Obesity incidence has increased dramatically during the last two to three decades among people in all industrialised countries and has also become an emerging health problem in developing countries where Western food habits and lifestyle are being adopted(Reference Cornier, Despres and Davis1, Reference Hossain, Kawar and El2). Weight gain and obesity are major risk factors for pathologies such as insulin resistance, type 2 diabetes mellitus, the metabolic syndrome, atherosclerosis and stroke(Reference Cornier, Despres and Davis1). These conditions are most clearly correlated with a fat distribution pattern characterised by an excessive accumulation of visceral (intra-abdominal) fat. This abdominal obesity is associated with a local low-grade inflammatory response with an increased production of pro-inflammatory cytokines, e.g. TNF-α, IL-1β and IL-6. It is also associated with the production of chemokines, such as monocyte chemotactic protein-1 (MCP-1), attracting monocytes, which in turn settle in the tissue around dead or dying adipocytes, giving rise to the so-called crown-like structures (CLS) in immune histological sections(Reference Cinti, Mitchell and Barbatelli3). Increasing evidence has demonstrated a mechanistic link between low-grade inflammation in adipose tissue and insulin resistance(Reference Hotamisligil, Shargill and Spiegelman4–Reference Gregor and Hotamisligil6).
Lifestyle interventions such as increased exercise and energy restriction are important measures for preventing obesity. In addition, it has been shown that a change of food patterns towards a diet with an increased percentage of less refined carbohydrates, as well as MUFA and PUFA, has beneficial effects on cardiometabolic risk factors, independent of weight loss(Reference Pereira, Kottke and Jordan7). In recent years, marine source-derived macro- and micronutrients (oils, proteins/peptides, polysaccharides, vitamins and minerals, as well as phytosterols and antioxidants) have gained increasing attraction as natural products with health benefits(Reference Larsen, Eilertsen and Elvevoll8). Although Calanus finmarchicus is the most abundant crustacean in the North Atlantic Ocean(Reference Melle, Ellertsen, Skjoldal and Skjoldal9), its potential health-promoting effects have not been well explored. The biochemical composition of the oil from C. finmarchicus is notably different from that of other marine oils commonly referred to for their health benefits. This oil contains MUFA and PUFA, which are bound to aliphatic long-chain monounsaturated alcohols as wax esters. This is in contrast to fish and krill oils, where fatty acids are primarily bound as TAG and phospholipids, respectively. Calanus oil also contains components that are not found, or found in very small quantities, in the majority of other marine oils, such as phytosterols and antioxidants (astaxanthin). In a recent study, Eilertsen et al. (Reference Eilertsen, Maehre and Jensen10) have shown that supplementation with Calanus oil decreased plaque formation in apoE-deficient mice. The aim of the present study was, therefore, to examine the effects of Calanus oil supplementation in a mouse model of diet-induced obesity and glucose intolerance, using both a preventive and a therapeutic approach.
Materials and methods
Study design and animals
Diet-induced obese mice were obtained by feeding 5–6-week-old C57BL/6J male mice (Charles River) a lard-based high-fat diet (HFD, no. 58V8, Test Diet; IPS Limited) containing 18, 36 and 46 % of energy from protein, carbohydrate and fat, respectively. There were three groups of obese mice: the first receiving the HFD throughout the whole 27-week feeding period (HFD); the second receiving the HFD supplemented with 1·5 % (w/w) Calanus oil from the start and throughout the entire 27-week feeding period (preventive treatment, CAP); the third receiving the HFD (without supplementation) for 7 weeks, followed by the HFD with 1·5 % (w/w) Calanus oil supplementation for the remaining feeding period (therapeutic treatment, CAT). Addition of Calanus oil was compensated for by the removal of 1·5 g lard/100 g diet, so that the total fat content was similar and the diets remained isoenergetic. Mice fed a normal chow diet containing 18, 72 and 10 % of energy from protein, carbohydrate and fat (CTR, no. 58Y2, Test Diet; IPS Limited) served as lean controls. To phenotype mice before the start of the CAT treatment, some mice were killed at the 7-week time point (Supplementary data, available online).
All animal experiments were approved by the local authority of the National Animal Research Authority in Norway (FOTS id 2365/2010). Mice were treated in accordance with the guidelines on accommodation and care of animals formulated by the European Convention for the Protection of Vertebrate Animals for Experimental and Other Scientific Purposes. They were housed at 21°C under a reversed light/dark cycle, three to four mice per cage. The mice received food ad libitum and had free access to drinking-water. Body weight was recorded weekly, and food intake was monitored at several time points throughout the feeding period. The data on food intake from both groups of Calanus oil-supplemented mice were combined. On the day of killing, organs from the fed mice were carefully dissected out, weighed, snap-frozen, fixed in Zn-based fixative (ZBF) and immersed either in McDowell's medium or in RNA-protecting agents for later analysis.
Calanus oil
The zooplankton C. finmarchicus was harvested in the North Atlantic Ocean off the Norwegian shore. The crude oil fraction was extracted by a conventional industrial process technology without further removal of any compounds (Calanus AS). Table 1 summarises the chemical composition of this oil.
Table 1 Calanus oil composition

FAOH, fatty alcohol.
† The major part (60 %) of the n-6 PUFA is linoleic acid (18 : 2n-6), while γ-linolenic acid (18 : 3n-6) and arachidonic acid (20 : 4n-6) account for 25 and 15 %, respectively.
‡ The major part of the FAOH consists of monounsaturated 20 : 1n-9 and 22 : 1n-11 alcohols.
* α-Linolenic acid (18 : 3n-3), stearidonic acid (18 : 4n-3), EPA (20 : 5n-3) and DHA (22 : 6n-3) account for 5, 37, 35 and 23 % of the n-3 PUFA fraction, respectively.
§ Others include TAG, NEFA and phospholipids.
Glucose tolerance tests and blood samples
A glucose tolerance test (following 4 h of fasting) was performed 7 weeks into the feeding period as well as 5-6 weeks before killing. Blood was collected from the saphenous vein immediately before (0 min) and 15, 30, 60 and 120 min after intraperitoneal administration of a glucose solution (1·3 g/kg). Glucose concentration was measured using a glucometer (Ascensia Contour; Bayer Healthcare). The trapezium rule was used to determine the AUC(Reference Matthews, Altman and Campbell11). During the last 2 weeks of the feeding period, blood samples were taken from both fasted (4 h) and fed mice at 13.00 hours by puncture of the saphenous vein. Plasma insulin, NEFA and glycerol concentrations were analysed using commercial kits from DRG Diagnostics, Wako Chemicals and Sigma-Aldrich, respectively, using a plate reader (Victor2 1420 Multilabel Counter; PerkinElmer).
Adipocyte size
Formalin-fixed perirenal adipose tissue was embedded in paraffin and deparaffinised, and sections of 4 μm thickness were stained with haematoxylin and eosin. The histological sections were viewed at 10 × magnification (Leica DM 2000 microscope; Leica Microsystems CMS GmbH). Squares of 0·23 mm2 were randomly selected, and adipocyte cross-sectional surface area was measured by manual tracing of each individual adipocyte in a square, using Image J (http://imagej.nih.gov/ij/).
Immunohistochemistry
Immunohistochemistry was performed on dewaxed 4 μm-thick perirenal adipose tissue sections that were incubated with rabbit anti-mouse F4/80 primary antibody (1:75; Mybiosource) according to the avidin–biotin complex method(Reference Xu, Barnes and Yang12). Endogenous peroxidase was inactivated using 3 % H2O2, followed by treatment with 10 % normal goat serum to reduce non-specific staining, and biotinylated horseradish peroxidase-conjugated goat anti-rabbit IgG was used as the secondary antibody (1:200, Biotinylated antibody Vectastain® ABC Elite kit, PK-6101; Vector Laboratories). Histochemical reactions were performed using Vector's Vectastain ABC Kit (Vector Laboratories) and substrate (Vector® NovaRED™ Substrate kit for peroxidase, SK-4800; Vector Laboratories). Sections were counterstained with haematoxylin, and images were obtained using a Leica DM 2000 microscope (Leica Microsystems CMS GmbH). Digital pictures were taken using a Leica DFC425 camera (Leica Microsystems CMS GmbH). F4/80-positive cells were considered macrophages, and their total number in each tissue section was determined. CLS were defined as one adipocyte surrounded by at least three macrophages. A tissue section of 1·28 mm2 was randomly selected, and quantification of the macrophages and CLS was executed at 40 × magnification.
Tissue TAG content
Hepatic TAG was extracted by the method of Folch, dried and thereafter dissolved in a tert-butyl and Triton X-100/methyl alcohol mixture. TAG concentration of the extracts was measured with the ‘Triglyceride 25’ kit from ABX Diagnostics according to the protocol, using a plate reader (Victor2 1420 Multilabel Counter; PerkinElmer).
Quantitative real-time PCR
The expression of mRNA in adipose tissue was determined using quantitative real-time PCR. Adipose tissue samples were immersed in Allprotect Tissue Reagent (Qiagen Nordic), and total RNA was extracted according to the Rneasy Lipid Tissue kit Protocol (Qiagen Nordic). Quantitative real-time PCR was performed in an ABI PRISM 7900 HT Fast real-time thermal cycler as described previously(Reference Hafstad, Khalid and Hagve13) (Applied Biosystems). Fast SYBR© Green master mix (Applied Biosystems) was used, and genes were normalised to the housekeeping gene glyceraldehyde-3-phosphate dehydrogenase (GAPDH). Forward and reverse primer sequences (5′–3′) were as follows: TNF-α – forward CAT-CTT-CTC-AAA-ATT-CGA-GTG-ACA-A, reverse TGG-GAG-TAG-ACA-AGG-TAC-AAC-CC; IL-6 – forward GCT-ACC-AAA-CTG-GAT-ATA-ATC-AGG-A, reverse CCA-GGT-AGC-TAT-GGT-ACT-CCA-GAA; adiponectin – forward TGT-TCC-TCT-TAA-TCC-TGC-CCA, reverse CCA-ACC-TGC-ACA-AGT-TCC-CTT; MCP-1 – forward TTA-AAA-ACC-TGG-ATC-GGA-ACC-AA, reverse GCA-TTA-GCT-TCA-GAT-TTA-CGG-GT; GAPDH – forward TCA-CCA-CCA-TGG-AGA-AGG-C, reverse GCT-AAG-CAG-TTG-GTG-GTG-CA.
Statistical analysis
Data are presented as means with their standard errors. Statistical analysis was performed using Sigma Plot 12.0 (Systat Software; Alfasoft AS). Two-way ANOVA followed by Holm–Sidak post hoc tests was used for comparing means. P< 0·05 was considered significant.
Results
Model of diet-induced obesity
The average daily energy intake of the HFD-fed mice (after food intake had plateaued) was significantly higher than that of the lean controls (CTR) (Fig. S1, available online). This increased energy intake resulted in a model of diet-induced obesity, characterised by increased body weight and excessive intra-abdominal fat accumulation (indicated by significantly increased perirenal white adipose tissue (pWAT) mass), hepatic steatosis, hyperinsulinaemia and impaired glucose tolerance, as well as increased levels of inflammatory markers and macrophage infiltration in intra-abdominal fat (Figs. 1–3 and Table 2).

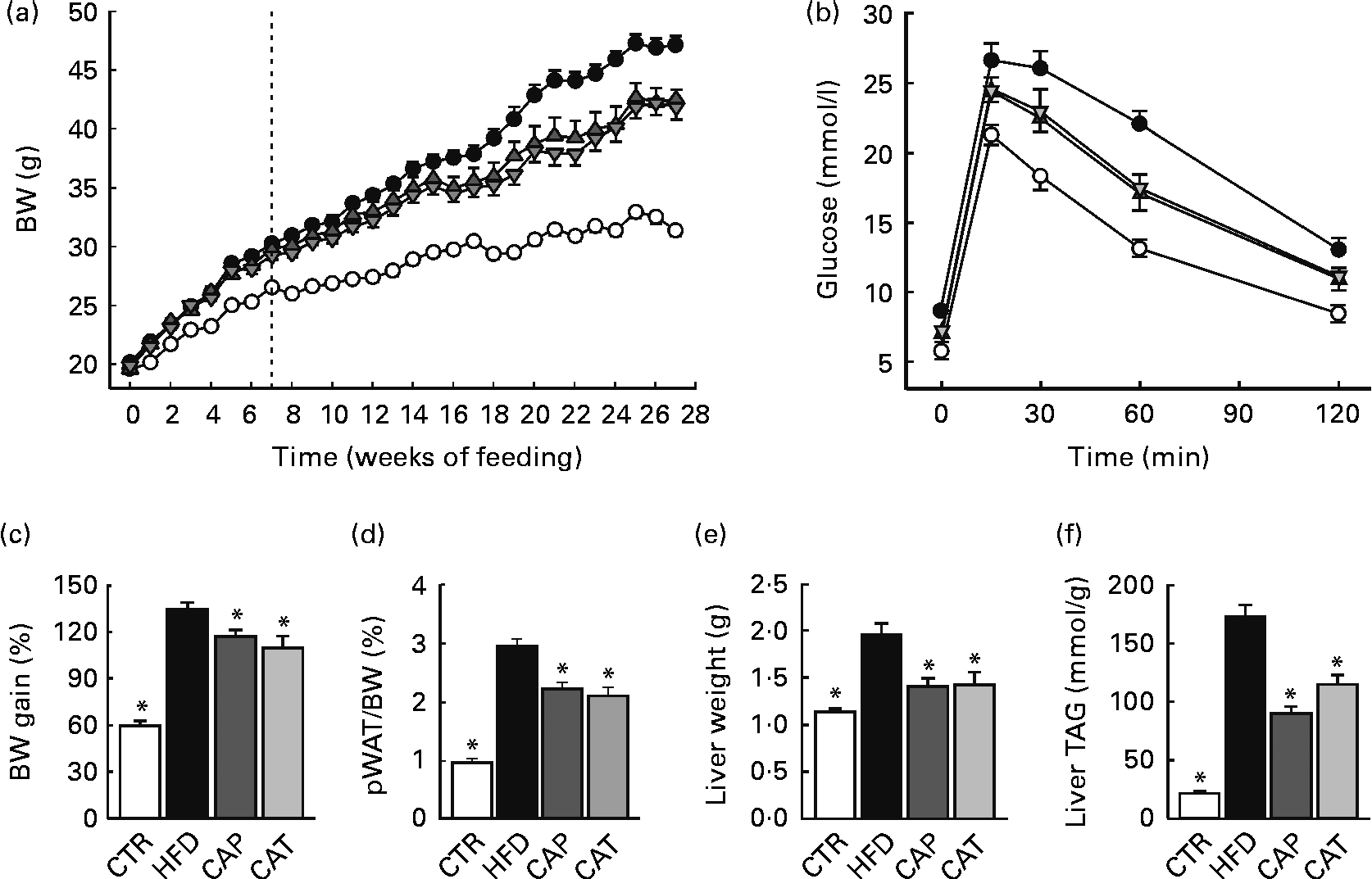
Fig. 1 (a) Changes in body weight (BW) in mice fed the normal chow diet (CTR (○), n 19), the high-fat diet (HFD (●), n 16) or the HFD supplemented with 1·5 % Calanus oil from the start (CAP (![]() ), n 11) or from the 7-week time point (CAT (
), n 11) or from the 7-week time point (CAT (![]() ), n 11), as indicated by the dotted line. (b) Glucose tolerance test performed 5–6 weeks before killing (n 11–15 per group). (c) BW gain in percentage from baseline, (d) perirenal white adipose tissue (pWAT) mass as a percentage of BW, (e) liver weight and (f) liver TAG content, determined at the end of the 27-week feeding period (n 9–19 per group). Values are means, with their standard errors represented by vertical bars. * Mean values were significantly different from that of HFD-fed mice (P< 0·05).
), n 11), as indicated by the dotted line. (b) Glucose tolerance test performed 5–6 weeks before killing (n 11–15 per group). (c) BW gain in percentage from baseline, (d) perirenal white adipose tissue (pWAT) mass as a percentage of BW, (e) liver weight and (f) liver TAG content, determined at the end of the 27-week feeding period (n 9–19 per group). Values are means, with their standard errors represented by vertical bars. * Mean values were significantly different from that of HFD-fed mice (P< 0·05).

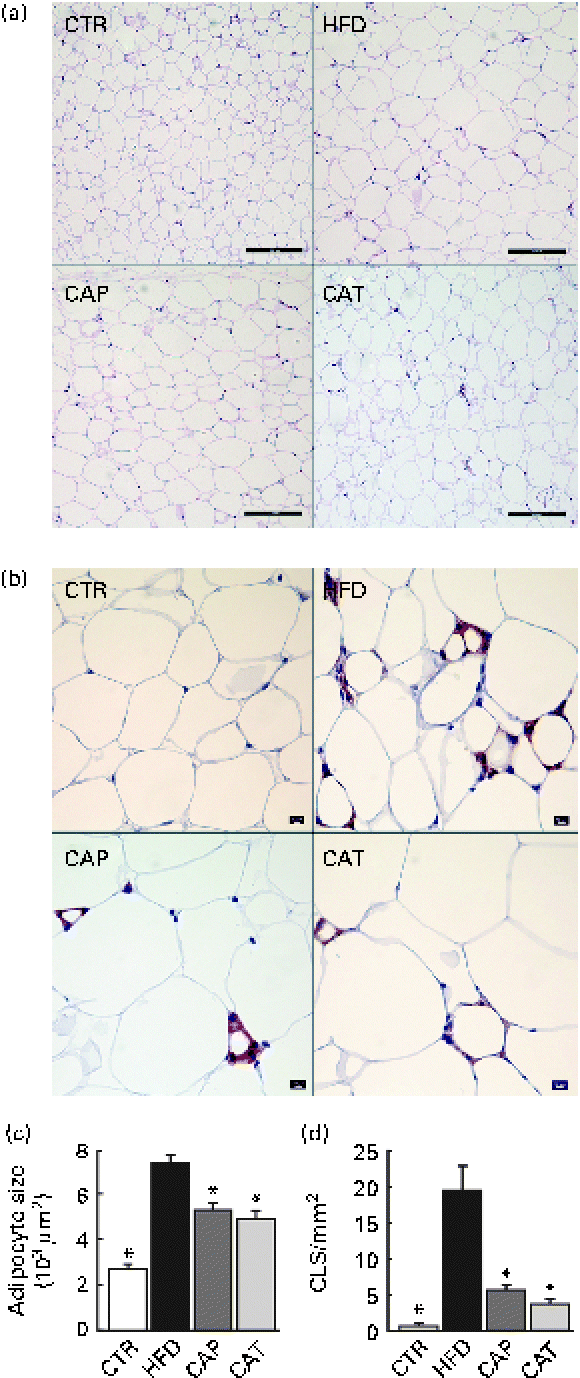
Fig. 2 (a) Haematoxylin and eosin staining of perirenal adipose tissue (scale bar 200 μm), (b) F4/80 staining for the identification of macrophages in perirenal adipose tissue (scale bar 50 μm), (c) adipocyte size (n 11–18 per group) and (d) number of crown-like structures (CLS) (n 5–8 per group). The groups are described in Fig. 1. Values are means, with their standard errors represented by vertical bars. * Mean values were significantly different from that of HFD-fed mice (P< 0·05). CTR, control mice fed the normal chow diet; HFD, mice fed the high-fat diet; CAP and CAT, mice fed the high-fat diet supplemented with Calanus oil in a preventive or a therapeutic approach, respectively.

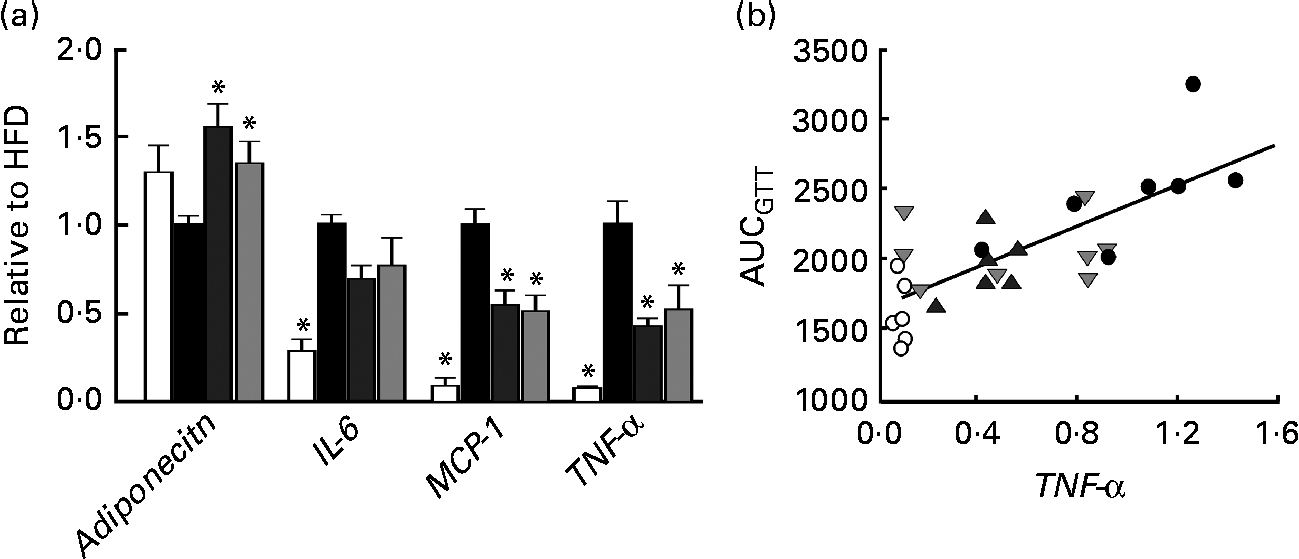
Fig. 3 (a) mRNA expression of adiponectin, IL-6, monocyte chemotactic protein-1 (MCP-1) and TNF-α in perirenal adipose tissue obtained from mice in groups described in Fig. 1 (n 5–8 per group). All groups are normalised to HFD. Values are means, with their standard errors represented by vertical bars. * Mean values were significantly different from that of HFD-fed mice (P< 0·05). CTR (□), control mice fed the normal chow diet; HFD (■), mice fed the high-fat diet; CAP (![]() ) and CAT (
) and CAT (![]() ), mice fed the high-fat diet supplemented with Calanus oil in a preventive or a therapeutic approach, respectively. (b) Correlation between TNF-α mRNA expression and AUC following the glucose tolerance test (GTT) (from Fig. 1). R 2 0·56, P< 0·001. ○, CTR; ●, HFD;
), mice fed the high-fat diet supplemented with Calanus oil in a preventive or a therapeutic approach, respectively. (b) Correlation between TNF-α mRNA expression and AUC following the glucose tolerance test (GTT) (from Fig. 1). R 2 0·56, P< 0·001. ○, CTR; ●, HFD; ![]() , CAP;
, CAP; ![]() , CAT.
, CAT.
Table 2 Plasma levels of NEFA, glycerol and insulin and calculations of AUC from the glucose tolerance test (GTT) at the end of the experimental period (Mean values with their standard errors)

CTR, control mice fed the normal chow diet; HFD, mice fed the high-fat diet; CAP and CAT, mice fed the high-fat diet supplemented with Calanus oil in a preventive or a therapeutic approach, respectively.
* Mean values were significantly different from that of HFD-fed mice (P< 0·05).
† The GTT was performed in eleven to fifteen mice per group.
Effect of Calanus oil on obesity and glucose tolerance
The HFD was supplemented with Calanus oil (1·5 % w/w) either from the start of the feeding period (preventive treatment, CAP) or after 7 weeks on the HFD (therapeutic treatment, CAT), where obesity and glucose intolerance were already established (Fig. S2, available online). Both the preventive and therapeutic treatment strategies significantly reduced body weight (Fig. 1(a)) and the calculated body-weight gain (Fig. 1(c)) as well as pWAT mass (Fig. 1(d)). Energy intake was slightly lower (not statistically significant) for mice receiving the Calanus oil-supplemented HFD compared with mice receiving the unsupplemented HFD (Fig. S1, available online). Measurements of tibia length did not differ between the four groups (data not shown), demonstrating that different amounts of body fat account for the differences in body weight. The diet-induced increases in liver weight (Fig. 1(e)) and hepatic steatosis (Fig. 1(f)) as well as plasma levels of NEFA and glycerol (Table 2) were significantly reduced in both the Calanus oil-supplemented groups when compared with the untreated HFD-fed mice.
At the end of the experimental period, significantly improved glucose tolerance and reduced plasma insulin levels were observed in both the CAP and CAT groups (Fig. 1(b) and Table 2). It should be noted that glucose tolerance was already significantly improved after 7 weeks of (preventive) Calanus oil treatment (Fig. S2, available online), as indicated by a reduced AUC (1907 (sem 54) v. 2241 (sem 95) for the CAP and HFD groups, respectively P< 0·05).
Effect of Calanus oil on adipose tissue macrophage infiltration and cytokine expression
Histological and immunohistochemical examinations of pWAT (Fig. 2) revealed that Calanus oil supplementation significantly attenuated the diet-induced increase in adipocyte size (Fig. 2(a) and (c)), accompanied by reduced macrophage infiltration (number of CLS; Fig. 2(b) and (d)). Calanus oil was also found to attenuate the diet-induced up-regulation of TNF-α, IL-6 and MCP-1 mRNA expression (Fig. 3(a)) in pWAT. We also found that TNF-α mRNA expression in pWAT correlated negatively with glucose tolerance (Fig. 3(b)), regardless of whether CTR was included or not (R 2 0·56, P< 0·001 and R 2 0·42, P= 0·002, with and without CTR, respectively). These data clearly demonstrate that Calanus oil attenuates the obesity-induced inflammatory response. Finally, the reduced expression of the adipocyte-derived insulin-sensitising hormone adiponectin in HFD-fed mice was found to be restored in both the treatment groups (Fig. 3(a)).
Discussion
Sedentary lifestyle and increased energy intake have resulted in a dramatic rise in the prevalence of obesity, the metabolic syndrome and insulin resistance, conditions correlating strongly with abdominal obesity(Reference Cornier, Despres and Davis1). Abdominal obesity represents a much higher risk factor for mortality and morbidity in humans than general obesity(Reference Kuk, Katzmarzyk and Nichaman14), and individuals accumulating large amounts of abdominal fat are particularly prone to developing diabetes and CVD(Reference Cornier, Despres and Davis1, Reference Wang, Rimm and Stampfer15). Abdominal obesity is also associated with a local low-grade inflammatory response, and there is robust evidence for a mechanistic link between inflammation and insulin resistance(Reference Hotamisligil, Shargill and Spiegelman4–Reference Gregor and Hotamisligil6, Reference Xu, Barnes and Yang12). The main findings of the present study are that oil from the zooplankton C. finmarchicus, given as a minor food supplement to diet-induced obese mice, attenuates the deposition of intra-abdominal fat, reduces liver steatosis and improves glucose tolerance. Calanus oil also decreases the inflammatory status in intra-abdominal adipose tissue.
The amount of fat in the body is regulated by mechanisms controlling anabolic and catabolic processes. Higher intake of marine fatty acids has received much attention as a natural way of readjusting this fat homeostasis and modulating obesity-related disorders. In the present study, we found that dietary supplementation with Calanus oil reduced obesity, expressed as marked reductions in body weight, abdominal fat stores, adipocyte size and hepatic steatosis. Although the present study cannot identify the impact of the different components of the oil, it is highly possible that n-3 PUFA play an important role, as several studies in rodents have reported anti-obesity effects following administration of n-3 PUFA(Reference Belzung, Raclot and Groscolas16–Reference Arai, Kim and Chiba22). More than 80 % of Calanus oil consists of wax esters in which n-3 PUFA (but also other PUFA and MUFA) are bound to long-chain fatty alcohols. Gorreta et al. (Reference Gorreta, Bernasconi and Galliani23) have demonstrated a similar bioavailability of n-3 PUFA, regardless of whether they were bound as wax ester, TAG or ethyl ester. In addition, Eilertsen et al. (Reference Eilertsen, Maehre and Jensen10) detected higher levels of EPA and DHA in the blood of Calanus oil-fed mice compared with mice fed a diet with concentration-matched EPA and DHA ethyl esters, indicating that wax ester-bound fatty acids are readily metabolised in mice. In addition to EPA and DHA, the n-3 PUFA fraction of Calanus oil contains a high amount of stearidonic acid (SDA; 18 : 4n-3). SDA is a precursor of EPA and has received interest as a means of n-3 PUFA enrichment in the human diet(Reference Whelan, Gouffon and Zhao24). A rapid conversion of SDA to EPA has also been shown in rodents(Reference Voss and Sprecher25), and the elevated plasma levels of EPA and DHA in mice following dietary supplementation with Calanus oil(Reference Eilertsen, Maehre and Jensen10) could, therefore, also reflect the conversion of SDA to EPA.
It should be noted that while an anti-obese effect of n-3 PUFA in rodents is usually shown in a preventive setting(Reference Belzung, Raclot and Groscolas16–Reference Sato, Kawano and Notsu19), Calanus oil exerted its anti-obesity effect in both a preventive and a therapeutic fashion. Suggested mechanisms for n-3 PUFA-mediated anti-obesity effects include decreased energy intake(Reference Perez-Matute, Perez-Echarri and Martinez20) and suppression of lipogenesis(Reference Raclot, Groscolas and Langin18, Reference Arai, Kim and Chiba22). The difference in energy intake between Calanus oil-supplemented mice and those given the HFD alone was not statistically significant. Nevertheless, since we observed a slightly lower food intake in Calanus oil-supplemented mice, we cannot rule out that reduced energy intake has contributed to decreased adiposity. In which way n-3 PUFA alter lipolysis is less clear from the literature(Reference Perez-Matute, Perez-Echarri and Martinez20, Reference Parrish, Pathy and Parkes26–Reference Lorente-Cebrian, Bustos and Marti28). At first glance, the observed reduction in the plasma levels of NEFA and glycerol in Calanus oil-treated mice can be interpreted as reduced adipose tissue lipolysis, which is in line with previous studies reporting reduced basal lipolysis in abdominal fat following administration of n-3 PUFA(Reference Parrish, Pathy and Parkes26, Reference Lorente-Cebrian, Bustos and Marti28, Reference Rustan, Hustvedt and Drevon29). It should be noted, however, that the plasma levels of NEFA and glycerol were measured at the end of the treatment period and do not reflect lipolytic activity throughout the whole feeding period. As enlarged adipocytes(Reference Berger and Barnard30), hyperglycaemia(Reference Ren, He and Jiang31) and increased levels of pro-inflammatory cytokines(Reference Lorente-Cebrian, Bustos and Marti28) have been reported to have a pro-lipolytic action, we suggest that the reduced plasma levels of NEFA and glycerol are a result of the reduced obesity and/or inflammation.
Even though it is tempting to assume that EPA and DHA are responsible for the observed anti-obesity effects, astaxanthin, a strong antioxidant found in Calanus oil, has also been shown to decrease the amount of abdominal fat in diet-induced obese mice(Reference Ikeuchi, Koyama and Takahashi32). It is important to emphasise, however, that the concentrations of EPA/DHA (including a potential conversion of SDA to EPA) and astaxanthin reported by the present study were extremely low compared with those reported by other studies. While the dietary content of n-3 PUFA in the present study was less than 0·3 % (w/w), other studies demonstrating the anti-obese effects of marine oils in rodents have used diets where their content varied from 2 % to more than 10 %(Reference Belzung, Raclot and Groscolas16–Reference Sato, Kawano and Notsu19), i.e. seven to thirty-five times the amount used in the present study. Likewise, the intake of astaxanthin in the study by Ikeuchi et al. (Reference Ikeuchi, Koyama and Takahashi32) was thirty times higher than the corresponding value in the present study (approximately 1 mg/kg per d).
Calanus oil also contains phytosterols, which have recently gained attention, not only for their cholesterol-lowering ability, but also for their weight-reducing effect(Reference Looije, Risovic and Stewart33, Reference Thornton, Warburton and Wasan34). However, again, the dietary content of phytosterols previously reported to induce anti-obesity effects(Reference Looije, Risovic and Stewart33, Reference Thornton, Warburton and Wasan34) was at least 100 times higher than the content in the Calanus oil-supplemented diet.
The present study confirmed that excessive fat accumulation in abdominal adipose tissue is accompanied by a state of low-grade inflammation, involving the infiltration of inflammatory cells and the activation of genes encoding pro-inflammatory cytokines(Reference Weisberg, McCann and Desai35). Calanus oil attenuated this local inflammatory response, as indicated by a marked reduction in macrophage infiltration as well as TNF-α, IL-6 and MCP-1 gene expression. Although both n-3 PUFA and astaxanthin are known to exert anti-inflammatory effects(Reference Perez-Matute, Perez-Echarri and Martinez20, Reference Calder36–Reference Yuan, Peng and Yin38), it is not clear whether the anti-inflammatory action is a direct effect of Calanus oil or whether it is a secondary consequence of the reduced adipocyte size. It has been suggested that limitation of oxygen delivery and induction of adipose tissue hypoxia are initiating events triggering the inflammatory response in expanded/obese adipocytes(Reference Ye, Gao and Yin39). Thus, one could argue that the anti-inflammatory action of Calanus oil supplementation is due to the reduction in adipocyte size, which occurred in parallel with the reduction in adipose tissue mass.
Calanus oil was also found to lower circulating insulin levels and improve glucose tolerance. As local pro-inflammatory mediators can create a systemic inflammation that adversely affects the metabolic function(Reference Gregor and Hotamisligil6), improved glucose tolerance in Calanus oil-supplemented obese mice could, at least in part, be explained in terms of the inflammatory status. This notion was indeed supported by the finding of a significant negative correlation between whole-body glucose tolerance and TNF-α expression in adipose tissue. Furthermore, the adipocyte-derived hormone adiponectin is known for its insulin-sensitising properties, and in line with the present results, adiponectin has been shown to negatively correlate with abdominal obesity(Reference Ouchi, Parker and Lugus40). Previous studies(Reference Bruun, Lihn and Verdich41) have also shown that TNF and IL-6 negatively regulate adiponectin. Hence, the decreased inflammatory status in the adipose tissue of Calanus-fed mice could have contributed to the increased production of adiponectin and thus to the observed improvement in glucose tolerance. Both n-3 PUFA(Reference Sato, Kawano and Notsu19, Reference Perez-Matute, Perez-Echarri and Martinez20, Reference Lamping, Nuno and Coppey42) and astaxanthin(Reference Arunkumar, Bhuvaneswari and Anuradha37, Reference Preuss, Echard and Yamashita43) have been shown to reduce hyperinsulinaemia and/or improve glucose tolerance/insulin sensitivity in diet-induced obese rodents. Again, it is worth noting that the improvement in glucose homeostasis by Calanus oil was obtained with concentrations of either n-3 PUFA or astaxanthin that were much lower than those reported previously. Finally, we cannot rule out the fact that MUFA play a role in the beneficial effects of Calanus oil, as they have been suggested to have favourable metabolic effects in both animal and human studies(Reference Yang, Miyahara and Mori44, Reference Gillingham, Harris-Janz and Jones45).
In summary, the present study has shown that dietary supplementation with as little as 1·5 % (w/w) Calanus oil (using either a preventive or a therapeutic approach) reduces intra-abdominal and hepatic fat deposition during high-fat feeding. It also attenuates low-grade inflammation in adipose tissue and improves systemic glucose tolerance. Although the present study does not reveal the active component(s) of the oil, it is tempting to suggest that the unique composition of Calanus oil plays a key role in the determination of its efficacy, and further investigations are required in order to clarify the role of the individual components of the oil.
Supplementary material
To view supplementary material for this article, please visit http://dx.doi.org/10.1017/S0007114513001839
Acknowledgements
The authors cordially thank Elisabeth Børde, Knut E. Steinnes, Marit Nilsen and the staff from the Department of Comparative Medicine of the University of Tromsø for their technical assistance. The present study was funded by the Norwegian Diabetes Association, the Norwegian Heart Foundation and Calanus AS. Calanus oil was provided by Calanus AS.
A. C. H., T. S. L. and E. A. designed the experimental protocol; A. C. H., W. S., A. M. K., A. D. H. and S. J. S. conducted the experiments; E. A., A. C. H., W. S. and S. J. S. analysed the data; A. C. H., E. A. and T. S. L. wrote the manuscript with the assistance of W. S. and J. R.
The partial funding by Calanus AS is a potential conflict of interest related to project support, according to ICMJE ‘Ethical Considerations in the Conduct and Reporting of Research’ (http://www.icmje.org/ethical_4conflicts.html). We would like to emphasise, however, that Calanus AS was not involved in any of the processes throughout the experiments, either in the study design and collection, analysis and interpretation of data or in the writing of the report.