Homocysteine (Hcy) is a sulphur-containing amino acid derived from S-adenosyl-Hcy produced from S-adenosyl-methionine, the major cellular methyl donor. Hcy is known as a powerful pro-oxidant( Reference Mouchabac 1 ) with multiple deleterious effects notably on the immune system in humans( Reference Grimble 2 ). In suckling piglets, there is a rapid postnatal development of homocysteinaemia( Reference Ballance and House 3 , Reference Simard, Guay and Girard 4 ). This condition is potentially harmful for these young animals as a model comparing two populations of piglets with high (24·7 µm) or low (16·7 µm) homocysteinaemia showed that indicators of cell-mediated immunity (proliferation of lymphocytes in response to the mitogen concanavalin A) were negatively affected in the former group of piglets, suggesting a weakened immune competence associated with high plasma concentrations of Hcy( Reference Audet, Girard and Lessard 5 ).
Blood plasma total Hcy (pHcy), the most common indicator of Hcy status, decreases in sows and piglets following administration of dietary supplements of folic acid and vitamin B12 to sows during gestation( Reference Simard, Guay and Girard 4 , Reference Barkow, Pietrzik and Flachowsky 6 , Reference Guay, Matte and Girard 7 ) and lactation( Reference Audet, Girard and Lessard 5 ). These responses in sows and piglets were associated with remethylation where vitamin B12-dependent methionine synthase transfers a methyl group from CH3-H4-folate to convert Hcy in methionine( Reference Bässler 8 ). Nevertheless, the lower concentrations of pHcy in these suckling piglets remain two to five times higher than in other species( Reference Simard, Guay and Girard 4 , Reference Audet, Girard and Lessard 5 ). Therefore, other metabolic pathways may be involved for Hcy homeostasis in these animals.
The demand for methyl groups which frequently results in accumulation of Hcy is particularly high in piglets for de novo synthesis of creatine( Reference Brosnan, Wijekoon and Warford-Woolgar 9 ). The same situation applies for endogenous phosphatidylcholine and choline( Reference Stead, Brosnan and Brosnan 10 ). Supplements of these preformed metabolites could reduce the need for methyl groups and prevent accumulation of Hcy. Besides the remethylation pathway via methionine synthase, the disposal of Hcy may also proceed through another remethylation pathway via betaine-Hcy-methyltransferase. Last, Hcy can be catabolised by the trans-sulfuration pathway where the vitamin B6-dependent cystathionine β-synthase and cystathionine-γ-lyase produce cystathionine and then cysteine and glutathione( Reference Bässler 8 ).
Therefore, it was hypothesised that pHcy in piglets can be regulated by exogenous oral supplements of betaine as a methyl group supplier, creatine for reducing the demand for methyl groups, choline as methyl group supplier and/or for reducing the demand for methyl groups and vitamin B6 for covering co-enzyme needs for Hcy catabolism.
Experimental methods
The experimental procedures followed the guidelines of the Canadian Council on Animal Care( 11 ) and were approved by the Institutional Animal Care Committee of the Dairy and Swine Research and Development Centre of Sherbrooke (QC, Canada). All animals were cared for and slaughtered according to the recommended code of practice of Agriculture Canada( 12 ).
Sow management
A total of seventeen Yorkshire–Landrace sows (second parity) were used for this experiment. The average body weights at insemination, farrowing and weaning were 205·8, 274·1 and 244·0 (sem < 7·0) kg, respectively. From 3 d after weaning, oestrus was detected twice per d when a boar was introduced near the pen for 1 h between 08.00 and 09.00 hours and from 16.00 to 17.00 hours. When oestrus was first detected in the morning, sows were inseminated twice, 8 and 24 h following detection, whereas when oestrus was first detected in the afternoon, the two inseminations were done 16 and 24 h later. Sows were artificially inseminated with 85 ml of semen (2 × 109 live sperm cells from pooled semen of three Duroc boars) provided by a local artificial insemination centre (Centre d'Insémination Porcine du Québec Inc., St-Lambert, QC, Canada). During gestation, sows were kept in individual stalls and individually fed 2·5 kg of a diet supplemented with folic acid (10 mg/kg) and vitamin B12 (150 µg/kg) (Table 1). These levels of dietary supplementation were chosen according to Simard et al. ( Reference Simard, Guay and Girard 4 ) and Guay et al. ( Reference Guay, Matte and Girard 7 ). At 1 week before expected farrowing, sows were transferred from the gestation (0·6 × 2·2 m, half-slatted concrete flooring) to the farrowing crate (1·5 × 2·2 m, cast iron and plastic-coated wire mesh flooring). The farrowing–lactating room housed five sows and was maintained at a temperature of 24°C. At 113 d of gestation, sows received an intravulvar infusion of 1·0 ml of dinoprost tromethamine (Lutalyse; Pfizer Animal Health) to induce delivery. After 24 h, if sows had not delivered, they received an intramuscular (neck) injection of 0·5 m of synthetic oxytocin (20 IU/ml, P.V.U., Oxyto-Sure; Vétoquinol). During lactation, the diet was also supplemented with folic acid and vitamin B12 at the same concentrations as for the gestation diet (Table 1). The feed allowance was 3·25 kg on day 1 and was increased by 0·5 kg/d until it reached ad libitum intake at the end of first week of lactation. Measures of body weight and back fat thickness were recorded at mating, on day 1 after farrowing and at weaning. Blood samples were taken when sows were transferred in the farrowing crate, on day 1 of lactation and at weaning. Milk samples were collected on days 1 and 21 of lactation as described by Simard et al. ( Reference Simard, Guay and Girard 4 ) and Audet et al. ( Reference Audet, Girard and Lessard 5 ).
Table 1. Composition of the basal diet (as-fed basis) for gestation and lactation periods

* The proximate analysis for crude protein, crude fat, crude fibre, Ca and P of the basal diet was 13·5, 3·8, 4·6, 1·2 and 0·7 %, respectively. Metabolisable energy was estimated at 12·2 MJ/kg. The calculated lysine, and lysine:methionine and lysine:methionine + cysteine ratios were 0·62 %, 2·58 and 1·19, respectively.
† The proximate analysis for crude protein, crude fat, crude fibre, Ca and P of the basal diet was 17·9, 3·2, 2·5, 1·0, and 0·6 %, respectively. Metabolisable energy was estimated at 12·3 MJ/kg. The calculated lysine, and lysine:methionine and lysine:methionine + cysteine ratios were 0·97 %, 3·34 and 1·62, respectively.
‡ Supplied (per kg of feed): Mn, 86·2 mg; Zn, 188·5 mg; Fe, 345·8 mg; Cu, 28·5 mg; I, 2·1 mg; Se, 0·3 mg; vitamin A, 14 564 IU; vitamin D, 1500 IU; vitamin E, 60 IU; vitamin K, 2·6 mg; vitamin B12, 0·03 mg; thiamine, 2·7 mg; riboflavin, 5·0 mg; niacin, 31·1 mg; pantothenic acid, 21·3 mg; folic acid, 10 mg; pyridoxine, 2·6 mg; biotin, 0·4 mg; choline, 520·7 mg.
§ Supplied (per kg of feed): Mn, 66·8 mg; Zn, 180·9 mg; Fe, 347·4 mg; Cu, 28·7 mg; I, 2·1 mg; Se, 0·3 mg; vitamin A, 14 564 IU; vitamin D, 1500 IU; vitamin E, 60 IU; vitamin K, 2·6 mg; vitamin B12, 0·03 mg; thiamine, 2·7 mg; riboflavin, 5·0 mg; niacin, 31·1 mg; pantothenic acid, 21·3 mg; folic acid, 10 mg; pyridoxine, 2·6 mg; biotin, 0·4 mg; choline, 520·7 mg.
|| Myco Curb®, Kemin Industries Inc.
¶ This premix was given as a top dressing which provided an equivalent of 116·4 µg/kg of vitamin B12 (analysed according to Girard et al.( Reference Girard, Robert and Matte 32 )).
Piglets and treatments
During farrowing, all piglets were placed in a box outside the crate to prevent access to mammary glands and colostrum intake until body weight measurement and a first blood sample were taken (day 0, within 1 h after birth). Teeth were cut and an Fe intramuscular injection (1 ml of iron dextran containing 100 mg of Fe/ml, Ironol, P.V.U., Lavaltrie) was given on day 1. A total of eight piglets of average body weight and condition (visual assessment) were chosen and assigned randomly to their respective treatments while the four remaining piglets did not receive any treatment but were kept with their dams in order to maintain the litter size at twelve piglets. On day 1, when needed to reach a litter size of twelve piglets, piglets (n ≤ 4) were fostered but those animals were not treated. Eight different treatments were compared within each litter: (1) control (0·9 % NaCl); (2) betaine; (3) choline; (4) creatine; (5) vitamin B6; (6) betaine and vitamin B6; (7) choline and creatine; and (8) betaine, vitamin B6, choline and creatine. Each treatment consisted of commercially available forms of these nutrients and was given as a liquid oral bolus, every day at the same time (13.00 hours) from day 0 until day 21 (weaning). Piglets were weighed and blood samples were collected from the jugular vein by venepuncture( Reference Matte, Girard and Brisson 13 ), on day 0 and then before the administration of treatments on days 1, 7, 14 and 21 of lactation. Before weighing and blood sample collection, all piglets were separated from their mother for 1 h. This period, which is slightly longer than the normal natural interval between two suckling episodes( Reference Pond and Maner 14 ), allowed us to standardise for the effect of previous milk intake on body weight and plasma metabolite concentrations.
Solutions for oral administration
Solutions were prepared with betaine hydrochloride (B7045, Sigma-Aldrich), choline chloride (70 % liquid; Chinook, Nutreco) and pyridoxine hydrochloride (P9755; Sigma-Aldrich). For each nutrient, stock solutions were prepared in water and frozen at −20°C. These stock solutions were further diluted in water as working solutions providing the target daily amounts for each nutrient (Table 2). For creatine treatments, creatine monohydrate (C3630; Sigma-Aldrich) was used and as the solubilisation was not complete, the daily allowance was weighed in a tube and diluted directly in water as a working solution for treatment 4 or within the appropriate working solutions for treatments 7 and 8. Daily allowances of betaine, choline, creatine and pyridoxine were adjusted every week based on intakes per kg body weight derived from studies of Eklund et al. ( Reference Eklund, Bauer and Wamatu 15 ), Brosnan et al. ( Reference Brosnan, Brosnan and Bertolo 16 ), Donovan et al. ( Reference Donovan, Mar and Zeisel 17 ) and Matte et al. ( Reference Matte, Giguère and Girard 18 ), respectively (Table 2). Piglets received a volume of 1·5 ml of each treatment during the first period (0–7 d of age) except for creatine treatments where the volume was 3·0 ml. During the second period (8–14 d of age), piglets received 3·0 ml of each treatment except for creatine treatments (6 ml). Finally, during the third period (15–21 d of age), piglets received 4·5 ml of each treatment except for creatine treatments (9·0 ml). For oral boluses containing creatine, the complete delivery was insured by continuous mixing during administration.
Table 2. Amounts of each nutrient administered orally to piglets according to age
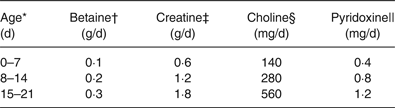
* These daily allowances were adjusted for expected body weights of 2, 4 and 6 kg at 0–7, 8–15 and 15–21 d of age. The actual average body weights were 2·2, 4·3 and 6·5 kg.
† Based on the average supplementation per kg body weight in growing–finishing pigs according to Eklund et al. ( Reference Eklund, Bauer and Wamatu 15 ) and adjusted for expected body weights of piglets.
‡ Based on total daily accretion per kg body weight in piglets according to Brosnan et al. ( Reference Brosnan, Brosnan and Bertolo 16 ) and adjusted for expected body weights of piglets.
§ Based on the total amount of choline provided by sows’ milk per kg body weight in piglets according to Donovan et al. ( Reference Donovan, Mar and Zeisel 17 ) and adjusted for expected body weights of piglets.
|| Based on optimal dietary provision per kg body weight in piglets after weaning according to Matte et al. ( Reference Matte, Giguère and Girard 18 ) and adjusted for expected body weights of piglets.
Sampling and analytical measurements
Blood samples were collected in EDTA-containing tubes (7 ml; Becton Dickinson and Co.). The tubes were placed on ice until all samples were collected. Then, the tubes were mixed gently for 5 min before determination of packed cell volume as described by Matte et al. ( Reference Matte, Girard and Brisson 13 ) The remaining blood was centrifuged during 12 min at 4°C (1800 g ). Plasma and erythrocytes were separated and frozen at −20°C until analysed.
Concentration of total pHcy and cysteine were measured by HPLC according to the method validated by Simard et al. ( Reference Simard, Guay and Girard 4 ). Blood plasma concentrations of creatine, choline and sarcosine were determined by commercial colorimetric kits (Biovision Research Products K615-100, K636-100 and K635-100, respectively) according to the manufacturer's instructions. Validation tests for measurement of creatine, choline and sarcosine in blood plasma showed satisfactory parallelism (CV < 9·7 %) and recovery test (98·5–102·5 %). Intra- and interassay CV were lower than 6·1 and 13·0 %, respectively. Pyridoxal-5-phosphate (P-5-P) was determined in erythrocytes and milk using the sample preparation and method adapted for erythrocytes by Matte et al. ( Reference Matte, Ponter and Sève 19 ) from Srivastava & Beutler( Reference Srivastava and Beutler 20 ).
Statistical analyses
Data were analysed according to a randomised arrangement of treatments in blocks (n = 16–17, except for plasma concentrations of choline, creatine and sarcosine where n = 7–9) and piglets within litter were considered as experimental units. Main effects of treatments (n = 8) and ages at sampling (n = 5) along with the interaction treatment × age were analysed using the repeated option of the SAS procedure for mixed models (SAS Institute Inc.)( Reference Littell, Miliken and Stroup 21 ). Several covariance structures were compared and the Ante-dependence covariable (ANTE) structure was retained as it yielded the best-fit statistical value. Differences were considered to be significant at P < 0·05 and a tendency at 0·05 ≤ P ≤ 0·10. A Tukey–Kramer correction was applied for all pairwise comparisons when main effects tended to be or were significant. All results are expressed as least-squares means with standard errors of the mean.
Results and discussion
Body condition of sows and growth of piglets
Average body weight and backfat thickness of sows were, respectively, 205·8 (sem 3·1) kg and 16·0 (sem 0·8) mm at mating, 251·0 (sem 3·9) kg and 16·9 (sem 1·0) mm on day 1 after parturition, and 244·0 (sem 4·1) kg and 15·1 (sem 1·0) mm at weaning. Measurements from nine piglets were missing at some stages of the lactation period due to weakness or crushing by their mother but these losses were not related to treatments. In one litter, treatments 1 and 8 were missing for the whole experimental period.
Growth performance of piglets was monitored during the lactation period. No treatment effect was observed on body weight (P > 0·54) or average daily gain (P > 0·13) of piglets during the lactation period. Average body weights were 1·71 (sem 0·01) kg at birth and 1·75 (sem 0·02), 3·17 (sem 0·04), 5·34 (sem 0·08) and 7·53 (sem 0·12) at 1, 7, 14 and 21 d of age, respectively whereas average daily gain was 0·18 (sem 0·01), 0·24 (sem 0·01) and 0·26 (sem 0·01) kg from 1 to 7, 7 to 14 and 14 to 21 d of age, respectively. Although growth of suckling piglets was not influenced by the different treatments, average daily gain and body weight of these animals during lactation reflect only partially the overall growth performance because no data are available on DM intake and feed conversion from milk consumption for each piglet.
Age and treatment responses of plasma homocysteine and cysteine
Overall plasma concentration of pHcy was very low at birth (2·48 (sem 0·06) μm), increased sharply to 8·27 (sem 0·44) μm within 24 h, and to 14·30 (sem 0·45) and 18·32 (sem 0·43) μm at 7 and 14 d of age, respectively, and plateaued during the last week of lactation with a value of 17·96 (sem 0·34) μm at 21 d of age (age effect; P < 0·01) (Fig. 1). This main age effect is consistent with that reported, under similar conditions, in other studies( Reference Ballance and House 3 – Reference Audet, Girard and Lessard 5 ). According to Simard et al. ( Reference Simard, Guay and Girard 4 ), such high concentrations of pHcy were probably due to piglet metabolism because Hcy is undetectable in milk. Further analyses of the present milk samples with mass spectrophotometric detection confirmed the absence of Hcy in milk from all sows whereas methionine corresponded to 1·2 (sem 0·06) g/l.

Fig. 1. Plasma concentrations of homocysteine according to treatment and age of piglets: ○, control (treatment 1); □, betaine (treatment 2); ∆, choline (treatment 3); ×, creatine (treatment 4); ◦, pyridoxine (treatment 5); ■, betaine and pyridoxine (treatment 6); ▲, choline and creatine (treatment 7); ●, all nutrients (treatment 8). Values are least-square means (n 16, 17, 17, 17, 17, 17, 17 and 16 piglets for treatments 1, 2, 3, 4, 5, 6, 7 and 8), with standard errors represented by vertical bars. There was an age effect (P < 0·01). There was a tendency for a treatment effect (P = 0·09). Pairwise comparison (Tukey–Kramer test) between treatments 1 and 8 was significant (P = 0·03).
In humans, pHcy concentrations increase from 20 to 40 d of age in breastfed infants (6·7–9·1 µm) but not in formula-fed infants( Reference Fokkema, Woltil and Van Beusekom 22 ). Nutritional factors have been raised to explain this phenomenon, in particular, the differences in B-vitamin intakes which were three (vitamin B6) to five times (vitamin B12, vitamin B2 and folates) higher in formula-fed than in breastfed infants. Such difference was probably amplified by a depressed B-vitamin status in lactating women during this period( Reference Fokkema, Woltil and Van Beusekom 22 ). In the present experiment, it is unlikely that milk folates or vitamin B12 were limiting because sow diets during gestation and lactation were supplemented at levels of 10 mg/kg of folic acid and 150 µg/kg of cyanocobalamin. Such supplements in sow feed are known to maximise milk transfer of folates( Reference Matte and Girard 23 , Reference Matte, Girard and Brisson 24 ) and vitamin B12 ( Reference Audet, Girard and Lessard 5 ) to piglets during lactation and reduce the postnatal rise of pHcy in piglets, probably via the cellular methionine synthase remethylation pathway as stated by Simard et al. ( Reference Simard, Guay and Girard 4 ). Other metabolic pathways for Hcy synthesis or disposal were addressed with the different nutrients used in the present experiment and concentrations of pHcy tended to be lower (P = 0·09) in treated than in control piglets (Fig. 1). However, the highest and sole pairwise significant decrease (23 %) was observed between control and treatment 8 (14·33 (sem 0·70) v. 11·05 (sem 0·70) μm, respectively; P = 0·03). None of the other pairwise comparisons between control (treatment 1) and each nutrient given alone (treatments 2, 3, 4 and 5) or in combination of two (treatments 6 and 7) reached significance (P > 0·15). There was no treatment × age interaction (P > 0·11). As mentioned earlier, two approaches, besides the methionine synthase remethylation pathway, were chosen to prevent the acute increase of pHcy in suckling piglets. The first one, using dietary betaine through the betaine-Hcy-methyltransferase pathway and pyridoxine through vitamin B6-dependent trans-sulfuration, aimed to enhance the disposal of Hcy while the second one provided preformed methylated nutrients (creatine and choline) to limit the need for methyl groups and, then, prevent Hcy formation. It seems that combining both approaches was necessary to attenuate the rapid rise of homocysteinaemia in neonatal piglets. However, although the response to treatment 8 represented a decrease of pHcy of 23 %, the lowest average concentration achieved from 1 to 21 d of age, at 13·1 µm, was similar to the mean comparable value of 13·2 µm observed during the suckling period after one intramuscular injection of vitamin B12 administered at 1 d of age to piglets( Reference Audet, Girard and Lessard 5 ). It appears that supplements of vitamin B12 given to piglets by injection, which had probably activated the cellular methionine synthase remethylation pathway for disposal of Hcy, were as efficient as the present dual approaches pursued with the cocktail of nutrients (treatment 8) to reduce pHcy increase during the suckling period. Additional studies are needed to know if a synergy among all those approaches (present cocktail of nutrients and intramuscular injections of vitamin B12 used by Audet et al. ( Reference Audet, Girard and Lessard 5 )) can enhance the clearance rate of Hcy in suckling piglets because even these lowest concentrations of pHcy in suckling piglets remain substantially higher than typical levels in other species (<10 µm), as stated by Simard et al. ( Reference Simard, Guay and Girard 4 ).
As for pHcy, plasma cysteine increased with age (P < 0·01) from 65·5 (sem 1·2) μm at birth to 99·3 (sem 1·7), 159·8 (sem 2·4), 198·3 (sem 2·2) and 209·4 (sem 2·1) μm on days 1, 7, 14 and 21 of age but it was not affected by treatments (P > 0·67; Table 3). This age response is similar to that reported by Ballance & House( Reference Ballance and House 3 ) and Simard et al. ( Reference Simard, Guay and Girard 4 ). In this last study, an increase of vitamin B6-dependent enzyme activities in the trans-sulfuration pathway (cystathione-β synthase and cystathionine-γ-lyase) was also reported between birth and 26 d of age. Nevertheless, in spite of these age-related enzymic changes, it cannot be ruled out that the consumption of cystine from sows’ milk( Reference Wu and Knabe 25 ) may be a main contributing factor to cysteine homeostasis in piglets during the suckling period and may have masked plasma cysteine responses to treatments with vitamin B6 supplements.
Table 3. Plasma concentrations of cysteine, choline and sarcosine in piglets during the first 21 d of age according to treatments
(Least-square mean values with their pooled standard errors)

a,b Mean values within a column with unlike superscript letters were significantly different (P < 0·05).
* Values are least-square means of 16, 17, 17, 17, 17, 17, 17 and 16 piglets for treatments 1, 2, 3, 4, 5, 6, 7 and 8.
† Values are least-square means of nine piglets for each treatment.
‡ Values are least-square means of seven piglets for each treatment.
§ Age effect (see text; P < 0·01).
|| Tendency for a treatment effect (P = 0·09) but no pairwise comparison (Tukey–Kramer test) was significant (P > 0·17).
¶ Treatment effect (P = 0·01).
Creatine, choline and vitamin B 6 homeostasis in piglets during the suckling period
Except for betaine, blood plasma concentrations of each studied nutrient in the present experiment were monitored during the experimental period.
As mentioned earlier, both creatine and choline were provided to reduce the demand for methyl groups and eventually the formation of Hcy. For choline, it has a dual role on Hcy metabolism because betaine, produced by oxidation of choline, could also promote remethylation of Hcy. Due to technical reasons, it was not possible to measure the plasma concentration of betaine in the present experiment. Plasma concentrations of creatine in creatine-supplemented treatments (4, 7 and 8) peaked at 1 d of age and decreased thereafter to be no longer different from in other treatments from 14 to 21 d of age (interaction treatment × age; P < 0·01) (Fig. 2). Such response suggests that the provision of exogenous preformed creatine exceeds the metabolic utilisation of this nutrient in piglets during their first days of life but not later in lactation. Nevertheless, it cannot be ruled out that this response could also be related to the efficiency of excretion of creatine in creatinine influenced by the postnatal development of renal function in piglets during the first weeks of life( Reference Friis 26 , Reference Alt, Colenbrander and Forsling 27 ). In the case of plasma choline, for all treatments, concentrations increased (age effect; P < 0·01) from 9·88 (sem 0·53) and 10·97 (sem 0·52) μm at birth and 1 d of age, respectively, to 15·50 (sem 0·67), 15·94 (sem 0·43) and 14·97 (sem 0·40) μm at 7, 14 and 21 d of age. A treatment effect on plasma choline was detected (P = 0·01) with the lowest concentrations for oral supplementations of betaine and pyridoxine (treatments 2 and 5) and the highest one with the cocktail of all treatments (treatment 8) (Table 3). It is noteworthy that, in spite of this treatment effect, none of the pairwise comparisons between individual or combined nutrients v. the control treatment was significant (P > 0·31). There was no treatment × age interaction (P > 0·94) on plasma choline concentrations.

Fig. 2. Plasma concentrations of creatine according to treatment and age of piglets: ○, control (treatment 1); □, betaine (treatment 2); ∆, choline (treatment 3); ×, creatine (treatment 4); ◦, pyridoxine (treatment 5); ■, betaine and pyridoxine (treatment 6); ▲, choline and creatine (treatment 7); ●, all nutrients (treatment 8). Values are least-square means of seven piglets for each treatment, with standard errors represented by vertical bars. There was an age effect (P < 0·01). There was a treatment × age interaction (P < 0·01).
Although not specifically measured in the actual study, creatine and choline are present in sows’ milk but their provisions are not considered sufficient to meet piglet requirements( Reference Brosnan, Wijekoon and Warford-Woolgar 9 , Reference Donovan, Mar and Zeisel 17 ). The present supplementations aimed to approximately double the estimated( Reference Brosnan, Wijekoon and Warford-Woolgar 9 , Reference Donovan, Mar and Zeisel 17 ) total daily provision of creatine and choline to piglets. They can be both synthesised by the animal, a process involving methylation brought by the conversion of S-adenosyl-methionine to S-adenosyl-Hcy. Three methyl groups are required for synthesis of phosphatidylcholine and one for creatine( Reference Stead, Brosnan and Brosnan 10 ). Phosphatidylcholine can be also derived from choline through the Kennedy pathway( Reference Stead, Brosnan and Brosnan 10 ). For creatine, the major proportion of accretion in piglets comes from de novo synthesis( Reference Brosnan, Wijekoon and Warford-Woolgar 9 ) which, in turn, accounted for the major part (approximately 77 %) of the daily requirement for labile methyl groups( Reference Brosnan, Wijekoon and Warford-Woolgar 9 , Reference Stipanuk 28 ). In terms of metabolic balance of methyl groups, if the present single (treatments 3 and 4) or combined (treatments 7 and 8) provisions of choline and creatine reduced the demand of methyl groups over their endogenous synthesis, this could have triggered metabolic mechanisms for the outflow of methyl groups such as sarcosine production and degradation( Reference Mudd, Brosnan and Brosnan 29 ). In this respect, plasma concentrations of sarcosine were measured in the present study. They increased from birth to 1 d of age (13·29 (sem 0·06) to 16·26 (sem 0·08) μm, respectively), reached a maximum at 7 d of age (19·73 (sem 0·05) μm) and then declined and remained stable from 14 to 21 d of age (16·92 (sem 0·03) and 16·25 (sem 0·04) μm, respectively) (age effect; P < 0·01). There was a tendency for a treatment effect (P = 0·09) but none of the pairwise comparison among treatments was significant (P > 0·17; Table 3). These results suggest, therefore, that none of the present treatments had generated an excess of methyl groups in piglets.
Another route for the disposal of Hcy is catabolism through the trans-sulfuration pathway where the vitamin B6-dependent cystathionine β-synthase and cystathionine-γ-lyase produce cystathionine and then cysteine and glutathione( Reference Finkelstein 30 ). In the present experiment, the fate of pyridoxine in the different treatments was assessed using P-5-P in erythrocytes as the indicator according to Matte et al. ( Reference Matte, Giguère and Girard 18 ). Globally, for all treatments, P-5-P concentrations decreased by 50 % from day 0 to day 7 of lactation and remained stable thereafter until day 21, though at greater concentrations with vitamin B6-supplemented treatments 5, 6 and 8 than with others (treatment × age interaction; P = 0·02) (Fig. 3). This response on P-5-P along with the lack of treatment 5 (vitamin B6 alone) effect on Hcy contrasts with Zhang et al. ( Reference Zhang, Kebreab and Jing 31 ) who showed that the contribution of the vitamin B6-dependent trans-sulfuration pathway is crucial for the variations of homocysteinaemia in a vitamin B6 depletion–repletion model with weaned piglets. These last authors observed the maximum effect of dietary vitamin B6 on pyridoxal -phosphate and pHcy concentrations with an intake of 2·3 mg/d. In the present experiment, during the last week of lactation, the total daily intake of vitamin B6 for the suckling piglets corresponded to the oral supplement (1·2 mg) along with the vitamin B6 intake from milk which can be estimated at 1·4 mg using an average daily milk consumption of 0·8 litres and a vitamin B6 concentration in sows’ milk of 1·7 (sem 0·06) mg/l. Thus, the total daily intake of vitamin B6 in vitamin B6-supplemented treatments 5, 6 and 8 was approximately 2·6 mg, an amount comparable with the dietary level used by Zhang et al. ( Reference Zhang, Kebreab and Jing 31 ) for a maximal reduction of pHcy. However, in Zhang et al. ( Reference Zhang, Kebreab and Jing 31 ), the basal diet was not supplemented with betaine or creatine but choline was provided in their vitamin premix for a daily intake of approximately 800 mg. In the present experiment, the combination of vitamin B6 and choline (560 mg per d) supplementations was present only in treatment 8 (all supplemented nutrients) where pHcy was significantly decreased as compared with treatment 1 (control). Therefore, it appears that the synergy between these two nutrients may be non-negligible contributors to the pHcy-lowering effect observed in treatment 8. In fact, as plasma sarcosine results probably indicate that no excess of methyl groups was produced with the present supply of nutrients reducing the demand of methyl groups it cannot be excluded that the present provisions of choline and possibly creatine were not optimal for a maximal lowering effect on the development of homocysteinaemia in suckling piglets.

Fig. 3. Pyridoxal-5-phosphate concentrations in erythrocytes according to treatment and age of piglets: ○, control (treatment 1); □, betaine (treatment 2); ∆, choline (treatment 3); ×, creatine (treatment 4); ◦, pyridoxine (treatment 5); ■, betaine and pyridoxine (treatment 6); ▲, choline and creatine (treatment 7); ●, all nutrients (treatment 8). Values are least-square means (n 16, 17, 17, 17, 17, 17, 17 and 16 piglets for treatments 1, 2, 3, 4, 5, 6, 7 and 8), with standard errors represented by vertical bars. There was an age effect (P < 0·01).There was a treatment × age interaction (P = 0·02).
Conclusion
Concentrations of pHcy were reduced by 23 % with the combination of the nutrients betaine, pyridoxine, choline and creatine supplied directly to suckling piglets during lactation. Nevertheless, minimum values of pHcy remained high (>13 µm) as compared with normal concentrations in other species (<10 µm) and the question remains as to whether a greater reduction of this intermediary amino acid is achievable and, eventually, worthwhile for the growth and immune function of piglets.
Acknowledgements
The authors are grateful to M. Guillette for her technical assistance, to the animal care team under the supervision of M. Turcotte and to S. Méthot for advice related to statistical analyses.
This study was supported by a core budget of Agriculture and Agri-Food Canada. M.-E. C.-R. was awarded a Master degree scholarship of La Fédération des producteurs de porcs du Québec. The study sponsors had no role in the design and conduct of the study; the collection, management, analysis and interpretation of the data; and the preparation, approval and submission of the manuscript. M.-E. C.-R., C. L. G., F. G. and J. J. M. contributed to the design of the study, interpretation of the results, writing of the manuscript, revision of its content and approval of the final version submitted for publication. J. J. M. was the principal investigator and supervised data collection and laboratory analysis; C. L. G. supervised the statistical analysis.
None of the authors had a financial or personal conflict of interest in relation to this study.










