Introduction
The majority of pet dogs and cats in the developed world are fed processed commercial pet foods throughout their lives(Reference Laflamme, Abood and Fascetti1). These foods are formulated and manufactured to support the pets' nutritional needs, health and vitality. During the manufacturing of most commercial pet foods, thermal treatments are used to improve the safety and nutritive properties of the foods(Reference Hullar, Fekete and Szocs2). These thermal treatments, including pre-conditioning, extrusion cooking, retorting and pelleting, can improve the digestibility of protein through denaturation and starch by gelatinisation. Moreover, vegetable ingredients such as legumes and cereals may contain anti-nutritional components (for example, trypsin inhibitors, lectins) that are inactivated by thermal treatments(Reference van der Poel3). Safety and shelf-life are improved by thermal destruction of viable spores and any bacterial contamination.
Besides the above-mentioned beneficial effects, thermal treatments can also negatively influence protein quality due to crosslinking, racemisation, oxidation of sulfur-containing amino acids, and the involvement of amino acids in the Maillard reaction(Reference Meade, Reid and Gerrard4, Reference Hodge5). The latter reaction is an important chemical reaction for food manufacturers as it contributes to desired flavour, colour and antioxidative properties in many foods(Reference van Boekel, Fogliano and Pellegrini6–Reference Manzocco, Calligaris and Mastrocola8). However, the Maillard reaction also has unfavourable consequences such as the loss of bioavailable essential amino acids(Reference Friedman9, Reference Hurrell and Carpenter10). During the Maillard reaction, a reducing sugar binds to a free reactive amino group of an amino acid. In food proteins, the reactive ɛ-amino group of lysine is the most important source of reactive amino groups(Reference Hurrell and Carpenter10, Reference Moughan and Rutherfurd11). Previous research has indicated that up to 61·8 % of the lysine in pet foods contains a bound ɛ-amino group, probably due to its involvement in the Maillard reaction(Reference Williams, Hodgkinson and Rutherfurd12, Reference Rutherfurd, Rutherfurd-Markwick and Moughan13). This complex, also referred to as early Maillard reaction products (MRP), may be absorbed from the gastrointestinal tract but cannot be utilised by the animal(Reference Hurrell and Carpenter10, Reference Moughan14, Reference Finot15). As lysine is the first or second limiting essential amino acid in commercial foods for cats and dogs(16), a reduced utilisation results in a reduced nutritive value of the food. In addition to the loss of essential amino acids, advanced MRP may have an influence on health. Some of these MRP are also endogenously formed, i.e. formed in the body, and have been associated with age-related diseases in humans and dogs(Reference DeGroot, Verzijl and Wenting-van Wijk17).
As commercially prepared pet foods are often routinely fed throughout the entire life of domestic cats and dogs, it is important to understand the factors that influence the availability of essential amino acids such as lysine and the formation of potentially bioactive MRP. The purpose of this review is to present an overview of the effect of the Maillard reaction on the nutritive value of pet foods in relation to the requirements of the animal. In addition, the potential health implications of MRP for dogs and cats are discussed. Factors influencing the formation of MRP in pet foods, including recipe ingredients and processing techniques used during pet food manufacture, will be presented in the context to minimising the formation of early and advanced MRP in complex matrices such as pet foods.
The Maillard reaction
The Maillard reaction is a non-enzymic browning and flavouring reaction that can occur during the processing and storage of foods(Reference Maillard18). Often free amino acids or amino acids in peptides and proteins are involved in the Maillard reaction. Free amino acids contain an α-amino (–NH2) group as well as a functional side chain that varies between amino acids and can react during the Maillard reaction(Reference Hurrell and Carpenter10). In peptides and proteins, amino acids form (poly)peptide chains through a polymerisation reaction, whereby the α-amino group links to an α-carboxyl group and becomes unavailable for the Maillard reaction. As a result, in peptides and proteins only the side chains of amino acids are reactive in the Maillard reaction. In a free as well as a protein-bound form, the ɛ-amino side chain of lysine is the most susceptible group to the Maillard reaction(Reference van Boekel7, Reference Hurrell and Carpenter10, Reference Moughan and Rutherfurd11, Reference Kwak and Lim19). Side chains of other amino acids, for example the guanidine side group of arginine, and side chains of histidine and tryptophan, are also known to be involved in the Maillard reaction but are less well studied(Reference Moughan and Rutherfurd11, Reference Silvan, van de Lagemaat and Olano20). Next to free amino acids and proteins, amino lipids and nucleic acids can also be involved in the Maillard reaction.
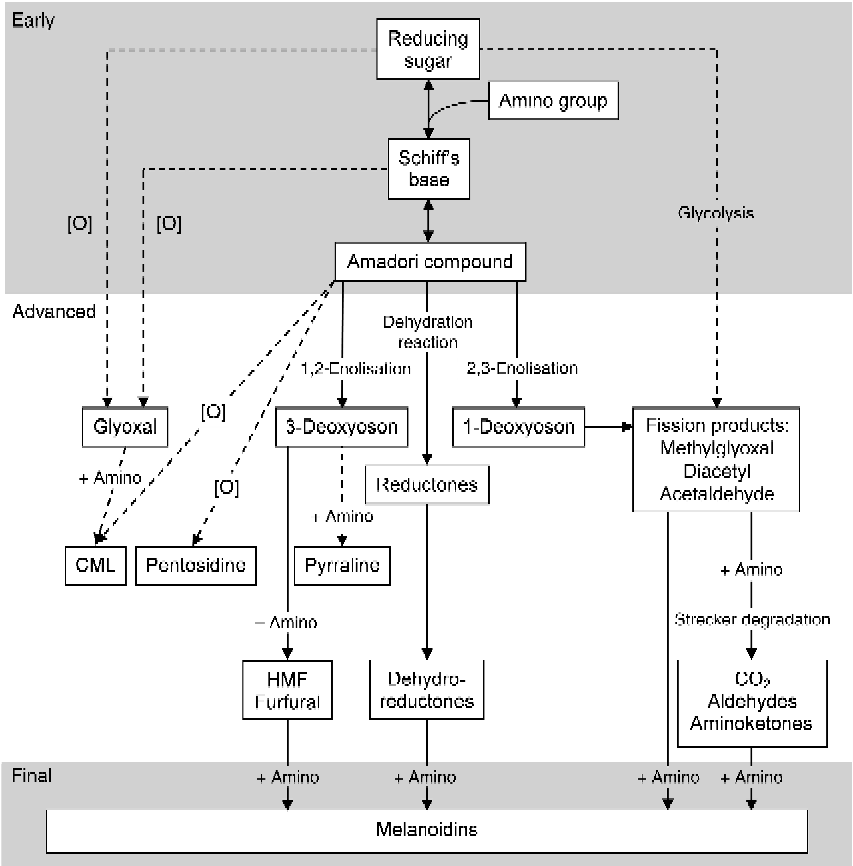
The Maillard reaction can be divided into early, advanced and final stages (Fig. 1). In the early stage, the carbonyl group of a reducing sugar reacts through a condensation reaction with the ɛ-amino group of lysine, resulting in the formation of a reversible Schiff's base. The Schiff's base can undergo an Amadori rearrangement resulting in the formation of the Amadori compound ɛ-N-deoxyketosyllysine(Reference Friedman21). It seems that the Amadori rearrangement can be reversible under certain conditions; however, the mechanism is not fully understood and it is unknown whether it is of quantitative relevance in the Maillard reaction(Reference Martins and Van Boekel22). In the advanced stage of the Maillard reaction, the Amadori compound can react further through several pathways, including rearrangement, condensation, oxidation and dehydration (and hydration) reactions, which lead to the formation of advanced MRP. Several α-oxoaldehydes including glyoxal, 1-,3-deoxyglucosones and fission products such as methylglyoxal are formed, either by non-oxidative rearrangements or by oxidation and glycolysation. These compounds are high in oxidative potential and, therefore, tend to be pro-oxidative(Reference Liu, Yang and Jin23–Reference Anese, Nicoli and Massini26). α-Oxoaldehydes and ɛ-N-deoxyketosyllysine react with proteins (or lipids) to generate oxidants such as N ɛ-(carboxymethyl)lysine (CML) or crosslink-forming endproducts such as pentosidine. Other advanced MRP derived from these precursors include pyrraline and hydroxymethylfurfural (HMF). These advanced MRP are the most common compounds and are used as markers to indicate the extent of the Maillard reaction in foods(Reference Erbersdobler and Somoza27). Amadori compounds that undergo a dehydration reaction result in reductones and dehydroreductones. In addition, the Strecker degradation converts fission products with an amino group into aldehydes, aminoketones and CO2. In the final stage, MRP further react with free amino groups to produce melanoidins which are responsible for the characteristic brown colour of heated foods(Reference Hurrell and Carpenter10, Reference Wang, Qian and Yao28). Increased antioxidant activity is observed during the formation of brown colouring(Reference Nicoli, Anese and Parpinel24, Reference Wang, Qian and Yao28), which can be mainly attributed to the reductones and dehydroreductones that can act as antioxidants in their reduced state(Reference Mogol, Yıldırım and Gökmen29).

Fig. 1 Scheme of the early, advanced and final Maillard reaction pathways and formation of Maillard reaction products and melanoidins (modified after Hodge(Reference Hodge5)). [O], oxidation; CML, N ɛ-(carboxymethyl)lysine; HMF, hydroxymethylfurfural.
Determining Maillard reaction products in processed foods and ingredients
Several methods have been developed to quantify MRP in processed foods and ingredients. Total lysine content of foods and ingredients is usually determined using traditional amino acid analysis. Proteins are heated in concentrated acid (for example, 6 m-HCl) at 110°C for 24 h, hydrolysing the peptide bonds and resulting in free amino acids that can be quantified using chromatographic separation. However, total lysine may not be suitable for quantifying nutritional available lysine in processed foods, feeds and ingredients as the early and some advanced MRP can revert back to lysine during the acid hydrolysis step(Reference Moughan and Rutherfurd11, Reference Hendriks, Moughan and Boer30–Reference Moughan32). Bioavailable lysine is defined as the reactive lysine that is digested, absorbed and potentially can be utilised for metabolism. Bioavailable lysine can be determined by either an animal growth assay where the ability of an animal to deposit protein or amino acids from a test diet is measured, or the true ileal amino acid digestibility assay(Reference Moughan14, Reference Rutherfurd and Moughan31). These methods are the most accurate but costly and time consuming. Alternative methods based on a reaction of chemical compounds with the free ɛ-amino group of lysine have been developed to measure reactive lysine in foods, feeds and ingredients. The most well-known method, and considered to be the reference method for determination of reactive lysine, was developed by Carpenter(Reference Carpenter33) and revised by Booth(Reference Booth34). Free amino groups of lysine in protein react with fluorodinitrobenzene (FDNB) to form the acid-resistant yellow compound dinitrophenol (DNP)-lysine. After hydrolysis of the protein, the α-DNP-amino acids are removed by ether extraction. The remaining ether-insoluble ɛ-DNP-lysine is detected spectrophotometrically. Variations on the FDNB-reactive lysine method have been developed. In the ‘Silcock’ method, reactive lysine in samples is determined as the difference between total lysine before the reaction with FDNB and the residual lysine present after the reaction(Reference Roach, Sanderson and Williams35). Determination of ɛ-DNP-lysine by HPLC results in higher values compared with the original method of Carpenter(Reference Carpenter33), possibly due to compounds formed during hydrolysis that interfere with the spectrophotometric determination of ɛ-DNP-lysine(Reference Peterson and Warthesen36). Trinitrobenzenesulfonic acid and sodium borohydride have also been used to form acid-stable complexes with the free ɛ-amino group of lysine; the formed complexes are measured after hydrolysis using a spectrophotometer or amino acid analyser(Reference Kakade and Liener37, Reference Couch and Thomas38). The ‘dye binding lysine’ method of Hurrell et al. (Reference Hurrell, Lerman and Carpenter39) is based on the difference between measurement of unmodified amino acids with Acid Orange 12 and after the ɛ-amino group of lysine has been blocked through a reaction with propionic anhydride; reactive lysine content is then calculated from the difference(Reference Hurrell, Lerman and Carpenter39). Another binding agent is ortho-phthaldialdehyde (OPA), which forms a fluorescent reaction product with the free amino groups in proteins(Reference Goodno, Swaisgood and Catignani40). The fluorescent intensity measured is corrected for the contribution of N-terminal amino groups to determine the reactive lysine content. In the above-mentioned methods, the chemical compounds bind to both the α- and ɛ-amino group of lysine and are, therefore, not suitable for accurate determination when free or synthetic lysine is present(Reference Rutherfurd and Moughan31). The guanidination method uses O-methylisourea (OMIU) as a reagent which is able to bind to only free ɛ-amino groups, converting lysine into homoarginine(Reference Rutherfurd, Moughan and van Osch41). Homoarginine is acid stable(Reference Moughan and Rutherfurd42) and, after acid hydrolysis via traditional amino acid analysis, allows for the accurate determination of the reactive lysine contents of foods, feeds and ingredients. A good correlation has been demonstrated between the FNDB- and OMIU-reactive lysine methods for a range of animal feedstuffs (r 0·996) and breakfast cereals (r 0·985), indicating that results obtained by the two methods are comparable(Reference Rutherfurd and Moughan31). According to Rutherfurd & Moughan(Reference Rutherfurd and Moughan31), the guanidination method is the most preferred method to analyse reactive lysine in processed foods and ingredients.
In terms of terminology, the present review will refer to total lysine as lysine molecules with a free ɛ-amino group and lysine that reverts back to lysine after standard acid hydrolysis. Reactive lysine is undamaged lysine that has a reactive ɛ-amino side chain. The difference between total and reactive lysine, therefore, is a measure of the lysine that reverts back to lysine after acid hydrolysis and includes lysine involved in the early Maillard reaction. Most lysine that has reacted to yield advanced Maillard products does not revert back to lysine during acid hydrolysis and as such the difference between total and reactive lysine is, therefore, not equal to the lysine that has undergone the Maillard reaction. It is, however, often used as an indication of heat damage of processed foods, feeds and ingredients(Reference Meade, Reid and Gerrard4, Reference Moughan and Rutherfurd11).
To be able to measure MRP in foods, feeds and ingredients, other chemical markers can be analysed. Furosine (ɛ-N-(furoyl-methyl)-l-lysine) is an amino acid formed during acid hydrolysis of the Amadori compound fructoselysine and is produced by reaction of the ɛ-amino groups of lysine with glucose. As such, furosine is a specific chemical marker of the Amadori compound generated during the early Maillard reaction(Reference Erbersdobler and Somoza27, Reference Finot, Bricout and Viani43). In the advanced stage, the extent of the Maillard reaction can be measured in several ways. The colour formation during this stage can be measured by absorbance at 420 nm. In addition, dehydration and fission reactions form fluorescent compounds that can be measured by fluorescence spectrophotometry at 347 nm excitation and 415 nm emission(Reference Delgado-Andrade, Rufian-Henares and Morales44). Specific markers of the advanced stage such as HMF and CML are often analysed by ultra-performance liquid chromatography or HPLC, sometimes combined with MS(Reference Assar, Moloney and Lima45).
From a practical point of view, procedures that can rapidly provide an indication of the occurrence of the Maillard reaction due to heat processing of foods, feeds or ingredients are necessary. Besides the furosine, colour and fluorescence methods, the total or reactive lysine:crude protein ratio seems to be a relatively rapid method to estimate heat damage as the concentration of lysine, but not the concentration of crude protein, reduces if samples are extensively heat processed(Reference González-Vega, Kim and Htoo46, Reference Fontaine, Zimmer and Moughan47). This method is, however, mainly tested in distillers dried grains with solubles (DDGS) and soyabean meal. An increase of dark colour in DDGS was related to an increase in acid-detergent fibre (r 0·62; P< 0·10) and acid-detergent insoluble N (r 0·79; P< 0·01) during heat processing, indicating that these components can be indicators of the Maillard reaction especially in fibrous feeds or ingredients(Reference Cromwell, Herkelman and Stahly48, Reference Van Soest and Mason49). Near-IR reflectance spectroscopy might be a future method to determine reactive lysine in foods, feeds and ingredients; however, data are limited.
The Maillard reaction in model systems
The Maillard reaction has been extensively studied using pure compounds as well as heat-treated food systems(Reference Hurrell and Carpenter10, Reference Moughan and Rutherfurd11, Reference Mauron50). Studies of pure compounds in model systems indicate that the type of reactions and the extent to which they occur depend on several reaction conditions. In terms of amino acid type, lysine had the highest reactivity among twelve investigated amino acids (aspartic acid, glutamic acid, alanine, leucine, isoleucine, valine, proline, serine, cysteine, phenylalanine, arginine and lysine) when heated with reducing sugars at 100°C for 3 h as measured by the formation of MRP with absorbance at 420 nm(Reference Kwak and Lim19). As the heating time was increased to 12 h, the colour intensity of the MRP from lysine became two to three times higher than that of the other amino acids(Reference Kwak and Lim19). In terms of reducing sugar type, glucose has been reported to be the most reactive reducing sugar when heated at 60°C in the presence of casein; OPA-reactive lysine content decreased by about 60 % within 10 h, followed by maltose (15 h), lactose (20 h) and fructose (35 h)(Reference Naranjo, Malec and Vigo51). Brands et al. (Reference Brands, Wedzicha and van Boekel52), however, observed contradictory results when casein was heated at 120°C for 90 min in the presence of glucose or fructose, with fructose being more reactive than glucose. After 60 min of heating, both reducing sugars induced an OPA-reactive lysine reduction of about 60 %. In a model system consisting of soyabean protein concentrate, glucose and microcrystalline cellulose, heated at 95°C for 75 min FDNB-reactive lysine content was reduced by 31·9 %(Reference Wolf, Thompson and Reineccius53). OPA-reactive lysine reduction appeared to be faster with increasing temperatures from 37 to 60°C(Reference Naranjo, Malec and Vigo51). Increasing the pH from 4 to 12 at a temperature of 100°C decreased total lysine content by up to 50 % after 2 h in the presence of fructose(Reference Ajandouz, Tchiakpe and Ore54). In addition, lowering the water content (w/w) of a sugar–amino acid model system from 100 to 20 % increased colour formation(Reference Eichner and Karel55). Based on these data, it appears that in model systems the type of amino acid and the type of reducing sugar influence the extent of the Maillard reaction. In addition, increasing heating temperature and time, pH level and decreasing water content increase the reaction of lysine.
Effects of the Maillard reaction on the nutritive value of pet foods
As described above, the early MRP of lysine may be partly absorbed but has no nutritional value(Reference Moughan14, Reference Moughan32, Reference Faist and Erbersdobler56, Reference van Barneveld57). This impaired utilisation of lysine that has undergone the Maillard reaction was recently confirmed in a kitten growth study(Reference Larsen, Calvert and Rogers58, Reference Larsen, Fascetti and Calvert59). Kittens fed an experimental diet including a heated casein–dextrose mixture containing a difference between total and reactive lysine of 32·9 % resulted in a lower growth rate (mean daily gain of 2·7 g) compared with kittens fed a control diet including unheated casein containing a difference between total and reactive lysine of 0·4 % (mean daily gain of 14·9 g). Adding 4·0, 5·5 and 7·0 g of synthetic lysine to the heated casein diet increased mean daily gain of the kittens to 5·4, 11·2 and 20·1 g, respectively. This study clearly indicates that the bioavailability of lysine can be significantly impaired by heating. Quantification of reactive lysine is therefore important for the evaluation of the nutritive value of pet foods.
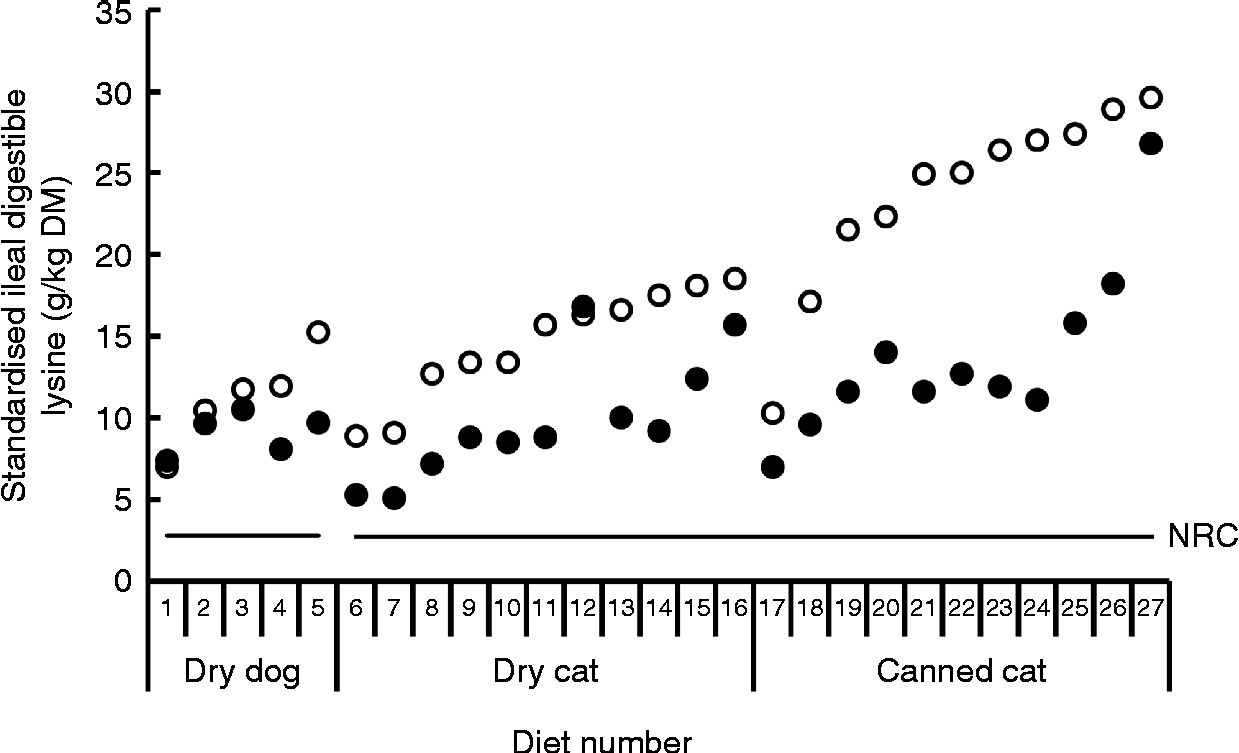
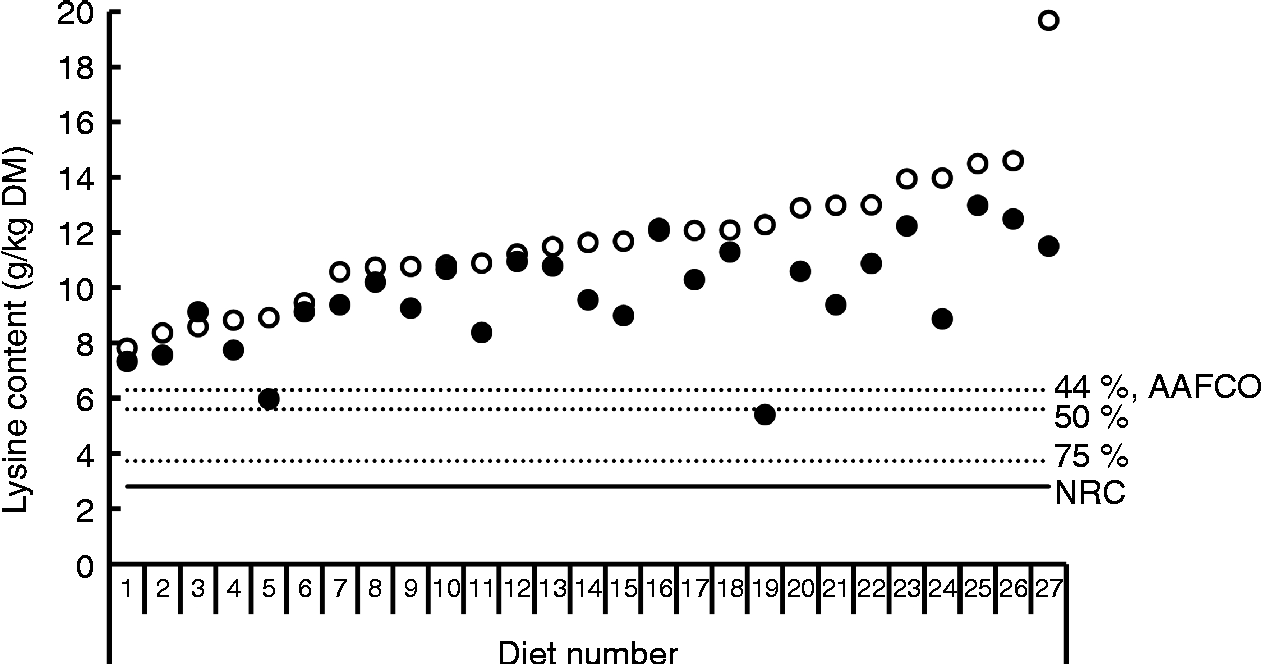
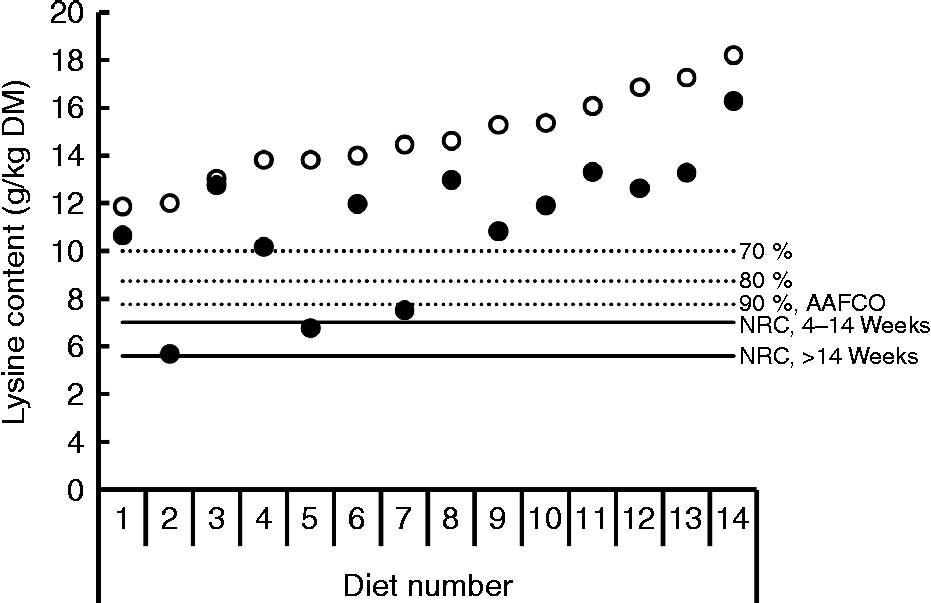
Bioavailability of nutrients, including lysine, is taken into account in the nutritional recommendations for the formulation of complete pet foods. The recommended allowances of lysine for dogs at maintenance based on scientific principles presented by the National Research Council (NRC)(16) and the European Pet Food Industry Federation (FEDIAF)(60) include a bioavailability factor of 0·80 and 0·67, respectively. The Association of American Feed Control Officials (AAFCO)(61) is a regulatory organisation that sets standards for the quality and safety of animal feed and pet food in the USA. AAFCO provides higher recommended lysine allowances compared with NRC, i.e. 6·3 v. 3·5 g/kg DM, including a bioavailability factor of 0·44 when calculated using the NRC minimal requirement of 2·8 g/kg DM(16). The fraction of the lysine in foods that is actually bioavailable depends on the extent of the fraction of lysine that has undergone the Maillard reaction and the ileal digestibility of the reactive lysine. The difference between total and reactive lysine of commercially produced canned and dry dog and cat foods can be considerable, with values reported of up to 61·8 %(Reference Williams, Hodgkinson and Rutherfurd12, Reference Rutherfurd, Rutherfurd-Markwick and Moughan13, Reference Tran, van Lin and Hendriks62). Furthermore, apparent ileal crude protein digestibility has been shown to be highly variable among 141 dog foods, with values ranging from 51·1 % up to 90·5 % and a mean digestibility of 73·5 %(Reference Hendriks, van Baal and Bosch63). As variability in amino acid digestibility can be expected to be similar to crude protein digestibility, these results indicate that lysine digestibility is likely to be highly variable as well. Data on ileal reactive and total lysine digestibility in dogs are scarce. However, recently Hendriks et al. (Reference Hendriks, Thomas and Bosch64) reported standardised ileal OMIU-reactive lysine digestibility values in dogs fed five commercial dry dog foods containing varying protein contents (24·4 to 32·7 % DM) of 79·5 to 93·7 % with a mean of 88·2 %. Corresponding standardised ileal total lysine digestibility values of these foods were 64·2 to 87·2 %, with a mean of 80·0 %. In another study involving dogs(Reference Hendriks and Sritharan65), the apparent ileal total lysine digestibility of a commercial dry dog food was reported to be 83·6 %. The bioavailable reactive lysine, i.e. standardised ileal digestible OMIU-reactive lysine, contents of the five commercial foods(Reference Hendriks, Thomas and Bosch64) met the minimal lysine requirement of 2·8 g/kg DM for dogs at maintenance(16) when a dietary energy density of 16·7 MJ (4000 kcal) metabolisable energy (ME)/kg is assumed (Fig. 2). The OMIU-reactive and total lysine contents of twenty-seven commercial maintenance foods for dogs(Reference Williams, Hodgkinson and Rutherfurd12, Reference Tran, van Lin and Hendriks62) are reported in Fig. 3. These foods varied considerably in terms of differences between total and reactive lysine, with values up to 56·0 % and an overall mean of 15·4 %. Assuming a dietary energy density of 16·7 MJ (4000 kcal) ME/kg, these foods were well above the minimal lysine requirement. The two foods with the lowest OMIU-reactive lysine contents would have been deficient in lysine if their ileal reactive lysine digestibilities were 51·7 and 46·9 %, which may be considered unlikely. The OMIU-reactive and total lysine contents of fourteen commercial growth foods for dogs(Reference Williams, Hodgkinson and Rutherfurd12) are reported in Fig. 4. For growing dogs dietary total lysine contents were all above the recommended allowance as set by the NRC(16) (Fig. 4). The OMIU-reactive lysine contents of two foods, however, were below the minimal total lysine requirements of 7 g/kg DM for growing dogs between 4 and 14 weeks of age(16), assuming a dietary energy density of 16·7 MJ (4000 kcal) ME/kg. If these foods for growing dogs were used as weaning diets, the minimal requirement for growing dogs between 4 and 14 weeks would not be met. No ileal reactive lysine digestibility data are available for growing dogs. Assuming that the ileal reactive lysine digestibility was within the range presented by Hendriks et al. (Reference Hendriks, Thomas and Bosch64) (79·5 to 93·7 %), three commercial foods would have had reactive lysine contents below the minimal total lysine requirements for 4- to 14-week-old growing dogs. It should be noted that growing dogs (and cats) have lower gastric pepsin secretion(Reference Fahey, Barry and Swanson66), so digestibility values would probably be lower than those observed in adult dogs. As long as the reactive lysine digestibility for the other eleven foods were above 70·0 %, these foods would have met the minimum lysine requirements for 4- to 14-week-old growing dogs. Interpretation of these results should take into account that most studies use commercially available single batch pet foods. Batch variation in ingredients as well as processing conditions (see further in the present review) can result in variation in the difference between total and reactive lysine content between batches of pet foods.

Fig. 2 Standardised ileal digestible total lysine (○) and standardised ileal digestible O-methylisourea (OMIU)-reactive lysine (●) contents for five commercial dry maintenance foods for dogs using ileally cannulated dogs(Reference Hendriks, Thomas and Bosch64) and twenty-two commercial maintenance (SM Rutherfurd, personal communication) foods for cats using the rat as the model animal(Reference Rutherfurd, Rutherfurd-Markwick and Moughan13, Reference Rutherfurd and Moughan31). Horizontal solid lines indicate the minimal lysine requirement for maintenance for dogs and cats presented by the National Research Council (NRC)(16), being, respectively, 2·8 and 2·7 g/kg DM, assuming a dietary energy density of 16·7 MJ (4000 kcal) metabolisable energy/kg.

Fig. 3 Total lysine (○) and O-methylisourea (OMIU)-reactive lysine (●) contents for twenty-seven commercial dry maintenance foods for dogs(Reference Williams, Hodgkinson and Rutherfurd12, Reference Tran, van Lin and Hendriks62) (reanalysis of original data of Williams et al. (Reference Williams, Hodgkinson and Rutherfurd12)). The horizontal solid line indicates the minimal lysine requirement for maintenance for dogs presented by the National Research Council (NRC)(16), being 2·8 g/kg DM, assuming a dietary energy density of 16·7 MJ (4000 kcal) metabolisable energy/kg. Dotted lines indicate bioavailability thresholds of 75 and 50 % for meeting minimal lysine requirements. The recommended lysine allowance for dog maintenance foods of the Association of American Feed Control Officials (AAFCO)(61) equals a dietary lysine bioavailability of 44 %.

Fig. 4 Total lysine (○) and O-methylisourea (OMIU)-reactive lysine (●) contents for fourteen commercial growth foods for dogs (reanalysis of original data of Williams et al. (Reference Williams, Hodgkinson and Rutherfurd12)). Horizontal solid lines indicate the minimal lysine requirement for growing dogs between 4 and 14 weeks old and older than 14 weeks presented by the National Research Council (NRC)(16), being, respectively, 7·0 and 5·6 g/kg DM, assuming a dietary energy density of 16·7 MJ (4000 kcal) metabolisable energy/kg. Dotted lines indicate bioavailability thresholds of 90, 80 and 70 % for meeting minimal lysine requirements. The recommended lysine allowance for dog maintenance foods of the Association of American Feed Control Officials (AAFCO)(61) equals a dietary lysine bioavailability of 90 %.
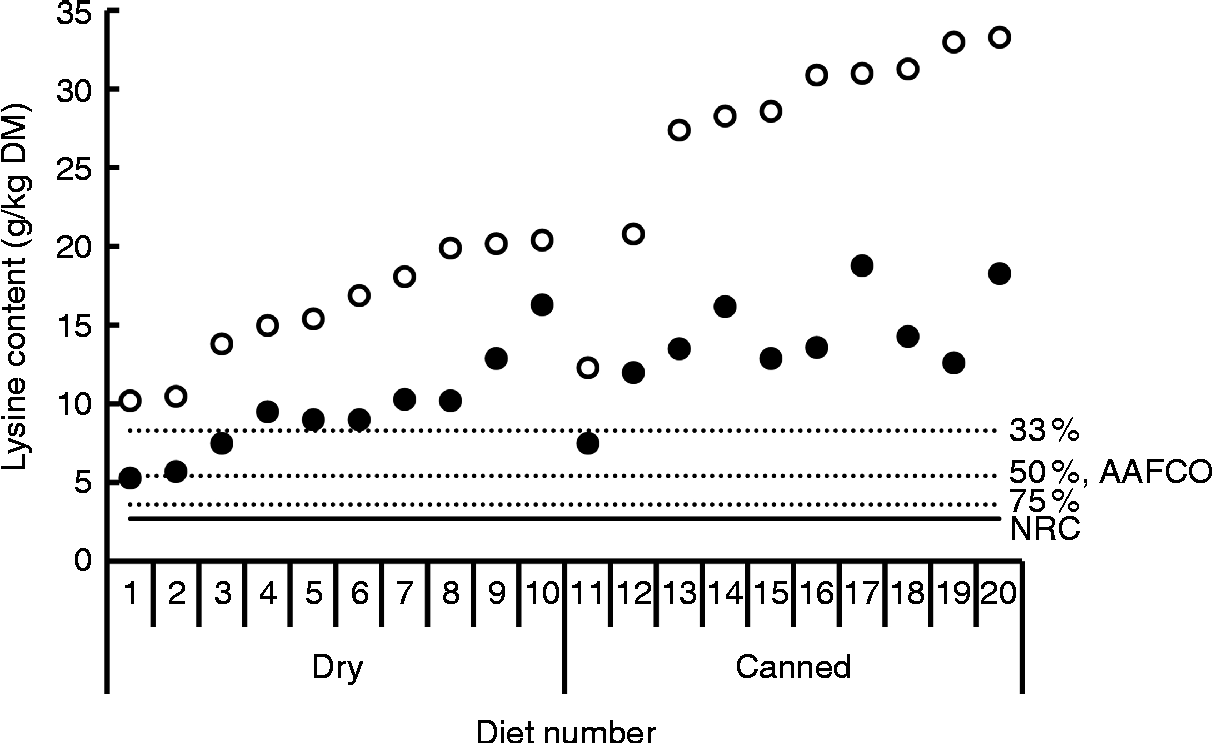
The difference between total and reactive lysine appears to be greater in commercial cat foods than in dog foods. Rutherfurd et al. (Reference Rutherfurd, Rutherfurd-Markwick and Moughan13) reported average differences between total and reactive lysine values in canned cat foods (n 10) of 48·6 % (range 39·0–61·8 %) and in dry cat foods (n 10) of 41·2 % of total lysine (range 20·1–48·7 %) values (Fig. 5). As long as the reactive lysine digestibilities are above 50·0 %, these foods will meet the minimum lysine requirements for adult cats(16). Using the rat as model animal, standardised ileal OMIU-reactive lysine digestibility of the canned cat foods ranged from 79·9 to 97·1 % with a mean of 88·1 % while for the dry cat foods values ranging from 89·9 to 97·7 % with a mean of 94·8 % were reported(Reference Rutherfurd, Rutherfurd-Markwick and Moughan13). These values are higher than a previously reported value of 71·5 % for the apparent ileal total lysine digestibility of a canned cat food using the rat as the model animal(Reference Hendriks and Emmens67). The latter is probably due to the difference in method (standardised v. apparent) and the presence of early MRP (OMIU-reactive only v. OMIU-reactive plus Amadori compounds). Assuming a dietary energy density of 16·7 MJ (4000 kcal) ME/kg(16), the approximated ileal digestible OMIU-reactive lysine content of these cat foods was higher than the minimal lysine requirements for maintenance in cats (Fig. 2).

Fig. 5 Total (○) and O-methylisourea (OMIU)-reactive lysine (●) contents of twenty commercial maintenance (SM Rutherfurd, personal communication) foods for cats(Reference Rutherfurd, Rutherfurd-Markwick and Moughan13). The horizontal solid line indicates the minimal lysine requirement for maintenance of cats presented by the National Research Council (NRC)(16), being 2·7 g/kg DM, assuming a dietary energy density of 16·7 MJ (4000 kcal) metabolisable energy/kg. Dotted lines indicate bioavailability thresholds of 75 and 50 % for meeting minimal lysine requirements. The recommended lysine allowance for dog maintenance foods of the Association of American Feed Control Officials (AAFCO)(61) equals a dietary lysine bioavailability of 33 %.
Overall, these data indicate that the difference between total and reactive lysine in commercial dog and cat foods can be considerable, in particular in canned foods. Factors such as ingredients used and processing conditions applied which contribute to the difference between total and reactive lysine in pet foods are described below. The standardised ileal digestibility of reactive lysine in analysed pet foods appears to be 80·0 % or higher. However, the number of pet foods evaluated is limited. In particular, the lysine supply of certain commercial foods consumed by growing dogs, and possibly also growing cats, can be compromised due to differences between total and reactive lysine. Further prospective studies are required to identify the effects of early MRP during growth.
Effects of glycated Maillard reaction products on health
The Maillard reaction does not only occur during heat treatment of pure compounds or food systems but is also a naturally occurring process in body tissues. Biological interest in the endogenously occurring Maillard reaction was initiated during the late 1960s with the identification of non-enzymically glycated Hb in the blood of diabetic patients(Reference Rahbar, Blumenfeld and Ranney68, Reference Henle69). During normal metabolism at homeostatic concentrations of glucose, endogenous proteins can be glycated resulting in advanced glycation endproducts (AGE)(Reference Thorpe and Baynes70, Reference Somoza71). A large number of AGE have been identified in vivo including CML, HMF, pentosidine and pyrraline. These AGE are the most widely studied and used as biomarkers for in vivo formation of AGE(Reference Thorpe and Baynes70, Reference Luevano-Contreras and Chapman-Novakofski72).
AGE have a variety of predominantly adverse biological effects. AGE can be pro-oxidants that can modify physico-chemical properties of proteins by covalently crosslinking to proteins and thereby modifying structural and functional properties of the proteins. As the crosslinks of AGE and proteins are resistant to degradation, turnover rate is delayed and tissue repair hindered(Reference Schalkwijk, Stehouwer and van Hinsbergh73–Reference Vlassara and Striker75). This results in accumulation of AGE in tissues with amounts depending on the turnover rate of the protein. As a result, proteins with a slow turnover such as collagens in connective tissue, eye lenses and nerve myelin are sensitive to AGE accumulation(Reference Baynes76, Reference Turk77). Due to the modified physico-chemical properties of tissue proteins, AGE can become pathogenic. This could, for example, lead to stiffening of collagen fibres in the arterial wall leading to vascular complications(Reference Chappey, Dosquet and Wautier78). Next to physico-chemical protein modifications, AGE can elicit cell-mediated responses through interaction with several cellular AGE receptors and induce cellular signalling, activation of transcription factors and subsequent gene expression. These receptors are expressed on a wide range of cells including smooth muscle cells, monocytes, macrophages, endothelial cells, podocytes, astrocytes and microglia(Reference Singh, Barden and Mori79). The best-characterised receptor is RAGE (‘receptor for AGE’), which belongs to the Ig superfamily and is capable of interacting with a broad spectrum of ligands, including AGE. Binding of an AGE to the receptor leads to activation of transcription factor NF-κB which in turn can lead to oxidative stress, vasoconstriction and inflammatory responses(Reference Ramasamy, Vannucci and Yan80, Reference Nass, Bartling and Navarrete Santos81). Under certain pathological conditions, such as hyperglycaemia or oxidative stress in diabetes mellitus, AGE formation can be accelerated. AGE have been, therefore, proposed to contribute to the development of long-term complications of diabetes(Reference Singh, Barden and Mori79). In addition, AGE may contribute to the pathogenesis of age-related diseases such as atherosclerosis, nephropathy, retinopathy, osteoarthritis and neurodegenerative diseases such as Alzheimer's disease in humans(Reference DeGroot, Verzijl and Wenting-van Wijk17, Reference Singh, Barden and Mori79, Reference Basta, Schmidt and De Caterina82). Age-related diseases such as diabetes and renal, cardiovascular and neurodegenerative diseases are also seen in dogs, showing many similarities to these diseases in humans(Reference Cummings, Head and Ruehl83–Reference Borras, Ferrer and Pumarola85). RAGE are reported in dogs(Reference Yamka, Kitts and True86), and several studies have been conducted indicating that AGE also accumulate by binding to tissue proteins of ageing and diseased dogs. Comazzi et al. (Reference Comazzi, Bertazzolo and Bonfanti87) reported higher AGE in plasma from dogs suffering from canine diabetes mellitus compared with control animals. In addition, elevated levels of AGE in tissue proteins in dogs were seen during cataract in the canine eye lens(Reference Bras, Colitz and Kusewitt88), osteoarthritis(Reference DeGroot, Verzijl and Wenting-van Wijk17), neurodegenerative diseases such as canine cognitive dysfunction syndrome(Reference Weber, Schmahl and Münch89), vascular dysfunction(Reference Shapiro, Owan and Mohammed90), atherosclerosis(Reference Chiers, Vandenberge and Ducatelle91), and in skin collagen(Reference Sell, Lane and Johnson92) with increasing age.
As indicated above, AGE formation occurs naturally in the body and defence mechanisms have evolved to protect against the adverse effects of (endogenously) formed AGE. Effects of AGE are avoided or regulated by renal AGE elimination and detoxification, antioxidant systems and suppression of signalling via the AGE receptor AGER1(Reference Vlassara and Striker75). As in humans, the dietary intake of AGE, such as CML, HMF, pentosidine and pyrraline, formed during the processing of foods potentially provides an additional AGE load to dogs and cats. These dietary components appear to be, at least partially, digestible and transported through the general circulation. A significant correlation (r 0·8) between ingested MRP and serum concentrations were observed when comparing the ingestion of a high-MRP diet v. a low-MRP diet in human diabetes mellitus patients with or without kidney disease(Reference Koschinsky, He and Mitsuhashi93). The latter authors reported that about 10 % of the dietary AGE were observed in serum. This supports the idea that dietary MRP contribute to the body's AGE pool(Reference Uribarri, Cai and Sandu94). As in tissues where the crosslinks of AGE and proteins are resistant to degradation, the majority (about 70 %) of dietary AGE is not available either because the crosslinks are resistant to enzymic hydrolysis, or trypsin digestion is impaired due to the absence of a positive charge on lysine in the intestinal tract(Reference Koschinsky, He and Mitsuhashi93). To the authors' knowledge, no data are available in the scientific literature on the absorption and excretion of dietary MRP in dogs and cats.
There are large differences in absorption, metabolism and excretion between the different MRP. Advanced MRP such as CML, pyrraline and pentosidine seem to be absorbed by the gut. Ingested dietary CML in rats appeared to be approximately 26·0 to 29·0 % excreted in the urine, and 15·0 to 22·0 % excreted in faeces(Reference Ames95). Approximately 1·7 % of dietary CML accumulated in the circulation, kidney and liver and approximately 50·0 % of the ingested CML was not recovered. This was confirmed in a later study where 31·2 % of ingested dietary CML was excreted in the faeces, 14·4 % in the urine, and 54·4 % left unrecovered in human subjects(Reference Delgado-Andrade, Tessier and Niquet-Leridon96). Whether the unrecovered CML is deposited in organs, degraded by colonic microbiota or metabolised is unknown. Approximately 80 % of dietary pyrraline is absorbed and excreted via the kidneys in humans within 48 h(Reference Foerster and Henle97). Urinary pyrraline is almost exclusively of dietary origin, and pyrraline is most probably not metabolised post-absorption. Approximately 2·0 % of dietary pentosidine is recovered in urine in the peptide-bound form; however, approximately 60·0 % is recovered in the free form(Reference Forster, Kuhne and Henle98). The remainder may have been metabolised into unknown compounds and also excreted in the urine. Oral administration of HMF in rats resulted in rapid absorption. Excretion was primarily via urine, with between 66·3 and 80·0 % of the radioactivity excreted in urine in the first 24 h(Reference Godfrey, Chen and Griffin99). In faeces, 8·5–12·2 % was excreted within 48 h. The highest concentration of HMF in organs was observed in the liver and kidneys, the major organs for metabolism and excretion(Reference Godfrey, Chen and Griffin99). There was no evidence of prolonged accumulation in any other tissues. The MRP that are not absorbed can be metabolised by intestinal microbes(Reference Erbersdobler, Gunsser and Weber100, Reference Erbersdobler and Faist101), giving MRP including Amadori products and melanoidins biotic properties(Reference Tuohy, Hinton and Davies102–Reference Morales, Somoza and Fogliano104). The majority of the ingested MRP are not bioavailable; most of the absorbed MRP are excreted via the urine, however, some of the dietary MRP may accumulate in body tissues. In addition, it is seen that dietary MRP increase markers associated with an increased risk of type 2 diabetes and CVD in healthy individuals, and dietary MRP promote inflammatory mediators in diabetics, which may lead to tissue injury(Reference Vlassara and Striker75, Reference Birlouez-Aragon, Saavedra and Tessier105, Reference Vlassara, Cai and Crandall106); restriction of dietary MRP suppressed these effects. In contrast to human foods, there is at present only one study reporting concentrations of early MRP, and no data on the concentrations of advanced MRP in pet foods have been reported. The difference between total and reactive lysine reported in pet foods, and a furosine level of 0·91 mg/g reported in a dry dog food(Reference Chiang107) indicate that at least the early phase of the Maillard reaction has occurred. In addition, there is no information available on the absorption of MRP in the gastrointestinal tract of dogs and cats and the contribution of dietary MRP to the body's AGE pool. At this stage, it can only be hypothesised that the daily intake of thermally processed foods could provide an additional peak load of AGE that may exceed the natural capacity to protect against AGE. In addition, it is unknown whether endogenous AGE formation is stimulated due to a postprandial increase in blood glucose levels. Dry foods for cats and dogs can contain up to 60 % carbohydrates (as is), compared with a voluntary selection of a diet with a protein–fat–carbohydrate energy balance of 52:36:12 for cats(Reference Hewson-Hughes, Hewson-Hughes and Miller108) and of 30:63:7 for dogs(Reference Hewson-Hughes, Hewson-Hughes and Colyer109). A test diet with a carbohydrate content at 25 % ME resulted in a lower peak and postprandial glucose concentration compared with diets with a carbohydrate content at 45 and 55 % ME, although all measured glucose levels were within normal reference ranges(Reference Elliott, Rand and Fleeman110). Whether dogs and cats have the capacity to protect against increased blood glucose levels when fed commercial diets compared with their natural diet remains to be determined. It was recently demonstrated that the proportion of dry food intake may not be a risk factor for the development of type 2 diabetes mellitus in cats(Reference Slingerland, Fazilova and Plantinga111); however, the study did not take into account the composition of the food or specific absorption of dietary AGE. Whether increased AGE exposure contributes to the pathogenesis of the aforementioned diseases in dogs(Reference DeGroot, Verzijl and Wenting-van Wijk17, Reference Comazzi, Bertazzolo and Bonfanti87–Reference Chiers, Vandenberge and Ducatelle91) and possibly cats warrants further study. Given the key role of the kidney in the elimination and detoxification of AGE, cats and dogs with conditions such as chronic kidney disease will probably have an impaired capacity to eliminate AGE, which causes a build-up of AGE, formation of new AGE in the body and contributes to the pathogenesis of various health conditions. Whether or not dietary AGE intake also plays a pivotal role in the development of diabetes mellitus in dogs and cats, as suggested by Vlassara & Striker(Reference Vlassara and Striker75) in humans, is unknown, as is the potential of AGE restriction as a cost-effective strategy in the prevention and treatment of diabetes mellitus in dogs and cats.
Early Maillard reaction products in ingredients and during processing of pet foods
The amount of early (and advanced) MRP in pet foods may originate from three sources: first, the pet food ingredients may already contain early MRP due to processing; second, the processing conditions applied to produce the actual pet food; and third, lysine may react during coating and storage of the pet food.
Early Maillard reaction products in pet food ingredients
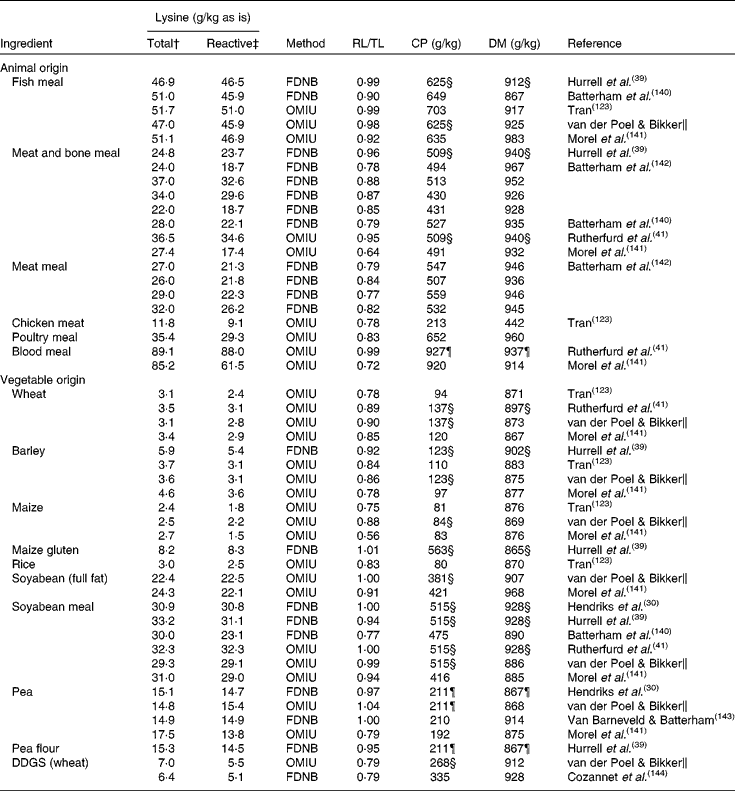
Most pet food manufacturers use co-products from other industries that may have been processed to varying degrees as primary ingredients in pet foods(Reference Williams, Hodgkinson and Rutherfurd12). Proteins in pet foods originate from both animal and vegetable ingredients(Reference Brown112). Animal protein sources in pet food include meat and bone meals of poultry, beef, pig, lamb and/or fish and other animal co-products, many of which are manufactured by a rendering-process(Reference Thompson113). Rendering separates fat, removes water and eradicates bacteria at temperatures as high as 130°C for several hours. These high temperatures and the duration of processing can influence protein quality. Using a chicken growth assay, total lysine availability in raw animal meals ranged from 86·9 to 107·7 % whereas in the rendered animal meals lysine availability ranged from 70·1 to 99·9 %(Reference Cramer, Greenwood and Moritz114). Differences between total and reactive lysine content in several ingredients of animal origin, mostly rendered meat meals, range from 1·0 % to up to 36·0 % (Table 1). The difference between total and reactive lysine content in fish meals is, on average, less (4·0 %) compared with meat and bone meal (16·0 %), poultry meal (17·0 %) and meat meal (20·0 %), although variation is also high within these ingredients of animal origin.
Table 1 Total and reactive lysine contents of common pet food ingredients*

RL, reactive lysine; TL, total lysine; CP, crude protein; FDNB, fluorodinitrobenzene; OMIU, O-methylisourea; DDGS, distillers dried grains with solubles.
* Pet food ingredients used in dog and cat foods according to the National Research Council(16).
† Total lysine was determined by conventional amino acid analysis.
‡ Reactive lysine was determined according to the given method.
§ Missing values for DM and CP were completed with the help of data from the National Research Council(16). Values were necessary to calculate results in g/kg from the original publications.
∥ AFB van der Poel and P Bikker, unpublished results.
¶ Missing values for DM and CP were completed with the help of data from the CVB(145).
Proteins of vegetable origin in pet foods originate from cereal grains and soyabean meal. These proteins are often considered to be of lower nutritional quality compared with animal proteins, because of a lower content of some essential amino acids and the presence of anti-nutritional factors(Reference Young and Pellett115). In particular, cereal proteins are relatively low in lysine (Table 1). The difference between total and reactive lysine content in several ingredients of vegetable origin ranges from 0 to 44·0 % (Table 1). The difference between total and reactive lysine content in peas and soyabean meal is, on average, less (0 and 6·0 %, respectively) compared with wheat (15·0 %), barley (15·0 %), maize (27·0 %) and rice (17·0 %). In cereals, differences between total and reactive lysine content are higher compared with non-cereal ingredients of vegetable origin, with an average difference of 17·0 v. 7·0 %, respectively. Vegetable ingredients are often dried and ground before being included in the pet food recipe. Both drying and grinding involve heat during the process.
The data in Table 1 indicate that part of the lysine in animal and vegetable protein sources has already gone through the early Maillard reaction before inclusion in pet food recipes. Given the use of meat meals as main protein sources in pet foods, it would, therefore, be of interest to evaluate where and how early MRP are formed in these ingredients, which may ultimately contribute to the reduction of the difference between total and reactive lysine content in pet foods.
Effect of processing of pet foods
Pet foods are produced utilising various process technologies. Dry pet foods are the most popular form of dog and cat food(Reference Laflamme, Abood and Fascetti1) and most often produced by extrusion cooking. Next to extrusion cooking, dry foods can also be manufactured by pelleting the ingredient mixture. Moist foods are manufactured using heat sterilisation using retorting processing. Every processing technology has its own characteristic process conditions, thereby having a greater or lesser impact on the rate and extent of the Maillard reaction. The effect of processing on the Maillard reaction is expressed in terms of a change in total and reactive lysine content. As the Maillard reaction can decrease total lysine content due to its conversion into advanced MRP, the calculated difference between total and reactive lysine content can give an underestimation of the effect of processing on the Maillard reaction. In addition, small changes in total and reactive lysine contents should be interpreted with care, as the CV in amino acid analysis can be as high as 3 % for total lysine(116). For reactive lysine, extra steps in the analysis can cause a higher CV compared with total lysine.
Extrusion
Extrusion cooking is a high-temperature, short-time treatment to improve the digestibility of raw ingredients and allows expansion, dehydration and shaping of the kibbles. The extruder consists of a feeder, preconditioner, extruder barrel, die and a knife assembly(Reference Rokey, Plattner and Souza117). The feeder controls the feed rate or throughput of the raw ingredients into the preconditioner. The main function of the preconditioner is to mix the ingredients with water and steam and to pre-cook the ingredients. The extruder barrel is a fixed metal barrel that contains one or two screws to transport the (pre-cooked) ingredient mix from the inlet zone to the die. The temperature inside the barrel is increased, resulting in cooking of the mix. Pet foods are generally extruded using temperatures between 80 and 200°C for 10 to 270 s, with moisture levels of 10 to 25 %(Reference Hand, Thatcher, Remillard and Roudebush118). At the end of the extruder barrel, a die is installed that restricts the product flow, causing increased pressure and shear. Moisture evaporates rapidly when the extrudate exits the die and encounters ambient pressure and temperature, which causes expansion and creates the characteristic texture of dry extruded pet food(Reference Rokey, Plattner and Souza117). In addition, the die and a knife assembly behind the die are responsible for the shape of the product. The kibbles are then dried to reduce the moisture content to less than 6–9 %. Dryers usually consist of a heating zone, where the kibbles are heated to about 80–100°C. The product is then moved into the drying zone with temperatures of about 120–150°C. Finally, the product is cooled to 80–100°C. This type of drying takes about 15 min for drying and 7 min for cooling(Reference Hand, Thatcher, Remillard and Roudebush118). Finally the pet food is coated with fat and/or a palatability enhancer and subsequently packaged. The extrusion process is controlled by several process parameters. Raw materials and screw configuration as well as die size are set before extrusion. Process parameters such as moisture content, screw speed and barrel temperature can be adjusted and continuously monitored during extrusion. Variables such as retention time, product temperature, pressure and mechanical energy change as a result of changing one or more of the process parameters(Reference Marsman119).
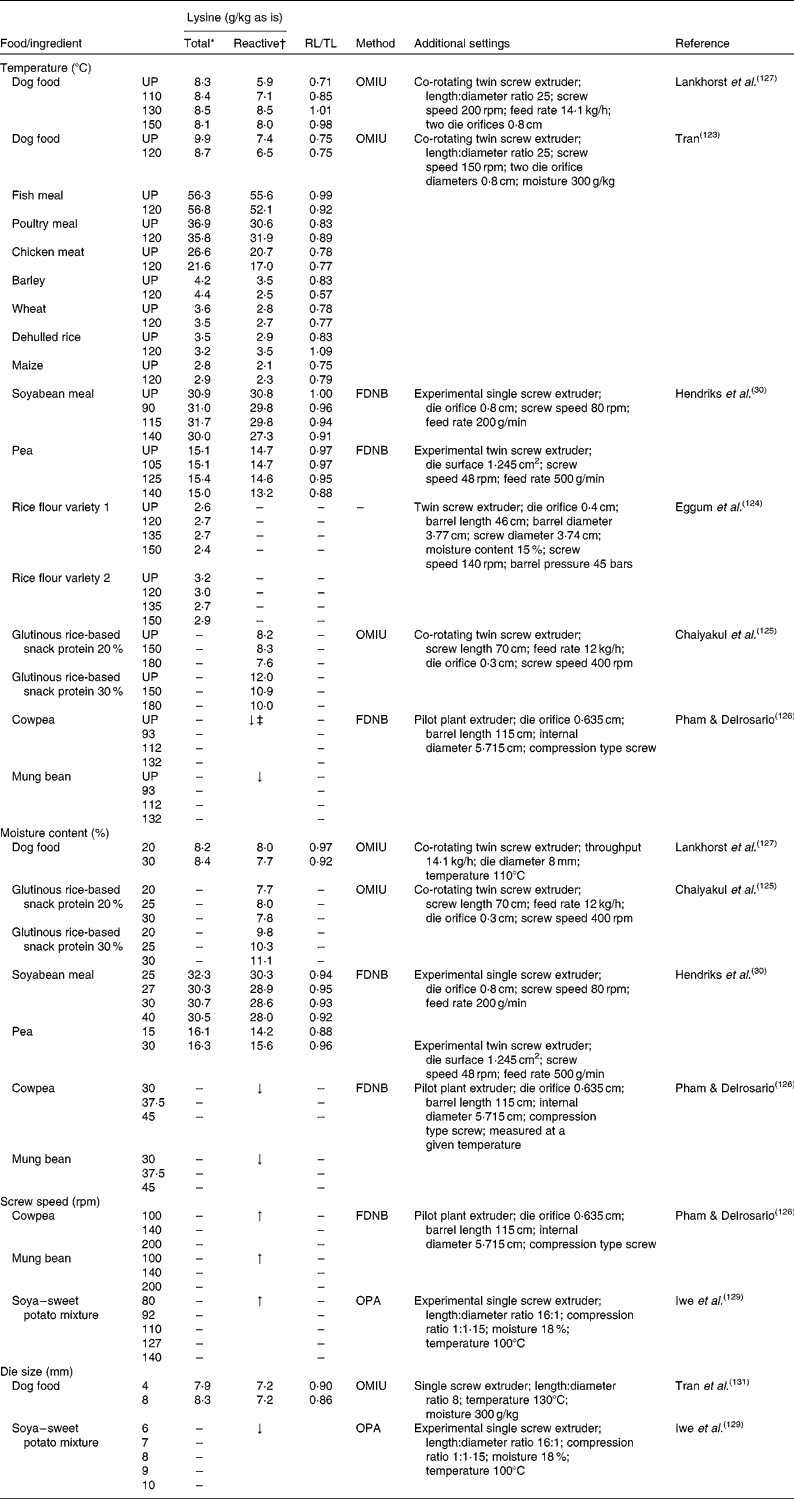
The effects of the process parameters, i.e. temperature, moisture and mechanical shear, on pet foods and pet food ingredients in relation to total and reactive lysine content are summarised in Table 2. It is commonly accepted that temperature is the most important process parameter for the Maillard reaction during extrusion(Reference Meade, Reid and Gerrard4, Reference Björck and Asp120–Reference Singh, Gamlath and Wakeling122). In general, the rate of Maillard reaction increases with temperature and time, resulting in a decrease in reactive lysine content and an increase in the formation of MRP as indicated in model systems(Reference Mauron50). Several studies have examined the effect of extrusion temperatures on total and reactive lysine content in food recipes and in various single ingredients. Tran(Reference Tran123) reported that extrusion at 120°C of an experimental dog food did not affect the difference between total and OMIU-reactive lysine content, which remained at 25·0 %, but resulted in a 12·1 and 12·2% reduction, respectively, of total and OMIU-reactive lysine content compared with the unextruded food mixture. Furthermore, this study reported that extrusion can have contradictory effects on total and OMIU-reactive lysine between ingredients used in this food mixture. Extrusion at 120°C of chicken meat and fish meal resulted in an OMIU-reactive lysine loss of 17·9 and 6·3 % whereas extrusion had little effect on OMIU-reactive lysine content of poultry meal. Furthermore, total lysine contents in chicken meat decreased by 18·8 % after extrusion, 3·0 % for poultry meal and slightly increased for fish meal (0·9 %). For ingredients of vegetable origin, total lysine contents were low overall and decreased after extrusion for wheat (2·8 %) and dehulled rice (8·6 %), whereas total lysine content increased for barley (4·8 %) and maize (9·5 %). OMIU-reactive lysine contents were decreased after extrusion for barley (28·6 %) and wheat (3·6 %), whereas increased contents were reported for dehulled rice (20·7 %) and maize (9·5 %). As the vegetable ingredients studied were low in lysine content, the contribution of a possible error of the assay to a relative change in lysine content would have been larger than that for the animal-derived ingredients that contained considerably more lysine. The data of Tran(Reference Tran123), however, do not show the effect of lower or higher extrusion temperature on total and reactive lysine content in foods or its ingredients. Extrusion of soyabean meal or peas had no effect on total lysine content and little effect on FDNB-reactive lysine content when extruded at 90 or 115°C and 105 or 125°C, respectively(Reference Hendriks, Moughan and Boer30). Extrusion at 140°C, however, decreased FDNB-reactive lysine content by 11·4 % for soyabean meal and 10·2 % for peas. Extrusion of two rice flour varieties at 120, 130 or 150°C had little effect on total lysine contents(Reference Eggum, Juliano and Ibabao124). Extrusion of a glutinous rice-based snack with 20 % protein at 150°C had no influence on the OMIU-reactive lysine content; however, it reduced OMIU-reactive lysine content by 9·2 % in a snack with 30 % protein(Reference Chaiyakul, Jangchud and Jangchud125). Increasing the extrusion temperature to 180°C reduced OMIU-reactive lysine content by 8·4 % for the 20 % protein snack, and by 16·7 % for the 30 % protein snack. Although absolute values were not reported, extrusion of cowpeas and mung beans at temperatures from 93 up to 132°C decreased FDNB-reactive lysine content by 25·4 and 21·3 %, respectively(Reference Pham and Delrosario126). Lankhorst et al. (Reference Lankhorst, Tran and Havenaar127) reported that extrusion of an experimental dog food had no effect on total lysine content, but OMIU-reactive lysine content was increased by 20·3 % after extrusion at 110°C and 35·6 % after extrusion at 150°C. At these temperatures, the difference between total and OMIU-reactive lysine content decreased from 29·0 % (unprocessed) to 15·0 and 2·0 %, respectively. The authors hypothesised that the bound ɛ-amino group of lysine regained its reactivity as a result of the extrusion process. The underlying mechanism is still unknown; however, based on these data, increasing extrusion temperatures generally reduced reactive lysine contents of single ingredients with little or no effect on total lysine content. The number of studies evaluating the change in total and reactive lysine in pet foods at varying extrusion temperatures is limited, and contradictory effects of temperature on reactive lysine were seen. Additional studies are, therefore, required to assess the importance of extrusion temperature to the formation of MRP in pet foods.
Table 2 Overview of extrusion parameters in relation to total and reactive lysine content of several foods and ingredients

RL, reactive lysine; TL, total lysine; UP, unprocessed; OMIU, O-methylisourea; FDNB, fluorodinitrobenzene; OPA, ortho-phthaldialdehyde.
* Total lysine is determined with conventional amino acid analysis.
† Reactive lysine is determined according to the given method.
‡ Data of reactive lysine content were not presented by authors. Arrows indicate a decrease ( ↓ ) and an increase ( ↑ ) in reactive lysine content with increasing parameter.
The moisture content of the ingredient or mixture can also be adjusted during extrusion. The moisture content is either the original moisture level of the ingredient or mix itself, or from added water or steam during extrusion. Moisture is necessary for the Maillard reaction to take place(Reference Mauron50, Reference Adrian128), but moisture also seems to inhibit the browning reaction if present in high concentrations in model systems. Increasing moisture content from 20 to 30 % during extrusion of an experimental dog food only slightly affected total lysine content (+2·4 %) and OMIU-reactive lysine content (–3·9 %)(Reference Lankhorst, Tran and Havenaar127) (Table 2). Moisture content had little effect on OMIU-reactive lysine content for a 20 % protein glutinous rice-based snack but for the 30 % protein snack the OMIU-reactive lysine content was increased by 5·1 and 13·3 % when moisture content was increased from 20 to 25 and 30 %, respectively(Reference Chaiyakul, Jangchud and Jangchud125). Increasing moisture content from 25 to 40 % during the extrusion of soyabean meal decreased total and FDNB-reactive lysine content by 5·0 and 6·5 %, respectively(Reference Hendriks, Moughan and Boer30). Also for cowpeas and mung beans, FDNB-reactive lysine content has been reported to be decreased with increasing moisture content from 30 to 45 % during extrusion(Reference Pham and Delrosario126). For peas, however, increasing moisture content from 15 to 30 % during extrusion slightly increased total lysine content (1·2 %) and increased FDNB-reactive lysine content by 9·9 %(Reference Hendriks, Moughan and Boer30). A high moisture content may induce a protective effect by increased or prolonged water evaporation, which keeps product temperature relatively low. In addition, water could reduce friction and, therefore, protects the ingredients from shear during processing.
Mechanical shear force may change during extrusion(Reference Marsman119). The amount of shear force developed during extrusion cooking depends on several process parameters such as screw configuration, compression ratio, screw speed and die size. Literature on the effect of these process parameters in relation to the Maillard reaction is scarce. There are only a few studies that have evaluated the effect of screw speed and die size in relation to reactive lysine content in ingredients or foods, and for most studies no quantitative data are reported (Table 2). There is no information available on the effect of screw speed on total and reactive lysine contents during the extrusion of pet foods. Increasing screw speed during the extrusion of cowpeas and mung beans from 100 to 200 rpm resulted in reduced loss of FDNB-reactive lysine content(Reference Pham and Delrosario126). Similarly, OPA-reactive lysine in a soya–sweet potato mixture resulted in less loss with increasing screw speed from 80 to 140 rpm during extrusion(Reference Iwe, van Zuilichem and Stolp129). At constant extrusion temperatures, a higher screw speed increases shear but reduces residence time and hence limits the exposure to heat treatment(Reference Mahungu, Drozdek and Artz130). Based on these data, shorter residence time and thus less exposure to heat treatment seem to be more important than the higher shear forces that occur when screw speed is increased. Shear and pressure are also affected by the die at the end of the extruder, which restricts the product flow. Increasing die size from 4 to 8 mm in a control sample dried at 40°C increased total lysine content in an experimental dog food by 5·1 % with no effect on OMIU-reactive lysine(Reference Tran, Hendriks and van der Poel131). Increasing die size from 4 to 9 mm in the same experimental dog food at four different drying temperatures (80, 120, 160 and 200°C) on average increased OMIU-reactive lysine content with no effect on total lysine. For extrusion of a soya–sweet potato mixture, an increase in die size from 6 to 10 mm at a temperature of 100°C reduced OPA-reactive lysine content(Reference Iwe, van Zuilichem and Stolp129).
Drying and storage
The heat applied during the drying process can also affect the difference between total and reactive lysine. Drying of a dog food at increasing temperatures of 80, 120, 160 and 200°C to an end moisture content of 6–9 % reduced total lysine content by 15·7 % as well as OMIU-reactive lysine content by 15·5 %(Reference Tran, Hendriks and van der Poel131).
Besides drying, addition of palatability enhancers can influence differences between total and reactive lysine in the final product. Flavour and texture are important properties that contribute to the overall palatability of the food, which determines how likely animals are to eat the food. Texture is created during processing; however, dry pet foods often have only moderate inherent flavour(Reference Nagodawithana, Nelles, Trivedi, Pasupuleti and Demain132). Therefore, palatability enhancers are regularly used during the production of dry pet foods. As well as palatability enhancers such as acidified yeast, hydrochloric acid, phosphoric acid, sugars and spices, most dry pet foods are coated with digests(Reference Hand, Thatcher, Remillard and Roudebush118). Digests are hydrolysed proteins from meats, offals and yeasts, produced using heat and enzymes. The digestion process releases amino acids such as lysine and dipeptides that enhance palatability. However, during this process, the hydrolysed proteins are available to participate in the Maillard reaction. The resulting MRP increase the palatability of the digest(Reference Nagodawithana, Nelles, Trivedi, Pasupuleti and Demain132), but simultaneously result in the formation of early and advanced MRP. The digest is applied as liquid sprayed on the dry food or applied as a powder. It is possible that the production process of digest adds to the differences in total and reactive lysine and to the amount of MRP in dry pet foods.
Little information has been reported on storage conditions and duration in relation to the Maillard reaction in pet foods. Chiang(Reference Chiang107) recorded an increase in furosine from 0·91 mg/g in a control dry dog food to 1·52 mg/g after storage of 12 weeks at 22·2°C. Storing the same control dry dog food for 12 weeks at 37·8°C increased furosine levels to 3·19 mg/g. These data are an indication that early Maillard products can be present in pet foods and that additional fructoselysine is generated during storage. No studies have been conducted to determine the stability of reactive lysine in pet foods during varying storage conditions. The data by Chiang(Reference Chiang107) indicate that as the furosine concentration increases during storage, the concentration of reactive lysine decreases.
Retorting
Retorting is a processing method in which food contents are sealed in airtight cans, containers, or flexible pouches and are subsequently heat-treated. Process temperatures sterilise the product, resulting in preservation of these high-moisture foods for extended periods of time(Reference Hand, Thatcher, Remillard and Roudebush118). Moist pet foods contain fresh or frozen meat and other animal tissues, which are ground and homogenised. Additional ingredients such as mash grains or other ground starch sources, vitamin and mineral premixes, and water are added and mixed to create a complete food recipe. The mixture is heated up to 85°C to start starch gelatinisation and protein denaturation(Reference Hand, Thatcher, Remillard and Roudebush118). The hot mixture is transported to machines that fill and seal the packages. A vacuum is created by either the hot product itself, or by injecting steam over the product just before sealing. The steam replaces air in the container, creating under-pressure as the steam condenses during cooling. After sealing, the packages are sterilised in a retort in a continuous or batch system. Retorting is a temperature/time-dependent process, using an F0-value as a unit to summarise the lethality of the heat employed on the product. The F0-value represents the time equivalent in minutes of a heating process to destroy micro-organisms at the reference temperature of 121·1°C(Reference Hendriks, Emmens and Trass133). In general, the process time should be at least 3 min at 121·1°C (F0-value of 3) to kill pathogenic bacteria(Reference Hand, Thatcher, Remillard and Roudebush118). However, higher values (F0-value >10) are employed in practical pet food production in order to destroy spores(Reference Hendriks, Emmens and Trass133).
As described above, the difference between total and reactive lysine can be considerable in moist canned foods, with values between 39·0 and 61·8 % (Fig. 5). This indicates that processing conditions applied during retorting of cans favour the Maillard reaction, but effects of processing conditions on total and reactive lysine content have been the subject of only a few experiments. Retorting a standard moist cat food recipe containing a low content of carbohydrates (maximum 6·7 %) at lethality values (F-values) of 5·3, 8·6, 17·2 and 24·3 (temperature set at 121°C, time periods between 80 to 120 min) had no effect on the total or OMIU-reactive lysine content(Reference Hendriks, Emmens and Trass133). In the unprocessed food, the difference between total and reactive lysine was 12·0 %. The difference between total and reactive lysine content in the pet food ingredients used in this particular food does not explain the high differences between total and reactive lysine reported in some moist canned foods. True ileal total lysine digestibility as measured using growing rats consistently decreased with increasing F-values from 84·2 % for the untreated food to 77·4 % for the food with the highest lethality value (24·3)(Reference Hendriks, Emmens and Trass133), indicating that part of the lysine was rendered indigestible. However, total as well as OMIU- and FDNB-reactive lysine did not change due to processing. It is therefore unclear whether the Maillard reaction was the cause of the reduced ileal total lysine digestibility. Results of other experiments indicate a decrease in reactive lysine during retorting. Retorting of tuna at 115°C for 55 and 90 min decreased FDNB-reactive lysine contents by 5·0 and 15·7 %, respectively(Reference Castrillón, Navarro and García-Arias134). Autoclaving a diet used for salmonid fish, including fish meal, barley protein concentrate and wheat flour as the main ingredients, at temperatures of 100, 110, 120 and 130°C reduced total lysine by 5·8 % and OMIU-reactive lysine by 18·3 %(Reference Morken, Moyano and Márquez L135). As FDNB- and OMIU-reactive lysine demonstrate a good correlation(Reference Rutherfurd and Moughan31), results of the effect of retorting on reactive lysine are inconsistent. It is unclear what the contribution is of the ingredient used or the retorting process on the high differences between total and reactive lysine contents reported in moist canned cat foods (Fig. 5). The effects of retorting temperature and its possible interaction with time on total and reactive lysine content have not been studied to date.
Pelleting
Pelleting dry pet foods is a processing technique that is less severe in terms of temperatures applied compared with extrusion (60 to 90°C v. 80 to 200°C). The pelleting process includes mash conditioning, pelleting and subsequent drying or cooling. For mash conditioning, heat, water and pressure with temperatures between 60 and 90°C(Reference Thomas, van Zuilichem and van der Poel136, Reference Mavromichalis and Baker137) are used to induce a wide range of physical and chemical changes including softening of the food, denaturation of proteins and gelatinisation of starch(Reference Thomas, van Zuilichem and van der Poel136). After conditioning, the mash is pressed through a die in a pellet mill. The die design is either a ring, which is revolving around fixed rollers, or flat, which is static and horizontal rollers rotate around a vertical axis. The rollers continuously press layers of mash inside the die hole, by which the pellet is built up. The extent of compression is dependent on the height of the layer and the gap distance between the die and the roller. Changing these conditions changes pellet quality. Die holes differ in their length:diameter ratio, influencing the amount of shear that the feed mash receives. After leaving the die, pellets are usually cooled by air flow(Reference Thomas, van Zuilichem and van der Poel136).
Despite the lower processing temperatures, the Maillard reaction occurs during pre-conditioning and/or pelleting, resulting in loss of reactive lysine and formation of MRP. Tran et al. (Reference Tran, van Lin and Hendriks62) reported a difference of 20·5 % between total and reactive lysine in pelleted commercial dog pellets. There are no reports from experiments in which pre-conditioning or pelleting conditions were evaluated in terms of influencing total and reactive lysine levels in pelleted pet foods. For pig feed, few studies are available but these only compared unprocessed feed mash with pellets under one fixed pelleting condition. Pelleting pig feed at 80°C did not affect total lysine content but slightly increased FDNB-reactive lysine content by up to 1·2 %(Reference Ginste and De Schrijver138). Pelleting a complex nursery pig diet (steam-conditioned at 60°C for 45 s through a 5 × 38 mm die) had no effect on lysine bioavailability as measured using a standard-curve bioassay with 8-d-old chicks(Reference Mavromichalis and Baker137). Pelleting a conventional pre-starter diet for suckling piglets (steam-conditioned at 40°C for 30 s; pelleted at 60 to 65°C through a 1·7 × 40 mm die) resulted in a 200 % increase of furosine, a marker for the presence of the Amadori compound of lysine (Fig. 1), from 11·71 to 35·18 mg/kg(Reference Delgado-Andrade, Rufian-Henares and Nieto139). Other MRP also increased; HMF increased by 32·6 % from 12·56 to 16·66 mg/kg, and furfural increased from non-detectable to 0·034 mg/kg.
Conclusions
Data indicate that significant proportions of the lysine in pet foods can be modified and may be unavailable for metabolism by dogs and cats. OMIU-reactive lysine content was below minimal requirement for growing dogs from 4 to 14 weeks in two out of fourteen analysed dry foods. As such, foods for growing dogs require more careful considerations in terms of lysine supply, as the requirement for growing animals is higher than that of adult animals. As batch-to-batch variation is unknown, the results may be different between batches of the same food due to variation in ingredients or processing. More knowledge of the bioavailability of lysine and other amino acids involved in the Maillard reaction in dog and cat foods is required. Advanced MRP have varying biological activities, and in dogs data indicate higher AGE in plasma from dogs suffering from canine diabetes mellitus compared with control animals. In addition, elevated levels of AGE in tissue proteins in dogs were observed for a number of diseases. It is unknown to what extent advanced MRP are present in pet foods. Most of the dietary ingested MRP are rapidly excreted via the kidneys although no data are available for cats and dogs. Whether or not the presence of dietary MRP in pet foods may result in the development of diseases such as diabetes and impaired renal function in pet animals requires further study. In the regulation of the levels of total and reactive lysine and possible advanced MRP in pet foods, control of ingredient processing and choice of ingredients may be a useful strategy as differences between total and reactive lysine are observed in several ingredients commonly used in pet foods. Effects of processing conditions on the difference in total and reactive lysine contents in pet foods are inconsistent and do not always correspond to model systems. Processing temperature is the most important factor followed by moisture level. Moist pet foods appear to have a higher difference between total and reactive lysine content compared with dry foods. Further study into choice of ingredients, extrusion, pelleting and retorting of pet food on the progression of the Maillard reaction is recommended, as are studies on the bioavailability of dietary MRP and their possible relationship with pet health.
Acknowledgements
The project for which the present review was conducted is jointly financed by the European Union, European Regional Development Fund and The Ministry of Economic Affairs, Agriculture and Innovation, Peaks in the Delta, the Municipality of Groningen, the Province of Groningen, MARS Petcare as well as the Dutch Carbohydrate Competence Center (CCC WP 5).
All authors contributed fundamentally to the present paper. C. v. R. contributed to all facets including data collection, calculations and writing the initial manuscript. Other authors contributed to data interpretation and manuscript preparation.
L. A. is an employee of Mars Petcare.