficacy compared with single PDox or PCur. In addition, the drug-loaded Pdots also exhibit higher biocompatibility compared with free drugs. This work provides a novel D–A/FRET bi-adjustment strategy for developing high efficiency imaging-guided cocktail DDSs in cancer therapy.
Keywords
- Type
- Article
- Information
- Copyright
- Copyright © The Author(s), 2020, published on behalf of Materials Research Society by Cambridge University Press
Footnotes
These authors contributed equally to this work.
References
- 1
- Cited by





