Introduction
The zoonotic infection taeniasis is most commonly caused by Taenia saginata, Taenia solium and Taenia asiatica, which are associated with ingesting raw or undercooked, infected beef meat, pork meat or pork viscera, respectively (Ito et al., Reference Ito, Nakao and Wandra2003; Eichenberger et al., Reference Eichenberger, Thomas, Gabriel, Bobic, Devleesschauwer, Robertson, Saratsis, Torgerson, Braae, Dermauw and Dorny2020). The life cycle of pathogenic Taenia species involves humans as definitive hosts, who harbour the adult stage in their small intestines. Cattle (T. saginata) or pigs (T. solium and T. asiatica) can act as intermediate hosts, who hold the larval stages as cysts that dwell themselves under the skin and in the muscles, nervous system or visceral organs such as the liver of the hosts (Qian et al., Reference Qian, Xiao, Li, Abela-Ridder, Carabin, Fahrion, Engels and Zhou2020). Tapeworm eggs or gravid proglottids are passed in feces, and the eggs can survive for days to months in the environment (Jansen et al., Reference Jansen, Dorny, Gabriël, Dermauw, Johansen and Trevisan2021). Humans can also serve as accidental intermediate hosts when exposed to embryonated eggs (this role is normally fulfilled by T. solium) released by themselves (autoinfestation) or by another tapeworm carrier living in close contact with the subject or involved in contaminated food and water, causing infection in various organs of the human body with the larvae also known as cysticerci (human cysticercosis) (Garcia et al., Reference Garcia, Gonzalez, Evans, Gilman and Cysticercosis Working Group in Peru2003; Garcia, Reference Garcia2018; Alroy and Gilman, Reference Alroy, Gilman, Ryan, Hill, Solomon, Aronson and Endy2020). While T. saginata is responsible for a significant number of cases of taeniasis in humans, it is not thought to be the aetiological agent of cysticercosis in humans because they do not appear to be an accidental intermediate host for this parasite (Garcia et al., Reference Garcia, Gonzalez and Gilman2020). It is not known whether T. asiatica causes cysticercosis in humans (Eom et al., Reference Eom, Rim and Jeon2020).
Twelve cases of T. saginata cysticercosis in humans had been described in the literature before 1972, and these diagnoses were based on autopsy and morphological examination by hookless scoleces (De Rivas, Reference De Rivas1937; Tanasescu and Repciuc, Reference Tanasescu and Repciuc1939; Asenjo and Bustamente, Reference Asenjo and Bustamente1950; Niiio, Reference Niiio1950; Bacigalupo, Reference Bacigalupo JADAB1956; Abuladze, Reference Abuladze1964; Goldsmid, Reference Goldsmid1966; Pawlowski and Schultz, Reference Pawlowski and Schultz1972). In addition, T. saginata infection in adults can occur simultaneously with human cysticercosis (Pawlowski and Schultz, Reference Pawlowski and Schultz1972). The infection of humans with the larvae (cysticerci) of T. saginata appears to be an exceptional situation (Pawlowski and Schultz, Reference Pawlowski and Schultz1972). Medical imaging testing, clinical/exposure criteria, serologic testing, histological pathology and molecular identification all help in the diagnosis of human cysticercosis (Del Brutto et al., Reference Del Brutto, Nash, White, Rajshekhar, Wilkins, Singh, Vasquez, Salgado, Gilman and Garcia2017). Due to the lack of molecular methods readily available in most hospital settings, proper species identification in all reported cases of human cysticercosis is impossible.
Numerous molecular procedures have been developed to identify Taenia spp. responsible for human taeniasis, using mitochondrial and ribosomal DNA, repetitive DNA sequences and/or genes encoding relevant antigens as specimens (Flores et al., Reference Flores, Gonzalez, Hurtado, Motta, Dominguez-Hidalgo, Merino, Perteguer and Garate2018). The cytochrome c oxidase I (COX1) gene is a widely used DNA barcoding marker because the rapid rate of evolution allows for the differentiation of not just closely related species, such as the Taenia genus, but also phylogeographic groups within a single species (Hebert et al., Reference Hebert, Cywinska, Ball and deWaard2003; Galimberti et al., Reference Galimberti, Romano, Genchi, Paoloni, Vercillo, Bizzarri, Sassera, Bandi, Genchi, Ragni and Casiraghi2012; Zhang et al., Reference Zhang, Chen, Yang, Liu, Jiang, Gu, Wang and Wang2014). Evidence suggests the HDP2 segment is a ribosomal DNA sequence useful for species-specific molecular diagnosis of human intestinal taeniasis (Gonzalez et al., Reference Gonzalez, Montero, Harrison, Parkhouse and Garate2000; Flores et al., Reference Flores, Gonzalez, Hurtado, Motta, Dominguez-Hidalgo, Merino, Perteguer and Garate2018). In this case study, we identified the case of a middle-aged Tibetan man with taeniasis owing to T. saginata and cysticercosis that had impacted his brain and muscles diagnosed by morphological and molecular methods. We also used COX1 to create a phylogenetic tree of Taenia.
Materials and methods
Clinical examinations
The electronic medical records of the study subject were retrospectively reviewed, and the following demographic and clinical information was collected: age, gender, past medical history, physical examinations, diagnosis, underlying conditions, drug use, iatrogenic procedures, laboratory tests, computed tomography (CT), enhanced magnetic resonance imaging (MRI), ultrasound scan and clinical outcomes.
Morphological evaluation
Two obvious cysts were detected in the muscles under the right anterior thoracic wall and right midaxillary line through palpation and ultrasound scan, respectively. The cyst biopsy of the right midaxillary line and the proglottids from the patient after deworming treatment was collected and observed morphologically. The gravid proglottids and cystic lesions were fixed in a 10% formalin solution, stained with the hydrochloric acid-carmine solutions and observed under a microscope for pathological diagnosis.
Amplification and sequencing
A portion of the cyst isolated from the right midaxillary line and proglottids was stored in 70% ethanol at −20°C until subsequent molecular diagnosis. Total genomic DNA was extracted by using the Trelief™ Animal Genomic DNA Kit (Tsingke Biotechnology, Beijing, China) in accordance with the manufacturer's instructions. Mitochondrial DNA was analysed by polymerase chain reaction (PCR) targeting COX1 using species-specific forward primers Tsag_COX1/F for T. saginata (positions 322–348) and a common reverse primer Tae_COX1/R for Taenia (positions 1148–1129) (Yamasaki et al., Reference Yamasaki, Allan, Sato, Nakao, Sako, Nakaya, Qiu, Mamuti, Craig and Ito2004), cestode forward primers JB3 (positions 1–24) and reverse primers JB4.5 (positions 444–421) (Bowles et al., Reference Bowles, Blair and McManus1992; Bowles and McManus, Reference Bowles and McManus1994; Tappe et al., Reference Tappe, Berkholz, Mahlke, Lobeck, Nagel, Haeupler, Muntau, Racz and Poppert2016). The HDP2 fragment was amplified with the forward primer PTs7S35F1 and the reverse primer PTs7S35R1 (Gonzalez et al., Reference Gonzalez, Montero, Harrison, Parkhouse and Garate2000). Details of the PCR primers and the reaction conditions are provided in Table 1. PCRs were run in a 30-μL reaction mixture composed of 15-μL I-5™ 2× High-Fidelity Master Mix (Tsingke Biotechnology), 1-μL DNA template, 10 pmol of each primer and 12-μL double-distilled water. The thermocycler parameters of the PCR amplification were as follows: 98°C for 3 min; 39 cycles composed of denaturation at 98°C for 10 s, annealing according to the primers and elongation at 72°C for 15 s. After 39 cycles, the reaction was terminated with an elongation step at 72°C for 5 min, followed by a final hold at 4°C. The amplicons were observed using a 1.5% agarose gel under ultraviolet light and then purified, after which Sanger sequencing methods were carried out at Tsingke Biotechnology or Sangon Biotech (Shanghai, China). The sequenced amplicons were compared to the GenBank database entries using NCBI BLAST (Johnson et al., Reference Johnson, Zaretskaya, Raytselis, Merezhuk, McGinnis and Madden2008), wherein the organism was limited to Taenia (taxid: 6202) and the query coverage was 100%.
Table 1. Primer pairs used for PCRs

a The per cent identity was obtained from the NCBI BLAST webpage and the organism was limited to the Taenia (taxid: 6202) genus and the query coverage was 100%.
b Only proglottids were successfully amplified.
c Both proglottids and cysts were successfully amplified.
Phylogenetic analysis
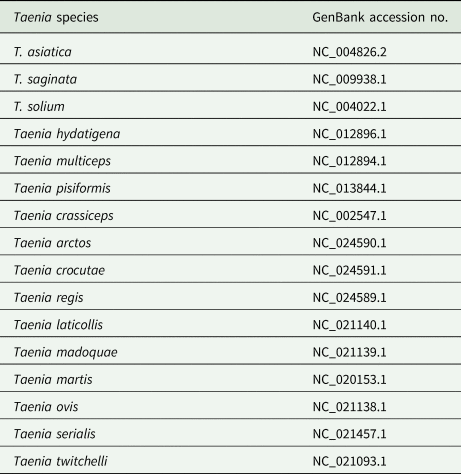
Multiple sequence alignment was constructed with Clustal W, Clustal Omega and SnapGene software. Phylogenetic analysis of the sequences of COX1 identified in this study and the reference sequences of Taenia species available in the GenBank database (Table 2) were constructed using the maximum-likelihood method by MAGA X. The bootstrap values were calculated with 2000 replications, and the nucleotide substitution of the Tamura–Nei model was adapted.
Table 2. Reference COX1 sequences of Taenia species in GenBank
Results
Case presentation
A middle-aged Tibetan man from China was admitted to our hospital with a 7-year history of recurring severe headaches and vomiting. The headache had started with vomiting, nausea, dizziness and chest tightness, especially on the right side, without any apparent predisposing cause. Weekly, he had 2 or 3 episodes of non-projectile vomiting without bile or coffee-like particles, and the headaches lasted for nearly a day each time. There were no other symptoms, such as fever, chills, convulsions, seizures, increased intracranial pressure or other discomforts. He had not been treated for these signs and symptoms before admitting to our hospital in December 2020. After being fully diagnosed in our hospital, his comorbidities included hypertension, fatty liver, liver insufficiency, paranasal sinusitis and intestinal bacterial infection. The immunosuppression was not found. Additionally, he spent a significant amount of time in a pastoral area that is an epidemic province for taeniasis, although no tapeworm infections were diagnosed before his admission to our hospital. He used to ingest raw beef, which might have led to the ingestion of the cysticerci of T. saginata. Moreover, as far as pork consumption is concerned, his family's eating habit, like most Han Chinese, were eating it cooked, ruling out the possibility of related parasites.
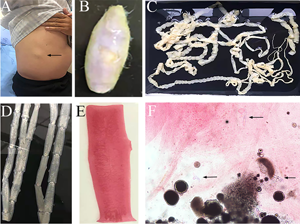
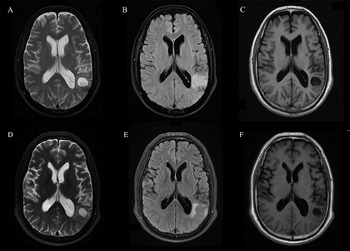
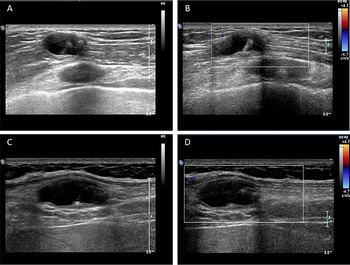
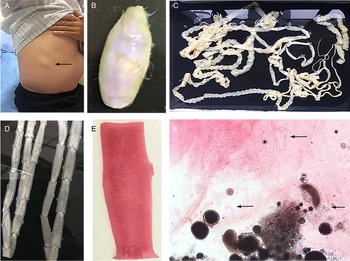
The routine laboratory blood tests, including eosinophil and lymphocyte count, were completely normal apart from the elevated inflammation markers (C-reactive protein and neutrophil count). Schistosomes, Clonorchis sinensis, Echinococcus granulosus and Toxoplasma gondii antibodies were negative in his serum, as were Cryptococcus capsular antigen and next-generation sequencing results from cerebrospinal fluid. Multiple intracranial nodules affecting the supratentorial and infratentorial cerebral parenchyma were shown in detail on CT and MRI of the head, indicating possible intracranial parasitic infection (Fig. 1). Ultrasound scan confirmed the presence of 2 palpable and soft masses located in muscles, which were approximately 19 × 8 × 15 mm3 under the right chest wall and 26 × 10 × 19 mm3 under the right midaxillary line (Fig. 2). A subsequent biopsy of the mass (Fig. 3) showed larval-like tissue, peripheral fibrous tissue hyperplasia, lymphocytic infiltration and hyaline degeneration. The patient was probably diagnosed with taeniasis and cysticercosis and treated with oral albendazole (400 mg, twice daily) over 2 weeks. Hydrocortisone 50 mg was provided 2 days after the first albendazole treatment to counteract any potential negative effects on the central nervous system. After only 2 days of this antiparasitic treatment, the adult tapeworm was eliminated through the patient's feces (Fig. 3). When compared with the first MRI (half a month before antiparasitic treatment), the second MRI (half a month after antiparasitic treatment) demonstrated a slightly smaller focus (Fig. 1). The headache and vomiting resolved, and the patient remained symptom free over a 3-month follow-up period.
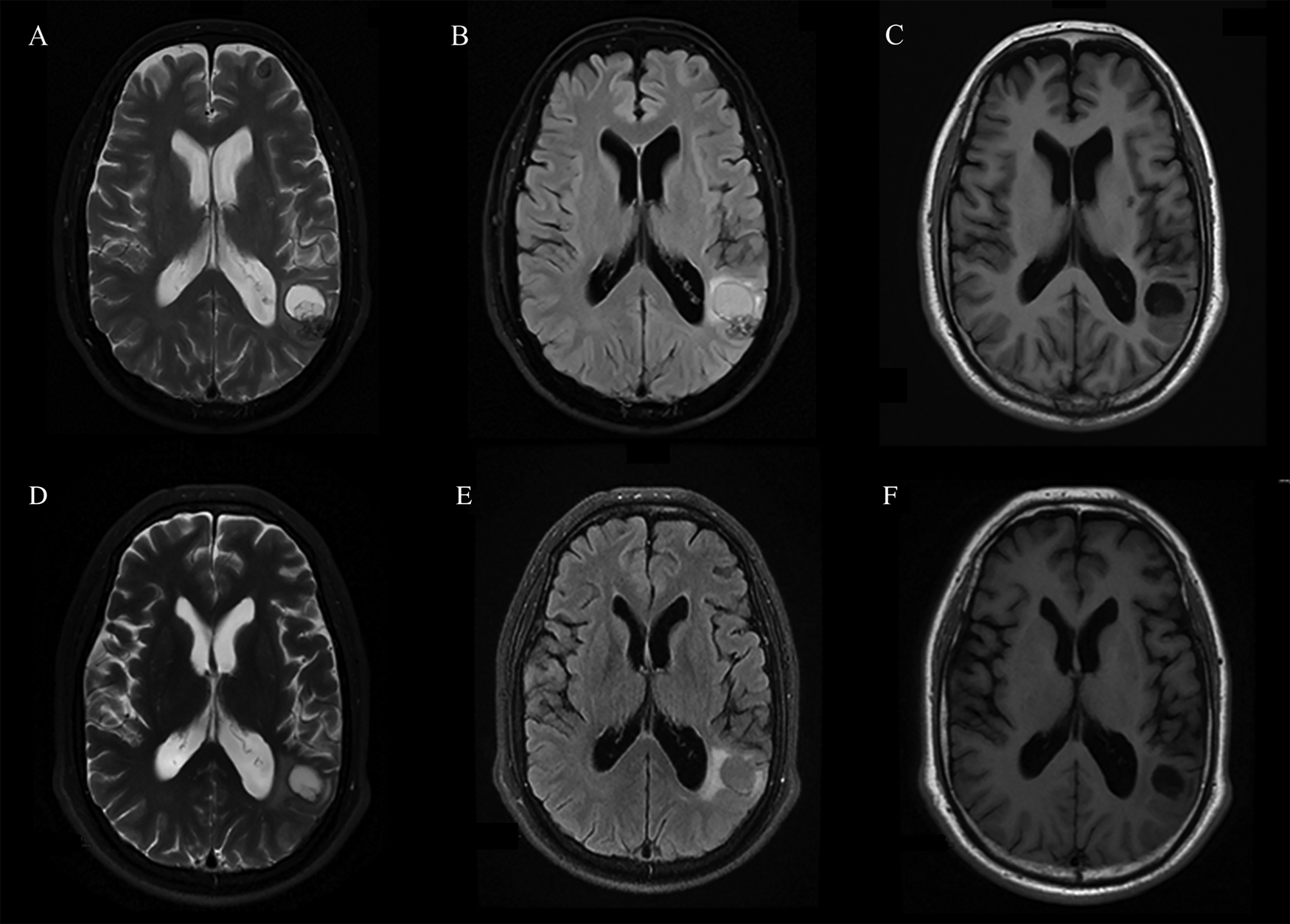
Fig. 1. MRI shows that the largest cystic nodule was located next to the left ventricular triangle: (A–C) half a month before antiparasitic treatment and (D–F) half a month after antiparasitic treatment.
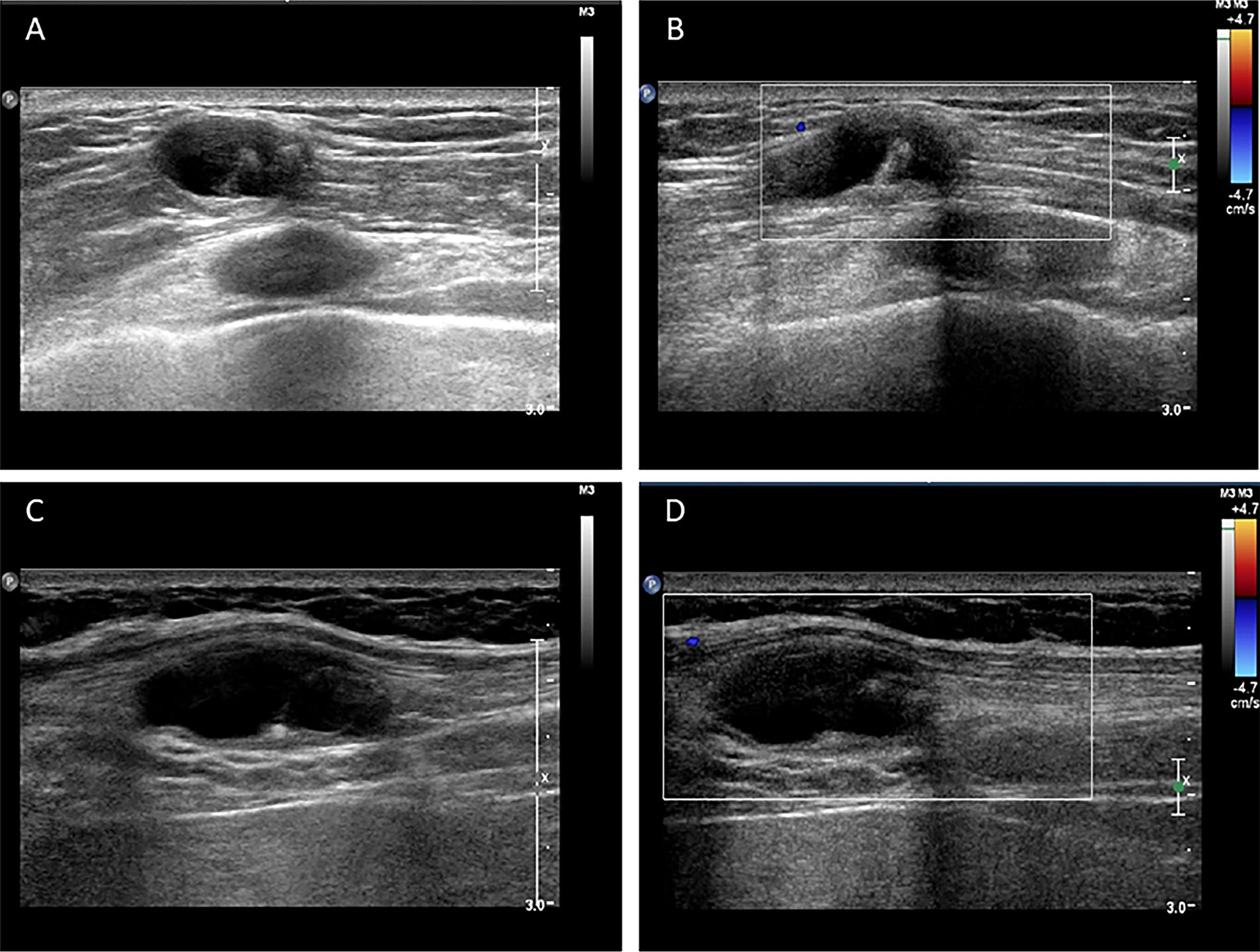
Fig. 2. Ultrasound scan shows hypoechoic mass containing cysticercus with calcification in the muscles of (A, B) right anterior thoracic wall (19 × 8 × 15 mm3) and (C, D) right midaxillary line (26 × 10 × 19 mm3).
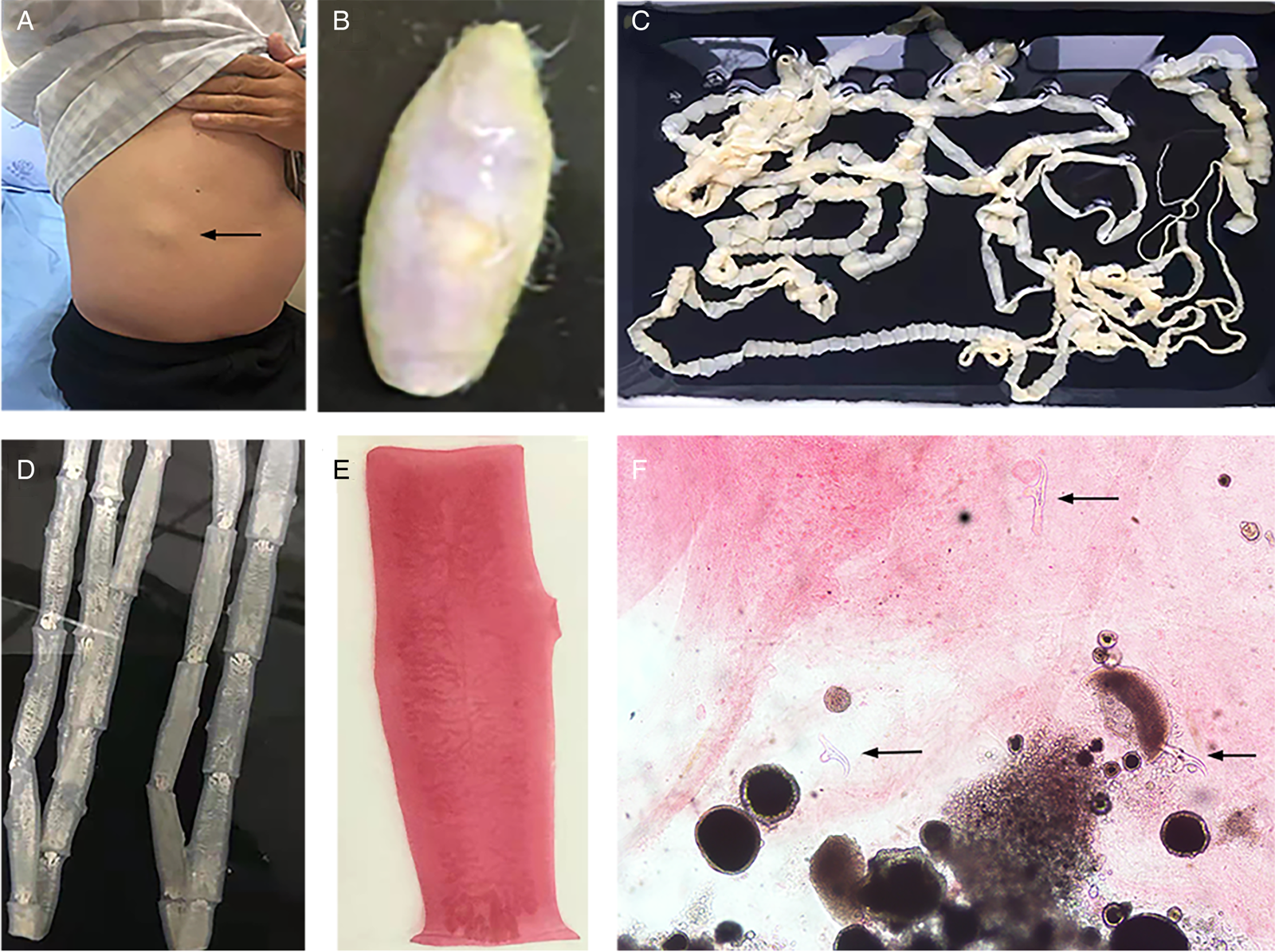
Fig. 3. Tapeworm materials from the patient: (A) subcutaneous mass; (B) cyst; (C) adult tapeworm; (D) unstained proglottids; (E) a stained proglottid and (F) cystic lesion with hydrochloric acid-carmine staining showing presumed deciduous hooks (black arrows).
Morphological observation
The adult tapeworm was milky white, flat and long like a belt, thin and translucent, and the main segments are shown in Fig. 3. The uterine branches in the gravid proglottids were regular, and each side had approximately 15–30 branches (Fig. 3). The slide of the cyst showed no obvious typical features; however, some presumed deciduous hooks were found in the same slide, leading to indistinguishable morphology (Fig. 3).
Molecular identification
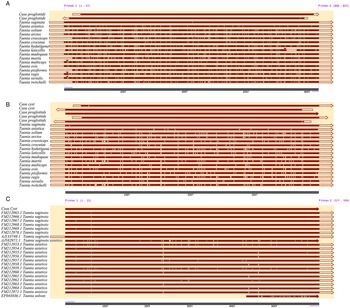
The proglottid from the patient was successfully amplified and sequenced using 2 pairs of the primers of the target COX1. The cyst was amplified with JB3 and JB4.5 primers, and the nuclear DNA HDP2 target fragment was also effectively amplified and sequenced. The 827-bp amplified products of proglottid using the T. saginata species-specific forward primer Tsag_COX1/F and Taenia common reverse primer Tae_COX1/R were 99.27–100% identical to T. saginata and 96.25–96.37% equivalent to T. asiatica without other species (Fig. 4). BLAST results indicated that the per cent identity of the 396-bp PCR products of the proglottid and cyst samples, which had trimmed JB3 and JB4.5 primer sequences, was 99.24–100% identical with T. saginata (Fig. 4). However, the 599 bp of the HDP2 target sequence revealed 99.67–99.83% identity with T. saginata, 95.66–99.67% with T. asiatica and 92.99–97.33% with T. solium under the same BLAST conditions (Fig. 4). Additional information regarding sequence alignment is provided in the Supplementary material. Based on the COX1 phylogenetic analysis (Fig. 5), the case is most likely T. saginata, next T. asiatica and finally T. solium.

Fig. 4. Sequence alignment analysis of (A) COX1 based on Tsag_COX1/F and Tae_COX1/R primers, (B) COX1 based on JB3 and JB4.5 primers and (C) HDP2 sequence.

Fig. 5. Phylogenetic tree was constructed based on the COX1 sequences of Taenia spp. using the maximum-likelihood method.
Discussion
Taeniasis coexisting with cysticercosis in humans is uncommon, especially for T. saginata infection. New insights into the pathogenicity and life cycle of T. saginata may be drawn from the case of the Tibetan patient with Taenia intestinal infection and cysticercosis (brain, muscles) reported in this paper. The life cycle of Taenia, involving the pathogenic mechanism of Taenia infection, is significant for ascertaining the disease classification (taeniasis or cysticercosis), epidemiological context, disease control and prevention (Garcia et al., Reference Garcia, Gonzalez and Gilman2020). Current medical evidence confirmed the proven diagnosis of human taeniasis caused by T. saginata and suggested the diagnosis of muscular cysticercosis and neurocysticercosis caused by the larval stage of uncertain Taenia species, which may belong to T. saginata, T. asiatica or hybrids of the 2 sibling species (Okamoto et al., Reference Okamoto, Nakao, Blair, Anantaphruti, Waikagul and Ito2010; Yamane et al., Reference Yamane, Suzuki, Tachi, Li, Chen, Nakao, Nkouawa, Yanagida, Sako, Ito, Sato and Okamoto2012). Studies have also reported that rare Taenia species (Taenia crassiceps, Taenia serialis and Taenia martis) also cause human cysticercosis, confirmed by molecular diagnostic tests (Tappe et al., Reference Tappe, Berkholz, Mahlke, Lobeck, Nagel, Haeupler, Muntau, Racz and Poppert2016; Mueller et al., Reference Mueller, Förch, Zustin, Muntau, Schuldt and Tappe2020). The potential pathogenetic mechanism of the larval stage of these species might be similar to T. solium, wherein embryos hatch in the small intestine and then may invade and spread haematogenously to the brain or muscle and other tissues (Garcia et al., Reference Garcia, Gonzalez and Gilman2020).
Three common Taenia species can be differentiated by examination of their morphological characteristics, such as the scolex, mature and gravid proglottids in the adult stage, and the scolex in the larval stage (Eom et al., Reference Eom, Rim and Jeon2020). However, the differential diagnosis between them is challenging when their morphology is not so typically visible. As far as morphological characteristics were concerned, the gravid proglottids (Fig. 3) from this patient were the same as T. saginata or T. asiatica, not T. solium, while the morphological evaluation of cysticercosis, that showed the presence of detached hooks (Fig. 3), referred to T. asiatica and T. solium rather than T. saginata. However, the traditional morphological taxonomy has some inherent limitations leading to possible false-species identification and can neglect cryptic or pseudocryptic species. That is the reason why integrated taxonomy approaches, wherein molecular studies combine detailed morphological information, are important in helping characterize pairing at the individual level resulting in the perfect characterization of cryptic biodiversity (Hebert et al., Reference Hebert, Cywinska, Ball and deWaard2003; Laakmann et al., Reference Laakmann, Blanco-Bercial and Cornils2020).
The most common method for molecular identification of Taenia tapeworms has been PCR coupled with the sequencing of the amplified PCR product (Ale et al., Reference Ale, Victor, Praet, Gabriël, Speybroeck, Dorny and Devleesschauwer2014). Other assays for the differential diagnosis of Taenia include multiplex PCR (Nunes et al., Reference Nunes, Lima, Manoel, Pereira, Nakano and Garcia2003; Yamasaki et al., Reference Yamasaki, Allan, Sato, Nakao, Sako, Nakaya, Qiu, Mamuti, Craig and Ito2004; Jeon et al., Reference Jeon, Chai, Kong, Waikagul, Insisiengmay, Rim and Eom2009; Ng-Nguyen et al., Reference Ng-Nguyen, Stevenson, Dorny, Gabriel, Vo, Nguyen, Phan, Hii and Traub2017), PCR coupled with restriction fragment length polymorphism analysis (Gonzalez et al., Reference Gonzalez, Montero, Morakote, Puente, Diaz De Tuesta, Serra, Lopez-Velez, McManus, Harrison, Parkhouse and Garate2004), random-amplified polymorphic DNA analysis (Jeon et al., Reference Jeon, Chai, Kong, Waikagul, Insisiengmay, Rim and Eom2009), loop-mediated isothermal amplification (Nkouawa et al., Reference Nkouawa, Sako, Nakao, Nakaya and Ito2009) and matrix-assisted laser desorption ionization-time-of-flight mass spectrometry (Wendel et al., Reference Wendel, Feucherolles, Rehner, Poppert, Utzinger, Becker and Sy2021). Different target sequences of Taenia genomic DNA, including mitochondrial DNA (e.g. COX1, CYTb, valine tRNA, NADH, 12S rDNA gene) (Yamasaki et al., Reference Yamasaki, Nakao, Sako, Nakaya, Sato, Mamuti, Okamoto and Ito2002; Okamoto et al., Reference Okamoto, Nakao, Blair, Anantaphruti, Waikagul and Ito2010; Roelfsema et al., Reference Roelfsema, Nozari, Pinelli and Kortbeek2016), ribosomal DNA (i.e. 28S rRNA, 5.8S rRNA and ITS rRNA gene) (von Nickisch-Rosenegk et al., Reference von Nickisch-Rosenegk, Silva-Gonzalez and Lucius1999; Praet et al., Reference Praet, Verweij, Mwape, Phiri, Muma, Zulu, van Lieshout, Rodriguez-Hidalgo, Benitez-Ortiz, Dorny and Gabriel2013) and nuclear DNA (HDP1, HDP2, cathepsin L-like cysteine peptidase, Ag2 gene) (Gonzalez et al., Reference Gonzalez, Montero, Harrison, Parkhouse and Garate2000; Gonzalez et al., Reference Gonzalez, Montero, Morakote, Puente, Diaz De Tuesta, Serra, Lopez-Velez, McManus, Harrison, Parkhouse and Garate2004; Flores et al., Reference Flores, Gonzalez, Hurtado, Motta, Dominguez-Hidalgo, Merino, Perteguer and Garate2018), have been used as markers in molecular diagnosis. In particular, the mitochondrial COX1 gene that has been used in the study is a universally accepted DNA barcoding marker for assessing the genetic variation and evolutionary biology of Metazoa, including helminth parasites (Hebert et al., Reference Hebert, Cywinska, Ball and deWaard2003; Galimberti et al., Reference Galimberti, Romano, Genchi, Paoloni, Vercillo, Bizzarri, Sassera, Bandi, Genchi, Ragni and Casiraghi2012; Zhang et al., Reference Zhang, Chen, Yang, Liu, Jiang, Gu, Wang and Wang2014; Rostami et al., Reference Rostami, Salavati, Beech, Babaei, Sharbatkhori and Harandi2015; Mioduchowska et al., Reference Mioduchowska, Czyz, Goldyn, Kur and Sell2018; Eberle et al., Reference Eberle, Ahrens, Mayer, Niehuis and Misof2020). Molecular characterization in our study based on COX1 and HDP2 indicated that the cyst seemed to be more inclined to T. saginata or T. asiatica. The analysis of HDP2 sequences in cysticercus samples ruled out the possibility of T. solium, but failed to reliably differentiate T. saginata from T. asiatica or their hybrids (Okamoto et al., Reference Okamoto, Nakao, Blair, Anantaphruti, Waikagul and Ito2010). High homologies were found between T. saginata and T. asiatica on mitochondrial COX1 gene, as shown by the phylogenetic dendrogram (Fig. 5) which is in line with a prior study showing a 4.6% difference between the whole mitochondrial genome sequence of T. saginata and T. asiatica (Jeon et al., Reference Jeon, Kim and Eom2007). However, the BLAST results of the cyst were inconsistent with its morphology, which encompassed dropping hooks in the slide (Fig. 3). These disagreements in morphology and molecular identification suggested the possibility of hybrids of T. saginata and T. asiatica.
For studies involving DNA sequences, whole-genome sequencing has become the standard method for collecting relevant data (Eberle et al., Reference Eberle, Ahrens, Mayer, Niehuis and Misof2020). Unfortunately, due to the scarcity of high-quality cyst samples, we have not been able to sequence the entire cyst genome. However, our study suggested that patients with taeniasis accompanied by cysticercosis may need whole-genome analysis to further ascertain whether T. saginata is also responsible for causing human cysticercosis. Despite the widespread availability of molecular identification methods, often tested on proglottids' samples, the sensitivity of detection in cyst samples may be lowered due to DNA degradation within cysts (Figueiredo et al., Reference Figueiredo, RA, Sato, da Silva, Pereira-Junior, Chigusa, Kawai and Sato2019). Many researchers have reported various mitochondrial/nuclear gene discordances in specimens, indicating potential hybrids between T. asiatica and T. saginata (Nkouawa et al., Reference Nkouawa, Sako, Nakao, Nakaya and Ito2009; Okamoto et al., Reference Okamoto, Nakao, Blair, Anantaphruti, Waikagul and Ito2010; Yamane et al., Reference Yamane, Suzuki, Tachi, Li, Chen, Nakao, Nkouawa, Yanagida, Sako, Ito, Sato and Okamoto2012; Ale et al., Reference Ale, Victor, Praet, Gabriël, Speybroeck, Dorny and Devleesschauwer2014). Therefore, a molecular sequence-based species characterization that relies on information encoded by a single gene of the mitochondrial genome can be deceptive. Hence, mitochondrial, ribosomal or nuclear marker-based multilocus sequence analysis of genetic heterogeneity within the Taenia spp. could be the more effective routine molecular identification in clinical laboratories (Pajuelo et al., Reference Pajuelo, Eguiluz, Dahlstrom, Requena, Guzman, Ramirez, Sheen, Frace, Sammons, Cama, Anzick, Bruno, Mahanty, Wilkins, Nash, Gonzalez, Garcia, Gilman, Porcella, Zimic and Cysticercosis Working Group in Peru2015). Therefore, it would be interesting to develop an easy, simple-to-handle and highly sensitive, multilocus sequences-based molecular diagnostic method for specimen identification of Taenia for validation in clinical settings.
In conclusion, the cause of human cysticercosis by non-solium Taenia species, such as T. saginata or T. asiatica, remains unclear. Correct diagnosis can be aided by utilizing whole-genome analysis and molecular identification approaches that focus on multilocus sequences. In order to better understand the comprehensive epidemiology and total life cycle of Taenia, the limitations of the existing molecular diagnostic applications require more research for the correct diagnosis and prevention of the disease.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S003118202200169X
Data availability
The data and materials information used and analyzed in the current study are available from the first author (Jie Hou) or corresponding author (Ying Ma) upon reasonable request.
Acknowledgements
We thank the patient and his family.
Author's contributions
Conceptualization: Jie Hou, Ying Ma and Junyan Qu; data curation: Jie Hou and Weilin Chen; formal analysis: Jie Hou, Weilin Chen and Rong Chen; investigation: Jie Hou, Weilin Chen and Chunlei He; methodology: Jie Hou; project administration: Ying Ma and Jie Hou; resources: Ying Ma and Junyan Qu; software: Jie Hou; supervision: Ying Ma and Junyan Qu; validation: Ying Ma and Junyan Qu; visualization: Jie Hou; writing – original draft: Jie Hou; writing – review and editing: Ying Ma and Junyan Qu.
Financial support
This research received no specific grant from any funding agency, commercial or not-for-profit sectors.
Conflict of interest
The authors declare there are no conflicts of interest.
Ethical standards
The patient's oral informed consent was obtained by phone, and written informed consent was waived with the approval of the West China Hospital, Sichuan University Biomedical Ethics Committee (approval number: 2022-472) because we met the criteria for an informed consent waiver.