Article contents
One-pot polyelectrolyte assisted hydrothermal synthesis of NiFe2O4-reduced graphene oxide nanocomposites with improved electrochemical and photocatalytic properties
Published online by Cambridge University Press: 26 September 2014
Abstract
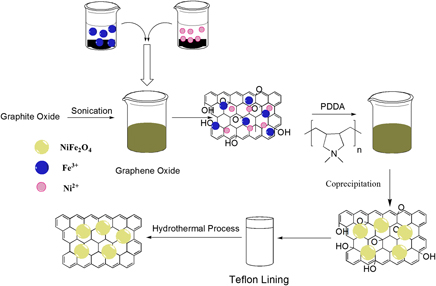
Reduced graphene oxide–nickel ferrite (RGO–NiFe2O4) has been successfully synthesized by the hydrothermal method in the presence of poly(diallyldimethylammonium chloride) (PDDA). PDDA is used both as a reducing agent and as a stabilizer. The prepared RGO–NiFe2O4 nanocomposites have been thoroughly characterized by spectroscopic (Fourier-transform infrared spectroscopy, Raman spectroscopy, and x-ray diffraction) and thermogravimetric analysis. Microscopy techniques (scanning electron microscopy, atomic force microscopy, and transmission electron microscopy) were used to probe the morphological structures as well as to investigate the exfoliation of RGO sheets. It is interesting to find that RGO–NiFe2O4 nanocomposites exhibited much better electrochemical capability than NiFe2O4. In addition, the as-prepared RGO–NiFe2O4 nanocomposites can effectively remove methyl orange from water under ultraviolet light irradiation, which can be used as novel photocatalysts for environmental protection.
- Type
- Articles
- Information
- Copyright
- Copyright © Materials Research Society 2014
References
REFERENCES
- 9
- Cited by




