To promote normal child growth, development and immune function, mothers must supply their children with adequate amounts of PUFA during pregnancy and lactation(Reference Das1, Reference Innis2). In particular, maternal fat stores and dietary intake must be adequate to allow for sufficient transfer of linoleic acid (LA), arachidonic acid (ARA), α-linolenic acid (ALA), EPA and DHA to the child. In resource-poor countries, breast milk composition is especially important, as children often depend on breast milk as an important source of fat for as long as they continue to breast-feed(Reference Brown, Black and Becker3, Reference Prentice and Paul4).
The 2008 Joint WHO/FAO Expert Consultation on Fats and Fatty Acids in Human Nutrition recommended that all women of reproductive age, including pregnant and lactating women, consume 20–35 % of total energy as fat, with 6–11 % of total energy from PUFA and at least 2 and 0·5 % of total energy from LA and ALA, respectively(Reference Brenna and Lapillonne5, Reference Elmadfa and Kornsteiner6). Estimated minimum intakes of LA and ALA that will prevent essential fatty acid (EFA) deficiency are 1 and 0·2 % of total energy, respectively(Reference Holman7, Reference Bjerve, Fischer and Alme8). Both the WHO/FAO Expert Consultation and the Perinatal Lipid Intake Working Group specifically recommend that pregnant and lactating women consume a usual daily intake of 200 mg DHA/d(Reference Brenna and Lapillonne5, Reference Koletzko, Lien and Agostoni9).
Maternal fat stores are another source of PUFA for transfer to the child, and there is consensus that both adequate pre-pregnancy weight (BMI ≥ 18·5 kg/m2) and appropriate gestational weight gain are important for maternal and child health(Reference Salihu, Mbah and Alio10, Reference Siega-Riz, Viswanathan and Moos11). Energy intake during lactation must adequately support milk production and prevent lean tissue loss and excessive depletion of adipose stores. During the first 6 months of lactation, the estimated additional energy needs for poorly nourished women are about 711·3 kJ/d (170 kcal/d) higher than those for well-nourished women(12). The energy requirements for lactation beyond 6 months differ depending on the amount of milk being produced(12).
The amount of PUFA delivered in human milk varies in different populations. Milk PUFA content is influenced by a number of factors, including maternal dietary intake, maternal fat stores, endogenous synthesis of fats and regulatory mechanisms affecting fatty acid transport and synthesis in the mammary gland(Reference Vuori, Kiuru and Makinen13–Reference Schmeits, VanderJagt and Okolo16). Bangladeshi women of child-bearing age may have inadequate fat intake and fat stores. Dietary surveys of households and lactating women in Bangladesh have broadly characterised the rural Bangladeshi diet as being very low in fat and high in carbohydrates, but specific intakes of PUFA have not been examined(Reference Vinoy, Rosetta and Mascie-Taylor17, Reference Hels, Hassan and Tetens18). Rural Bangladeshi women store very little fat during pregnancy and consistently lose weight during the first 2 years of lactation(Reference Vinoy, Rosetta and Mascie-Taylor17, Reference Alam, van Raaij and Hautvast19, Reference Sarkar and Taylor20). The median duration of breast-feeding in Bangladesh is 32·8 months, with 78 % of children still receiving some breast milk at 24 months of age(21).
The present study examined data from Bangladeshi mothers of children 24–48 months of age. Our aims were to examine the relationship between prolonged breast-feeding and maternal BMI, to compare the fat and energy intakes of breast-feeding and non-breast-feeding mothers, to assess the adequacy of fat intake in rural Bangladeshi mothers and to determine breast-milk fatty acid composition in a subsample of mothers. We also examined the relationships between maternal fat intake and several potentially associated factors, including season and maternal age, BMI, education level, socio-economic status (SES) and site of residence. These data are unique in that they characterise maternal dietary intake and breast-milk PUFA content during prolonged lactation; the vast majority of the work done previously on this topic has focused on the first 6 months of lactation. Based on the anthropometric status, dietary fat intake and prolonged lactation of these mothers, we hypothesised that the PUFA content of their breast milk would be lower than that observed for breast milk that was previously reported in the literature.
Subjects and methods
Study design
The study was designed as a cross-sectional, representative, multi-stage survey of mothers of children 24–48 months of age who resided in one of two rural districts of the northern poverty belt of Bangladesh. The present study was conducted according to the guidelines laid down in the Declaration of Helsinki, and all procedures involving human subjects were approved by the Institutional Review Board of the University of California, Davis and the Ethical Review Committee (ERC) of the International Centre for Diarrhoeal Disease Research, Bangladesh (ICDDR,B). Written informed consent was obtained from all study participants.
Study sites and selection of study participants
We selected two rural ‘upazilas’, Trishal and Pirgacha, in northern Bangladesh, based on their high prevalence of poverty and food insecurity and the presence of some existing research infrastructure established by the ICDDR,B.
Within each ‘upazila’, all villages or ‘mauzas’ (administrative subdivisions) identified by the 2001 census were included in the sampling universe. There were a total of 159 clusters (‘mauzas’ or villages) in Trishal and 169 clusters in Pirgacha. During the first sampling stage, twenty-five clusters were selected from each study site using systematic sampling with the probability of selection proportional to estimated population size. At the second stage of sampling, ten households within each cluster were selected using a global positioning system sampling method(Reference Grais, Rose and Guthman22). The study sites and our sampling methods have been previously described in detail(Reference Arsenault, Yakes and Hossain23).
Approximately 92 % of the originally selected eligible households agreed to participate in the study. Data were collected from 240 households in Trishal from October 2007 to May 2008 and from 240 households in Pirgacha from January to June 2008. Forms for one household in Trishal were lost during transit from the field site to the ICDDR,B. The present analysis excluded data from households in which the primary carer was the father (n 1) or a woman older than reproductive age (age ≥ 55 years; n 4). Thus, the total sample size was 474 women of reproductive age, 4 % of whom were grandmothers of the study children.
Data collection and processing
Dietary data were collected by fieldworkers posted in recruited households for 12 h periods on two non-consecutive days during the course of a 1-week period. A fieldworker observed food preparation and consumption, and used a frequently standardised food scale ( ± 1 g, MyWeigh KD7000; MyWeigh, Phoenix, AZ, USA) to measure the weights of all foods included in recipes, the weights of recipes before and after cooking and the weights of foods and mixed preparations served and leftover after consumption. Animal source foods included in mixed preparations (e.g. fish in a vegetable curry) were weighed separately at the point of consumption to estimate actual intake. On the morning after the 12 h observation period, a fieldworker returned to the household to elicit information on any food consumed during the 12 h period after the previous day's observation ended. A standardised plate, cup and spoon were used to assist with portion size estimation, and fieldworkers reviewed all information with the carer after the initial list of foods was completed to probe for food items that may have been forgotten. Child's breast milk intake was estimated by the test-weighing procedure, which has been previously described in detail(Reference Arsenault, Yakes and Hossain23).
Foods consumed were converted to nutrients using the USDA Nutrient Database for Standard Reference (Release 20)(24) and the International Minilist(Reference Kramer, Peterson and Rogers25). Food sources of nutrients were calculated by summing the total nutrient intakes for all observation days, summing the nutrient intakes from each food source and calculating the percentage of total nutrient intake from each food.
A trained field-worker measured maternal height using a portable stadiometer ( ± 0·1 cm, ShorrBoard; Shorr Productions, Olney, MD, USA) and maternal weight using a frequently standardised electronic scale ( ± 100 g, Seca 840 Digital Floor Scale; Seca, Hamburg, Germany). If two height measurements differed by more than 0·5 cm, a third height measurement was taken. Average weight and the average of the two closest height values were used to calculate BMI (kg/m2).
Information on SES and agricultural practices was gathered during interviews with the carer and the head of the household.
Biological samples were collected from six enrolled households in a cluster. Breast milk was collected from lactating mothers starting in December 2007 (2 months after the start of the study) due to a delay in obtaining Ethical Review Committee approval. Of the 474 carers of reproductive age enrolled in the study, 242 were breast-feeding a child between 24 and 48 months of age and 128 of these women were from households who participated in biological sample collection in the period after breast milk collection started. These women were asked to provide a breast milk sample, and ninety-eight (77 %) gave a sample. Reasons for not providing a sample included refusal and difficulties with producing a milk sample via hand expression.
Efforts were made to standardise both the time of day and the time elapsed since the last feeding for breast milk collection(Reference Ruel, Dewey and Martinez26). Carers were brought to a central collection site (health complex or school) early in the morning and were asked to breast-feed their children upon arrival and then again 60 min later. During this latter feed, a female fieldworker assisted the mother with hand expressing approximately 3·5 ml of human milk into a sterile conical vial from the last breast suckled during the previous feed. Breast milk samples were transported to the field laboratory on crushed ice and then gently inverted several times and aliquoted to storage vials. The breast milk aliquots were stored in a freezer ( − 20°C) with a back-up generator for 1 week to 6 months (depending on the date of collection) until being shipped to ICDDR,B and then to University of California, Davis on dry ice. At the ICDDR,B and University of California, Davis, the samples were stored at − 80°C until extraction and derivatisation.
A sample of 30 μl (approximately 0·03 g) human milk was used for fatty acid methyl ester (FAME) analysis. Both the human milk and an authentic internal standard (TAG (17 : 0); Avanti Polar Lipids, Alabaster, AL, USA) were measured by weight, and then the Folch method was used for lipid extraction(Reference Folch, Lees and Sloane Stanley27). The organic phase was then evaporated under N2. The dried organic extracts were methylated by adding 1 ml of 3 m-methanolic HCl and incubating in a sealed vial for 12 h at 60°C. FAME were extracted in hexane with 0·01 % butylated hydroxytoluene after neutralisation of the methanolic hydrochloric acid with 5 % potassium bicarbonate. FAME extracts were dried under vacuum and then dissolved in hexane for analysis by GC.
FAME were separated via capillary GC using an Agilent Technologies (Santa Clara, CA, USA) gas chromatograph model 7890 equipped with a 30 m DB225MS capillary column (J&W Scientific, Folsom, CA, USA) and a flame ionisation detector. An authentic GLC reference standard (461; Nu-Chek Prep, Elysian, MN, USA) and Agilent Technologies GC ChemStation software were used to identify and quantify the FAME peaks. Every chromatogram was reviewed to check for proper peak integration and identification. Percentage of fatty acids by weight was calculated by dividing the peak area for a particular fatty acid by the total sum of the peak areas for all identified fatty acids.
Data analysis
We used the National Cancer Institute method for episodically consumed foods to estimate distributions of usual food and nutrient intake(Reference Tooze, Midthune and Dodd28, 29). This method was used to better approximate the variance of the intake distributions by removing within-person variation and to improve estimates of intake for foods and nutrients that were episodically consumed during the 2 d of dietary data collection. Briefly, the National Cancer Institute method uses data from two or more 24 h estimates of intake in a two-part model that first estimates the probability of consumption using logistic regression and then estimates the amount consumed using linear mixed regression on a transformed scale(Reference Tooze, Midthune and Dodd28).
The covariates of interest for both the probability of consumption and the amount of consumption models included maternal age, BMI, education level, household access to electricity and housing quality (as proxies of household wealth), season and site of residence. The housing quality variable was a composite continuous measure assessing the type of floors, walls and sanitary facilities in the family home, and the type of cooking fuel used. Maternal education was a continuous variable that was categorised to minimise the effects of measurement error. All other continuous variables (maternal age, BMI and house quality score) were used as continuous variables in the models. The first model (probability of consumption) was of the form(Reference Tooze, Midthune and Dodd28):
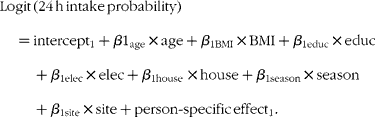
The second model (amount of consumption) was of the form(Reference Tooze, Midthune and Dodd28):

Both models were used to estimate distributions of intake for foods and nutrients that were episodically consumed, including oil, eggs, fish, meat/poultry, ARA, EPA and DHA. Only the amount of consumption model was used for all other foods and nutrients because they were consumed in some amount by all participants on all observation days. The percentage of energy derived from fat, carbohydrate and protein was calculated for each woman on each day; these distributions estimate the long-term mean of the daily ratio of intakes (the usual ratio of intakes)(Reference Freedman, Guenther and Dodd30). Distributions of intakes were estimated for the entire maternal population who had complete covariate information (n 455) and for the breast-feeding and non-breast-feeding subgroups. Covariates were assessed as being significantly associated with intake based on test statistics from the fitted models.
The SURVEYLOGISTIC procedure in SAS (version 9.2; SAS, Cary, NC, USA) was used to analyse the relationships between maternal characteristics and breast-feeding status, and the SURVEYREG procedure was used to analyse the relationships between maternal characteristics and total milk fat content. These procedures allow for correct estimation of standard errors and test statistics in survey samples.
Results
Characteristics of women and study households
Table 1 presents the characteristics of Bangladeshi mothers included in the study sample and their households. The women ranged in age from 16 to 50 (median 25) years, and their BMI ranged from 14·5 to 36·7 (median 19·1) kg/m2. Maternal weight ranged from 30·0 to 82·0 (median 43·1) kg, and height ranged from 131·8 to 168·6 (median 150·2) cm. Approximately, 40 % of the mothers had not completed any formal education, and 70 % did not have access to electricity. A small number of households (8 %) were surveyed before the major rice harvest (pre-Aman harvest; October–November); the remaining households were surveyed after the main harvest (50 %; post-Aman harvest; December–March) and during the secondary rice harvest (42 %; Aus/Boro harvest; April–June). A total of 274 women (58 % of total female carers of reproductive age) were currently breast-feeding, with 241 women breast-feeding a 24–48-month-old child and the rest (n 33) breast-feeding a younger sibling. About 76 % of 24–35-month-old children and 38 % of 36–48-month-old children nursed an average of ≥ 5 times/d on the observation days.
Table 1 Characteristics of rural Bangladeshi mothers included in the study and their households, by the women's current breast-feeding status
(Numbers of subjects and percentages)

CED, chronic energy deficiency.
* The P value for the effect in a logistic regression model, with characteristic as the independent variable and maternal breast-feeding status (yes/no) as the outcome.
† BMI categories defined according to Shafique et al. (Reference Shafique, Akhter and Stallkamp31) and WHO Expert Consultation(54).
‡ Three women in this category were pregnant. One was 3 months pregnant (BMI = 22 kg/m2), one was 7 months pregnant (BMI = 21 kg/m2) and one was 9 months pregnant (BMI = 19 kg/m2).
Relationships between breast-feeding status and covariates of interest
Bivariate relationships between breast-feeding status and the covariates of interest were examined (Table 1), and breast-feeding status was not significantly associated with access to electricity, housing quality, maternal age, maternal education level, season of data collection or district of residence. Maternal BMI, however, was significantly associated with lactation status. Approximately 42 % of breast-feeding mothers were underweight (BMI < 18·5 kg/m2) compared with 26 % of non-breast-feeding mothers (P = 0·0003). The significant relationship between breast-feeding status and maternal BMI persisted in a multivariate model that adjusted for access to electricity, housing quality, maternal education, maternal age and season. On average, mothers who were breast-feeding had a BMI 0·8 kg/m2 less than non-breast-feeding mothers (P = 0·001).
Energy intake and macronutrient profile of diet
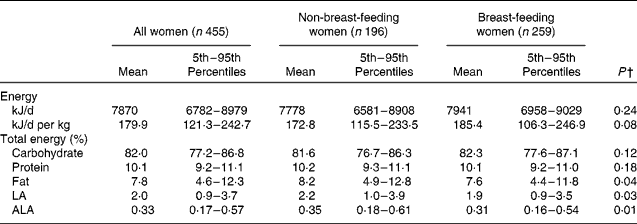
Table 2 shows estimated distributions of usual energy, macronutrient and EFA intake, expressed as a percentage of total energy. The mean (5th–95th percentile) daily energy intake for all women was 7870 (6782–8979) kJ/d. Almost all of the women in the study population consumed more than 75 % of their total energy from carbohydrates. Intakes from protein had a relatively narrow range, with most women consuming about 10 % of their energy from protein. The maternal diet averaged 7·8 % of total energy from fat and 2 and 0·33 % of total energy from LA and ALA, respectively.
Table 2 Estimated distributions of usual energy intake and percentage of energy derived from fat, carbohydrate and protein among rural Bangladeshi women, by current breast-feeding status*
(Mean values and 5th–95th percentiles)
LA, linoleic acid; ALA, α-linolenic acid.
* Percentage of energy derived from fat, carbohydrate and protein was calculated for each woman on each day; these distributions estimate the long-term mean of the daily ratio of intakes (the usual ratio of intakes)(Reference Freedman, Guenther and Dodd30).
† Reported P -values are for the effects of lactation status on intake after adjusting for maternal age, BMI, education, socio-economic status and site of residence in a linear mixed regression model.
After adjusting for maternal age, BMI, education level, SES and site of residence in separate linear mixed regression models, intakes of total energy, carbohydrates and protein as a percentage of total energy did not differ by lactation status. Intakes of total fat (β = − 0·07; P = 0·04), LA (β = − 0·10; P = 0·03) and ALA (β = − 0·12; P = 0·01), expressed as a percentage of total energy, were slightly lower in breast-feeding than in non-breast-feeding women in the same model. SES was significantly associated with higher intakes of protein (electricity access: β = 0·03; P = 0·01; better housing quality: β = 0·03; P = 0·002) and fat (better housing quality: β = 0·20; P < 0·0001; maternal access to formal education: β = 0·11; P = 0·003) and lower intakes of carbohydrates (better housing quality: β = − 1·7; P = 0·02) as a percentage of total energy in separate linear mixed regression models.
Based on the intake distributions, it is estimated that 99 % of the women in the study sample had usual fat intakes less than 20 % of total energy, and 84 % had usual fat intakes of less than 10 % of total energy. Almost all women (99 %) were estimated to consume less than 6 % of total energy from PUFA. About 60 % of women consumed less than 2 % of total energy from LA, and 90 % consumed less than 0·5 % of total energy from ALA. An estimated 7 and 13 % of mothers had a usual intake of LA and ALA less than 1 and 0·2 % of total energy, respectively, placing them at a potential risk of EFA deficiency.
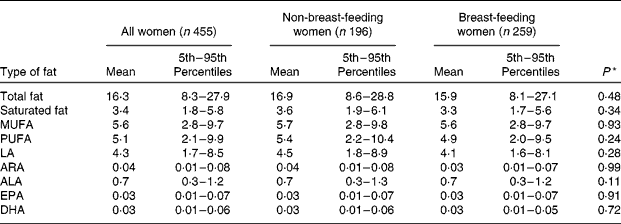
Maternal consumption of fatty acids
Table 3 presents the women's estimated distributions of usual fat intake expressed as g/d. The mean intake of total fat for all women was 16·3 g/d. The average ratio of LA:ALA intake in the maternal diet was 6·7, with a 5th–95th percentile range of 4·4–9·7. After adjusting for maternal age, BMI, education level, SES and site of residence in a linear mixed regression model, breast-feeding status was not a significant predictor of total fat intake or intake of any of the fat subclasses. Total fat intake was significantly associated with several other factors in the model, including season (lowest in the pre-Aman harvest: β = − 0·08; P = 0·04), maternal age (β = − 0·01; P = 0·02), maternal BMI (β = 0·02; P = 0·02), housing quality (β = 0·22; P = < 0·0001), maternal level of formal education (β = 0·11; P = 0·02) and site of residence (lower in Trishal: β = − 0·16; P = 0·001). Similar associations were observed for intake of the fat subclasses.
Table 3 Estimated distributions of usual fat intake (g/d) by rural Bangladeshi women, by current breast-feeding status
(Mean values and 5th–95th percentiles)
LA, linoleic acid; ARA, arachidonic acid; ALA, α-linolenic acid.
* Reported P-values are for the effects of lactation status on intake after adjusting for maternal age, BMI, education, socio-economic status and site of residence in a linear mixed regression model.
Food sources of fatty acids in the maternal diet
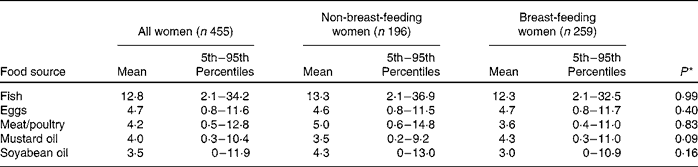
Vegetable oil was the source of about 50 % of total fat intake for the entire carer population. Rice was also a prominent source of fat, providing 27 % of the total fat in the maternal diet. Animal source foods, including freshwater fish, eggs, meat/poultry and dairy products, provided about 15 % of total maternal fat intake. These patterns did not differ for breast-feeding and non-breast-feeding women.
The dietary patterns for PUFA were similar to those for total fat, with vegetable oil accounting for 57 % of total PUFA intake and rice providing another 26 %. Vegetables and beans supplied about 6 % of the total PUFA. Oil, rice, beans and vegetables (particularly leafy vegetables) were the most important sources of both LA and ALA. The primary food sources of ARA included freshwater fish (49 %), eggs (42 %) and meat/poultry (7 %). Almost all of the EPA and DHA were provided by freshwater fish. Again, the pattern of food intake was similar in breast-feeding and non-breast-feeding women.
Estimated usual intake distributions for food sources of PUFA (g/d) are shown in Table 4. There were significant differences in the probability of consumption of food sources of PUFA between the two study sites in a linear mixed model that adjusted for lactation status, maternal age, BMI, education level and SES. The probabilities of consuming soyabean oil (β = 4·0; P < 0·0001), meat/poultry (β = 0·87; P = 0·002) and eggs (β = 1·2; P < 0·0001) were higher in Pirgacha, whereas the probabilities of consuming mustard oil (β = 2·1; P < 0·0001) and fish (β = 1·2; P < 0·0001) were higher in Trishal.
Table 4 Estimated usual consumption (g/d) of food sources of PUFA by rural Bangladeshi women, by current breast-feeding status
(Mean values and 5th–95th percentiles)
* Reported P -values are for the effects of lactation status on intake after adjusting for maternal age, BMI, education, socio-economic status and site of residence in a linear mixed regression model.
Breast-milk fatty acid composition
The characteristics of mothers who provided a breast milk sample and their households were similar to those of the overall maternal population. The mean BMI for breast-feeding women who provided a sample was 18·8 kg/m2, while the mean BMI for breast-feeding women who did not provide a sample because of lack of opportunity (n 114) or refusal/difficulty with producing a sample from hand expression (n 30) was 19·6 kg/m2 (P = 0·01).
Table 5 lists the fatty acid content (% wt) of the breast milk samples. The median total fat content for the samples was 3·5 g/100 g of breast milk (5th–95th percentile range, 1·4–7·3). Total milk fat content was not significantly associated with maternal BMI (P = 0·22) or with breast-feeding frequency (P = 0·42) or duration (P = 0·18). The majority of the fatty acids present in the human milk were SFA, followed by MUFA and then PUFA. The most abundant fatty acids were 16 : 0, 18 : 1, 14 : 0 and 18 : 2n-6 (LA). The median ratio of LA:ALA in the milk was 35, while the median ratio of long-chain n-6:long chain n-3 fatty acids was 1·6. Breast-milk ARA and DHA concentrations were highly correlated (r 0·7; P < 0·001).
Table 5 Fatty acid composition of breast milk from rural Bangladeshi mothers
(Median values and 5th–95th percentiles, n 98)

LA, linoleic acid; ALA, α-linolenic acid; LC, long-chain.
Discussion
Our data support the concerns raised by earlier studies regarding the adequacy of fat stores in lactating Bangladeshi women. Overall, the BMI patterns for the women in the present study were similar to those observed in the nationally representative 2000–4 Nutritional Surveillance Project(Reference Shafique, Akhter and Stallkamp31). However, we found that women who were breast-feeding a 24–48-month-old child had a considerably higher prevalence of underweight (BMI < 18·5 kg/m2) than non-breast-feeding women with a 24–48-month-old child.
As our data are cross-sectional, we cannot determine the postpartum period during which the weight discrepancy between these groups was established, or definitively establish that the energy demands of lactation were the cause of this weight discrepancy. Longitudinal studies in both well-nourished American women and poorly nourished Bangladeshi women found that breast-feeding women experienced the most weight loss from 3 to 6 months postpartum(Reference Vinoy, Rosetta and Mascie-Taylor17, Reference Dewey, Heinig and Nommsen32). For the American mothers, the weight differences that were observed between breast-feeding and formula-feeding mothers were no longer significant after 12 months, indicating that the breast-feeding mothers probably experienced some weight recovery as breast-feeding intensity decreased(Reference Dewey, Heinig and Nommsen32). The Bangladeshi mothers in the longitudinal study were not followed past 13 months postpartum. A cross-sectional study of rural Bangladeshi women in the Jhenaidah district found that the weights of lactating women were lower than those of non-lactating women throughout 48 months of lactation after controlling for height, education and food consumption; however, the differences were only significant up to 24 months postpartum(Reference Sarkar and Taylor20). Milk production was not quantified in that study, but the authors hypothesised that decreased milk production after 24 months may have allowed maternal weight to recover(Reference Sarkar and Taylor20).
There was no significant difference in energy intake between lactating and non-lactating women after adjusting for maternal age, BMI, education level, SES and site of residence. Based on estimated mean 24 h breast milk consumption for the children in the present study (145 g/d for 24–35-month-old children; 90 g/d for 36–48-month-old children) and assuming that maternal fat stores were not covering additional energy needs, the breast-feeding women in the present study needed about 301–502 kJ/d in excess to support milk production (range of maternal energy needs based on 1st and 99th percentiles of the child's breast milk intake: 29–1799 kJ/d in excess)(12, Reference Butte and King33). We may not have had the power to detect a difference in intake of 418 kJ between the two groups; a post hoc power analysis showed that the power to detect this difference in the present study is 48 %. It is also possible that we may have underestimated energy intake, although this would not affect our ability to find a difference in energy intake between the two groups unless it was differential between breast-feeding and non-breast-feeding women. The women in the present study spent most of the day engaged in household activities (cooking, cleaning, child care and grain processing). Bangladeshi women with similar activity profiles were found to have physical activity levels of 1·59 (lightly active) in a study that used the doubly labelled water method(Reference Rosetta, Kurpad and Mascie-Taylor34) and 1·76 (moderately active) in a study that used the FAO/WHO/UNU factorial method(Reference Kramer, Peterson and Rogers25). Based on these physical activity levels, the total estimated energy expenditures for a woman weighing 43 kg with an estimated BMR of 4700 kJ/d would be 7473 and 8272 kJ, respectively(12). These total estimated energy expenditures correspond to approximately the 25th and 75th percentiles of estimated energy intake for both lactating and non-lactating women in the present study.
Our data also support the concerns raised by earlier studies regarding the adequacy of fat intake in Bangladeshi women(Reference Vinoy, Rosetta and Mascie-Taylor17, Reference Hels, Hassan and Tetens18). We found that women in rural Bangladesh consumed a very low-fat, high-carbohydrate diet, with an estimated average of just 7·8 % total energy from fat. Intakes of fat were similar among breast-feeding and non-breast-feeding women. The main sources of fat in the diet were fairly limited, with vegetable oil and rice contributing the most to overall fat intake. There were significant geographical differences in the consumption of the animal source foods that are good sources of long-chain PUFA. These differences are probably due to the differences in availability and local preference and have important implications for programme planning. Based on distributions of usual food intake, Bangladeshi women of child-bearing age consume very small quantities of foods that are important sources of fat, and consequently, small quantities of PUFA.
The total fat and ALA intakes of Bangladeshi mothers, as a percentage of total energy, are estimated to be almost universally less than the respective 20 and 0·5 % minimums recommended by the 2008 Joint WHO/FAO Expert Consultation on Fats and Fatty Acids(Reference Brenna and Lapillonne5, Reference Elmadfa and Kornsteiner6). The estimated intake of LA was also below the minimum recommended intake levels in approximately 60 % of women. Approximately, 10 % of the women in our sample may have LA and ALA consumption below the levels considered to be necessary to prevent EFA deficiency. Although it is likely that most women with this low intake would be protected from EFA deficiency by their fat stores, women with low body fat may be at increased risk(Reference Hibbeln, Nieminen and Blasbalg35). Also, there should be particular concern for breast-feeding women with this low level of intake, as they are continuously transferring LA and ALA to their children in breast milk, and our BMI data suggest that depleted fat stores may be more common in breast-feeding women. A similar concern might be raised for DHA, as the women in the present study consumed far less DHA than the recommended usual daily intake of 200 mg/d for lactating women(Reference Brenna and Lapillonne5, Reference Koletzko, Lien and Agostoni9, Reference Koletzko, Cetin and Brenna36).
Overall, the pattern of fatty acids that we observed in the milk from rural Bangladeshi mothers reflected the dietary patterns in the population. Consistent with the findings in a number of other studies in populations with high-carbohydrate, low-fat diets, we observed a high level of myristic acid (14 : 0), a medium-chain SFA that is synthesised from acetyl-CoA in the mammary gland(Reference van Beusekom, Martini and Rutgers15, Reference Insull, Hirsch and James37–Reference Thiombiano-Coulibaly, Rocquelin and Eymard-Duvernay40). The breast milk from the Bangladeshi mothers also had low levels of oleic (18 : 1n-9) and stearic (18 : 0) acids; the concentrations of these fatty acids are generally lower in human milk with increased medium-chain SFA levels(Reference Vuori, Kiuru and Makinen13, Reference Schmeits, VanderJagt and Okolo16).
Levels of LA and ALA in the breast milk from the present study sample were among the lowest reported in the extensive literature characterising breast-milk fatty acid composition. Prentice & Paul(Reference Prentice and Paul41) summarised data on the breast milk composition of mothers from separate studies in eight African and South American countries with diets that could be generally characterised as high carbohydrate and low fat. When compared with women from those countries, the women from the present study delivered the lowest median LA levels (0·103 g/g fat; range for eight countries: 0·110–0·238) and the second lowest ALA level (0·003 g/g fat; range: 0·001–0·014)(Reference Prentice and Paul41). Breast-milk LA and ALA levels from the Bangladeshi mothers were also below those observed in mothers from more developed countries(Reference Xiang, Harbige and Zetterstrom42, Reference Yuhas, Pramuk and Lien43). Human milk from mothers in Nepal, the Philippines, the Congo and Pakistan exhibited comparably low LA levels, but the very low ALA level observed in the breast milk from Bangladeshi women was observed only in milk from the Pakistani women(Reference Yuhas, Pramuk and Lien43–Reference Rocquelin, Tapsoba and Kiffer46). As a result of the very low ALA levels, breast milk from the Bangladeshi mothers had a median LA:ALA ratio of 35, which is much higher than the recommended range of 5–15:1(Reference Rocquelin, Tapsoba and Kiffer46). The authors of the studies that included the Filipina and Pakistani mothers both concluded that inadequate LA and ALA intake might be the cause of the low EFA levels in the breast milk(Reference Yuhas, Pramuk and Lien43, Reference Smit, Oelen and Seerat44). The present study provides support for this conclusion, as we found that the Bangladeshi women were consuming low levels of EFA compared with international recommendations.
In contrast to the present findings regarding breast-milk LA and ALA, the breast-milk ARA and DHA concentrations for the Bangladeshi mothers fell in the middle of reported ranges for women from many different countries(Reference Prentice and Paul41–Reference Yuhas, Pramuk and Lien43, Reference Glew, Huang and VanderJagt45, Reference Marangoni, Agostoni and Lammardo47, Reference Peng, Zhang and Wang48). The median ARA and DHA percentage weights for breast milk from Bangladeshi mothers were very close to the mean concentrations of DHA (0·32 %) and ARA (0·47 %) that were determined in a descriptive meta-analysis of milk from 2474 women in sixty-five studies(Reference Brenna, Varamini and Jensen49).
It is possible that regulatory mechanisms play an important role in determining DHA and ARA levels in human milk, as the ARA and DHA levels in the breast milk of the Bangladeshi mothers were maintained at levels that fall near the middle of ranges observed in other populations, despite low intake of fat and prolonged lactation. There are several potential mechanisms that could be up- or down-regulated to control breast-milk PUFA content, including the release of fatty acids from lipoproteins by lipase, the transport of fatty acids from the blood into the mammary gland and the synthesis of milk TAG by acyl transferases(Reference Schmeits, VanderJagt and Okolo16). ARA, in particular, seems to be tightly controlled in breast milk, with a limited range of ARA concentrations observed across populations with very different diets(Reference Smit, Martini and Mulder39, Reference Sanders50–Reference Kuipers, Fokkema and Smit52). Future longitudinal studies that examine changes in white adipose tissue or erythrocyte fatty acid composition in mothers over the course of prolonged lactation could help to elucidate these issues.
The low LA intake of the women in the present study may have allowed for increased conversion of ALA to DHA. However, subjects in an intervention trial who consumed a diet with an EFA profile similar to the women in the present study (3 % of energy from LA and 0·4 % of energy from ALA) converted more dietary ALA into EPA but did not increase the absolute amount of DHA synthesised compared with subjects on a control diet (7 % of energy from LA and 0·4 % of energy from ALA)(Reference Goyens, Spilker and Zock53).
Fish is a regular part of the Bangladeshi diet, but the breast-milk DHA concentrations in the present study were not as high as those observed in other countries where fish is regularly consumed(Reference Lauritzen, Jorgensen and Hansen51). This may be explained by the fact that the vast majority of fish intake was from freshwater fish, which generally contain less DHA than ocean fish. In addition, the estimated amount of usual fish intake was low.
Several limitations of the present study deserve comment. Our observations about the relationship between PUFA intake and breast-milk concentrations would have been strengthened by presenting data on maternal biochemical PUFA status. However, we were unable to collect blood samples from the mothers, and there is no previous data on biochemical PUFA status available for a comparable Bangladeshi population. It is possible that we may have under- or overestimated the percentage of women who would fall below the stated fat intake cut-off values because we collected only 2 d of dietary data for each individual. However, we improved our estimates for the population intake distribution by using statistical methods to adjust for episodic consumption and remove within-individual variation(Reference Tooze, Midthune and Dodd28). Although the in-home observations allowed us to collect reliable quantitative data on actual food intake, it is conceivable that our presence may have influenced the women's food consumption on the observation days. However, if the women did include more desirable foods (e.g. oil and animal source foods) in their cooking during our observation days, we may have overestimated their true usual fat intakes. Another potential source of error is that at least one major meal (approximately 22 % of total energy) was generally obtained via recall, as it was consumed outside of the 12 h observation window. However, we did find that estimated intakes of energy were similar between recalled and observed meals. Additional error may have been introduced when using food composition tables to convert food to nutrients, as the nutrient composition of foods can vary significantly across seasons and geographical regions, and budgetary constraints prevented us from directly analysing foods from the study area to determine fatty acid composition. To partially address this limitation, we have presented quantitative data on food intake to support the nutrient intake data.
Conclusion
Overall, breast milk from Bangladeshi mothers provides 24–48-month-old children with easily absorbed medium-chain SFA, some LA and ALA, and adequate amounts of ARA and DHA. However, prolonged lactation combined with very low fat intake may lead to significant demands on the maternal body pool of fatty acids. Increasing the dietary intake of PUFA in these mothers thus has the potential to positively affect breast milk composition, and consequently, children's health and development. Research into this area needs to consider not only the fatty acid content of breast milk, but also to examine the effects of breast-feeding on maternal fat stores, maternal health outcomes and the amount of fatty acids supplied to subsequent offspring during pregnancy and lactation. Women who are breast-feeding at high frequency for an extended period of time may require greater fat intake to provide the fatty acids for milk and the additional energy needed to prevent lean tissue loss and to promote replenishment of maternal fat stores. Bangladeshi women in general, and especially those who breast-feed for more than 2 years, may benefit from an increased percentage of total energy consumed from lipids and greater intake of food sources of PUFA.
Acknowledgements
The authors thank the study participants who welcomed us into their homes and the staff from the ICDDR,B who were instrumental to the data collection process. We thank Dr Daniel Tancredi from the University of California (UC) Davis School of Medicine, Department of Pediatrics, for statistical consultation. We are also grateful to Diego Vargas and Andrea Eaton for their invaluable assistance during the laboratory analyses and to Dr Susan Ebeler from UC Davis Department of Viticulture and Enology, for generously allowing us to use the GC in her laboratory. The present study was supported by the Bristol-Myers Squibb Foundation, Inc., the Harvest Plus Challenge Program (coordinated by the Centro Internacional de Agricultura Tropical and the International Food Policy Research Institute), the National Institute of Environmental Health Sciences (NIEHS) grant R37 ES02710, the NIEHS Superfund Basic Research Program P42 ES04699, the UC Davis Center for Children's Environmental Health, NIEHS grant P01 ES11269 and the University of California Discovery Program. None of the authors had any financial or personal interest in the organisations sponsoring the present study. The authors' responsibilities were as follows: E. A. Y. participated in the study design, study implementation, data analysis, interpretation of results and prepared the manuscript; J. E. A. contributed to the study design, study implementation and critical review of the manuscript; M. M. I., M. B. H., A. S. R., T. A., K. M. J. and B. L. L. were involved in the study implementation and critical review of the manuscript; J. B. G. assisted in for funding, interpretation of results and critical review of the manuscript; L. A. G. was responsible for method development for the laboratory analysis; C. D. performed the critical review of the manuscript; K. H. B. had the primary responsibility for funding, study design, interpretation of results and critical review of the manuscript.









