Article contents
The advantage of alcohol–calcium method on the formation and the stability of vaterite against ethanol–water binary solvent method
Published online by Cambridge University Press: 17 January 2020
Abstract
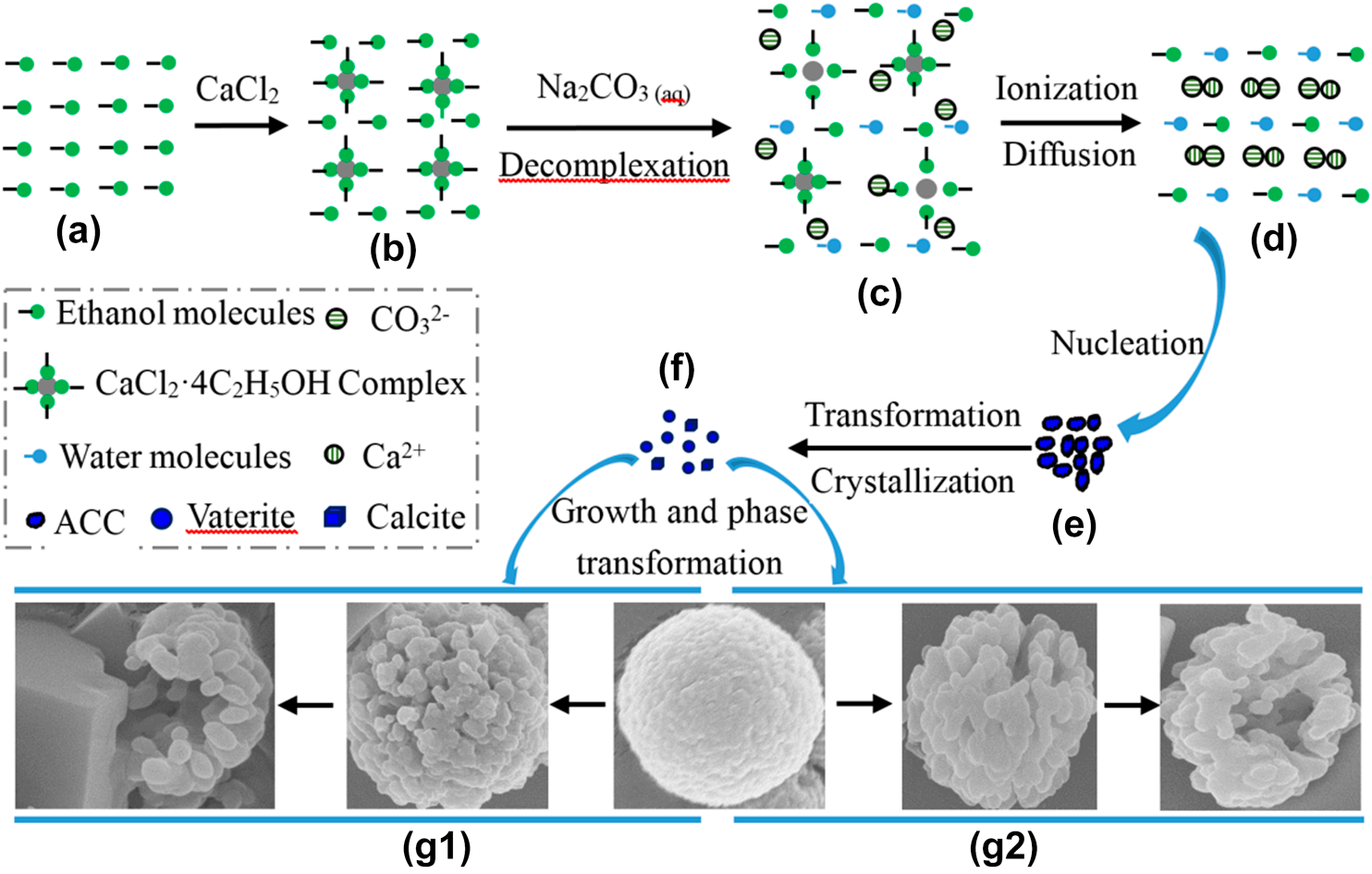
The advantage of alcohol–calcium method on the formation and the stability of vaterite against ethanol–water binary solvents (EWBS) method was studied through comparative experiment. The polymorphs and morphologies of CaCO3 were characterized by X-ray diffraction (XRD) and scanning electron microscopy (SEM). XRD results show that vaterite slowly decreases from 90.4 to 82.5% as increasing aging time from 0 to 1320 min in alcohol–calcium system, while quickly decreases from 46.5% to 0% at the same aging time in EWBS system. The similar variation as reaction temperature was found in both systems. SEM images indicate that calcite presents its typical rhombohedral morphology in both systems, while the morphologies of vaterite particles in two systems are different. In alcohol–calcium system, small vaterite nanoparticles aggregate into spherical microparticles, and these microparticles become porous, loose, and irregular, even incomplete, as increasing aging time and reaction temperature, while in EWBS system, vaterite nanoparticles aggregate into irregular microparticles. The advantage of alcohol–calcium method was discussed from the formation of the complex compound CaCl2·n(C2H5OH) in alcohol and its decomplexation in aqueous medium.
Keywords
- Type
- Article
- Information
- Copyright
- Copyright © Materials Research Society 2020
Footnotes
These authors contributed equally to this work.
References
- 6
- Cited by




