Nutritional and lifestyle factors are thought to be associated with a higher risk for cancer and other chronic conditions, but little is known whether guidelines from different agencies are related to indicators of lower disease risk. The World Cancer Research Fund and American Institute for Cancer Research (WCRF/AICR) emphasise recommendations to improve individual behaviour, including avoidance of tobacco products, maintaining a lean body mass, participating in moderate physical activity, consuming a primarily plant-based diet and minimising the consumption of energy-dense foods and drinks, red and processed meats and alcohol( 1 ). In two large cohort studies, participants experienced a 9 to 10 % lower mortality for each WCRF/AICR recommendation that was met( Reference Vergnaud, Romaguera and Peeters 2 , Reference Hastert, Beresford and Sheppard 3 ). Based on evidence that chronic inflammation plays a major role in cancer development( Reference Chaturvedi, Moore and Hildesheim 4 , Reference Hanahan and Weinberg 5 ), we evaluated the diet of 275 healthy premenopausal women who completed a FFQ and explored the association of adherence scores with levels of three biomarkers of antioxidant and inflammation status: serum C-reactive protein (CRP), urinary F2-isoprostane and serum γ-tocopherol. CRP represents a non-specific indicator of inflammation( Reference Albers, Antoine and Bourdet-Sicard 6 ) that has been associated with cancer incidence( Reference Ollberding, Kim and Shvetsov 7 ) and survival( Reference Cooney, Chai and Franke 8 ). Among markers of oxidative stress, F2-isoprostanes are considered the ‘gold standard’ because they are stable and specific and are only formed directly by chemical oxidation from NO x generated in vivo ( Reference Magalhaes, Segundo and Reis 9 – Reference Cracowski, Durand and Bessard 11 ). Owing to their antioxidant activity, tocopherol isomers may shield against oxidative damage( Reference Ju, Picinich and Yang 12 , Reference Constantinou, Papas and Constantinou 13 ). In particular, γ-tocopherol selectively protects cells from the DNA-damaging effects of NO x ( Reference Cooney, Franke and Harwood 14 – Reference Tanaka, Cooney, Preedy and Watson 16 ), possesses anti-inflammatory activity( Reference Jiang and Ames 17 ), and rises in response to inflammation( Reference Jiang, Lykkesfeldt and Shigenaga 18 , Reference Tanaka, Wood and Cooney 19 ). High circulating levels of γ-tocopherol, that is, >2·5 μg/ml, do not appear to reflect dietary intake, rather they represent a response to the presence of an inflammatory stimulus. Individuals with circulating levels of γ-tocopherol >2·5 μg/ml are considered hyper γ-tocopherolaemic( Reference Cooney, Franke and Wilkens 20 ); such elevated levels are associated with low vitamin D status( Reference Cooney, Franke and Wilkens 20 , Reference Chai, Bostick and Ahearn 21 ), obesity( Reference Chai, Maskarinec and Cooney 22 ), age( Reference Chai, Novotny and Maskarinec 23 ), smoking( Reference Cooney, Franke and Wilkens 20 ) and CRP( Reference Chai, Maskarinec and Cooney 22 ). Thus, γ-tocopherol may be an excellent overall marker of health risk. We hypothesised that women with high adherence to the WCRF/AICR recommendations have a more favourable inflammatory biomarker profile. In addition, we explored the association between BMI and the same biomarkers of antioxidant and inflammatory status.
Methods
Study design and population
The current analysis used baseline data from two dietary intervention studies (Fig. 1): the Breast, Estrogen, and Nutrition (BEAN1), which randomised 220 women to a 2-year clinical trial to examine the effects of two daily soya servings on sex steroids and mammographic densities( Reference Maskarinec, Franke and Williams 24 ), and BEAN2, which was conducted in a cross-over design with eighty-two women( Reference Maskarinec, Morimoto and Conroy 25 ). Only data collected at baseline before randomisation to an intervention were analysed. The protocols for both studies were approved by the University of Hawaii Committee on Human Studies and by the Institutional Review Boards of the participating hospitals. All participants signed an informed consent form before entry into the trials. As described in detail previously( Reference Maskarinec, Franke and Williams 24 , Reference Maskarinec, Morimoto and Conroy 25 ), eligibility criteria for both studies included a normal mammogram, no breast implants, no current oral contraceptive use or pregnancy, no previous cancer diagnosis, intact uterus and ovaries, regular menstrual cycles, and low soya intake. Additional criteria for BEAN2 included the ability to produce at least 10 μl nipple aspirate fluid, one of the study outcomes( Reference Maskarinec, Morimoto and Conroy 25 ). After exclusion of dropouts and women with incomplete data, the current analysis included 275 women who provided biological samples and complete nutritional information from the baseline FFQ that could be used to score adherence to the WICR/AICR recommendations (Table 1).

Fig. 1 Flow chart for recruitment and study population of the Breast, Estrogen, and Nutrition 1 (BEAN1) Study (a) and the Breast, Estrogen, and Nutrition 2 (BEAN2) Study (b). NAF, nipple aspirate fluid.
Table 1 Scoring for World Cancer Research Fund and American Institute for Cancer Research (WCRF/AICR) recommendations based on data from baseline FFQ (Number of women and percentages)

N/A, not available.
Data collection
All participants completed a previously validated FFQ at baseline that included questions on habitual dietary intake during the last year, physical activity, smoking status, medical history and reproductive characteristics( Reference Stram, Hankin and Wilkens 26 ). The FFQ had eight frequency categories for foods and nine for beverages. Respondents could choose from three typical serving sizes and photographs were included to help visualise proportions for selected foods. BMI was calculated from baseline weight and height. The FFQ were analysed utilising the Food Composition Table maintained by the Nutrition Support Shared Resource at the Cancer Center( Reference Murphy 27 ); the databases represent an extensive list of local foods consumed by the various ethnic populations of Hawaii and the Pacific. Food servings were defined according to the Food Guide Pyramid( Reference Sharma, Murphy and Wilkens 28 ).
Sample collection and assays
Serum and urine samples were collected during the midluteal phase using ovulation kits in BEAN1 and confirmation by serum progesterone levels( Reference Maskarinec, Franke and Williams 24 ) and self-reported menstruation information in BEAN2( Reference Maskarinec, Morimoto and Conroy 25 ). For this analysis, CRP and serum γ-tocopherol were available for BEAN1 participants only and urinary F2-isoprostane levels for BEAN2 women only. All specimens were stored at − 80°C after aliquoting. The CRP assay was based on a latex particle enhanced immunoturbidimetric method using a Cobas MiraPlus clinical autoanalyser and a kit from Pointe Scientific, Inc., with a detection limit of 0·1 mg/l( Reference Maskarinec, Steude and Franke 29 ). Serum samples were analysed for γ-tocopherol by reverse phase HPLC with photodiode array detection between 220 and 600 nm( Reference Franke, Custer and Cooney 30 , Reference Franke, Cooney and Henning 31 ). The assay was regularly validated during the analysis by inclusion of external standards within each sample batch and through successful participation in the quality assurance program organised by US National Institute of Standards and Technology. Levels of urinary 15-F2t-isoprostane were measured using an enzyme-linked immunosorbent assay kit (Oxford Biomedical Research) in six batches( Reference Sen, Morimoto and Heak 32 ).
Statistical analysis
All statistical analyses were performed using SAS, release 9.4 (SAS Institute, Inc.). A scoring system for eight of the ten recommendations by the WCRF/AICR for cancer prevention( 1 ) was modeled similar to a previous publication( Reference Hastert, Beresford and Sheppard 3 ). Participants were given a score of 1 or 0 depending on if they met or did not meet a recommendation (Table 1), and the adherence scores were classified as low ( < 5), moderate (5–6), and high ( ≥ 7). Analyses for BMI status were based on pre-determined BMI categories: normal ( < 25 kg/m2), overweight (25– < 30 kg/m2) and obese ( ≥ 30 kg/m2). Because only three women had a BMI < 18·5 kg/m2, these participants were excluded from the analyses. An indicator variable with a cut-off of 2·5 μg/ml for hyper γ-tocopherolaemic status was created. Means and standard deviations for dietary and lifestyle habits as well as biomarkers were computed by level of adherence and by BMI status. Non-normally distributed variables were log-transformed to obtain P-values for trend tests across categories using analysis of variance. In adjusted models, we included age, parity, ethnicity and timing of biospecimen collection within the luteal phase (yes or no) as covariates and expressed the differences as least square means with 95 % CI. Finally, we examined the distribution of hyper γ-tocopherolaemia by adherence category and computed OR and 95 % CI in a logistic regression model with low adherers as the reference group.
Results
The study population (Table 2) consisted of 41 % whites, 36 % Asians, 14 % Native Hawaiians and 9 % Others with a mean age of 41·9 (sd 4·5) years. The mean BMI was 26·1 (sd 5·7) kg/m2 with 27 % classified as overweight and 22 % as obese. Of all biospecimens, 87 % were collected during the luteal phase. The majority of participants (Table 1) were never smokers (65 %), met the physical activity guideline (65 %) and reported drinking one or fewer alcoholic beverages per day (90 %). As to nutritional recommendations, 88 % adhered to low red/processed meat intake, 71 % to low soda intake, but only 48 % limited salt intake to < 2400 mg/d, and 32 % consumed adequate amounts of vegetables, fruits and whole grains. The respective means for CRP, γ-tocopherol and F2-isoprostances were 2·1 mg/l, 1·7 μg/ml and 14·6 ng/ml. Levels of serum γ-tocopherol and CRP were significantly correlated 0·24 (P= 0·001).
Table 2 Baseline characteristics of 275 premenopausal women from two intervention studies* (Number of premenopausal women and percentages; mean values and standard deviations)

BEAN, Breast, Estrogen, and Nutrition.
* Percentages may not add to 100 due to rounding.
† Serum γ-tocopherol and C-reactive protein (CRP) were available for BEAN1 only and urinary F2-isoprostane levels for BEAN2 only. Data were missing for serum γ-tocopherol (n 23) and serum CRP (n 12).
Overall, seventy-two women were classified as low-adherers, 150 as moderate adherers, and fifty-three as high-adherers (Table 3). The low adherers had a mean BMI of 30·3 (sd 6·0) kg/m2, while the mean BMI of the high adhering women was 22·2 (sd 2·0) kg/m2. High adherers were also more physically active (P trend< 0·0001), consumed less red/processed meat (P trend< 0·0001), lower percentage total energy from fat (P trend= 0·002), less salt (P trend< 0·0001) and more dietary fibre (P trend= 0·01) and more fruits and vegetables (P trend= 0·002). The unadjusted means for CRP (BEAN1 only) were 2·7, 2·0 and 1·7 mg/l for low, moderate and high adherers (P trend= 0·03); this association was strengthened after adjustment for potential confounders with least square means for log-transformed CRP values of 1·12, 0·87 and 0·71 mg/l (P trend= 0·006). The respective values for serum γ-tocopherol (BEAN1 only) were 1·97, 1·63 and 1·45 μg/ml (P trend= 0·02) before and 1·10, 0·96, and 0·91 μg/ml (P trend= 0·03) after adjustment. However, for urinary F2-isoprostane (BEAN2 only), the higher values in low adherers (16·0, 14·5, and 13·3 ng/ml) did not reach statistical significance (P trend= 0·18). In the adjusted models, the respective F2-isoprostane values were 2·74, 2·66 and 2·60 ng/ml (P trend= 0·21).
Table 3 Characteristics of women by adherence to World Cancer Research Fund and American Institute for Cancer Research recommendations* (Number of women and percentages; mean values and standard deviations)
CRP, C-reactive protein.
* Percentages may not add to 100 due to rounding.
† P trend values were computed for continuous variables using log-transformed data except for age and % total energy from fat. P values for categorical variables were computed using χ2 or Fisher's exact test. Adjusted model included age, parity, ethnicity, and timing of biospecimen collection within the luteal phase (yes or no) as covariates.
‡ Serum γ-tocopherol and CRP were available for Breast, Estrogen, and Nutrition 1 (BEAN1) only and urinary F2-isoprostane levels for Breast, Estrogen, and Nutrition 2 (BEAN2) only. Data were missing for serum γ-tocopherol (n 23) and serum CRP (n 12).
The inverse associations for the biomarkers with higher adherence (Fig. 2) were more pronounced for CRP (P trend< 0·01) and γ-tocopherol (P trend< 0·01) than for F2-isoprostane (P trend= 0·27). Whereas the mean levels by adherence score were more or less flat for F2-isoprostane, serum γ-tocopherol showed a continuous decline from >2·0 to < 1·5 μg/ml across categories. We observed smaller proportions of women with hyper γ-tocopherolaemia among moderate and high adherers than in low adherers (17 and 12 v. 25 %, respectively). However, the respective OR of 0·60 (95 % CI 0·26, 1·41) and 0·41 (95 % CI 0·12, 1·42) for moderate and high adherers v. low adherers were not statistically significant. For CRP, the levels were up to twice as high for the three lower categories than for women scoring 6 to 8.
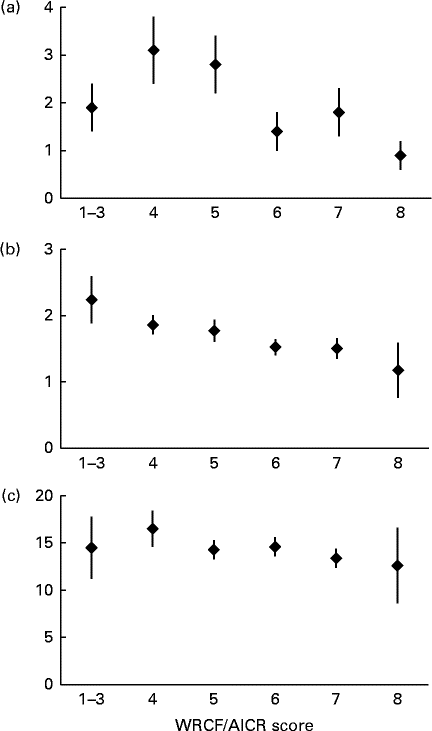
Fig. 2 Mean biomarker levels and standard errors by World Cancer Research Fund and American Institute for Cancer Research (WCRF/AICR) score. (a) Serum C-reactive protein (mg/l, P trend< 0·01), (b) serum α-tocopherol (μg/ml, P trend< 0·01) and (c) urinary F2-isoprostane (ng/l, P trend= 0·27). Missing data were recorded for (a) (n 23) and (b) (n 12). P trend values were computed using log-transformed biomarker levels.
When participants were grouped by BMI category (Table 4), red/processed meat, percentage of total energy from fat and Na intake were significantly higher and physical activity significantly lower in overweight and obese than normal weight women. AICR scores not including BMI were lowest in the obese group, followed by overweight and normal weight women with respective values of 4·3 (sd 1·0), 4·8 (sd 1·2) and 5·1 (sd 1·0). The levels of CRP, γ-tocopherol and F2-isoprostane were substantially higher in overweight and obese than normal weight women; these trends remained unchanged after adjustment for age, ethnicity, parity and luteal phase. However, the linear trend was only statistically significant for CRP and γ-tocopherol with log-transformed values of 0·56, 0·96 and 1·38 (P trend< 0·0001) and 7·20, 7·40 and 7·55 (P trend= 0·008), respectively. The respective least square means for F2-isoprostane were 2·64, 2·57 and 2·83 (P trend= 0·21) across BMI categories.
Table 4 Characteristics of women by BMI category* (Mean values and standard deviations; number of women and percentages)

CRP, C-reactive protein; WCRF/AICR, World Cancer Research Fund and American Institute for Cancer Research.
* Percentages may not add to 100 due to rounding. Three women with a BMI < 18·5 kg/m2 were not included in the analysis.
† P trend values were computed for continuous variables using log-transformed data except for age and % total energy from fat. P values for categorical variables were computed using χ2 or Fisher's exact test. Adjusted model included age, parity, ethnicity, and timing of biospecimen collection within the luteal phase (yes or no) as covariates.
‡ Serum γ-tocopherol and CRP levels are available for Breast, Estrogen, and Nutrition 1 (BEAN1) only and urinary F2-isoprostane levels are available for Breast, Estrogen, and Nutrition 2 (BEAN2) only. Missing data were recorded for serum γ-tocopherol (n 23) and serum CRP (n 12).
§ BMI individual score was removed from the AICR score.
Discussion
In the present study of multi-ethnic premenopausal women, many women met a high proportion of the eight recommendations operationalised according to the WCRF/AICR cancer prevention guidelines, in particular, limiting the consumption of energy-dense food such as sodas, red/processed meat and alcohol. Women with lower adherence scores had a higher BMI and reported higher intakes of unfavourable foods and behaviours. The results of this study suggest a weak inverse relation between adherence to nutritional and lifestyle recommendations and two of the three markers of antioxidant and inflammatory status examined. Specifically, γ-tocopherol and CRP, but not F2-isoprostane, were lower in women with higher adherence scores. In a similar pattern, overweight and obese women had substantially higher levels of all three biomarkers than normal weight women; in particular, CRP was nearly fivefold higher in obese women. In response to the inflammatory stress marked by low adherence scores and excess body weight, γ-tocopherol appears to rise as a physiological mechanism to protect the body against inflammation-related damage. Although a well-established marker of inflammation, urinary F2-isoprostane showed little association with adherence scores and BMI status.
To our knowledge, this is the first analysis to examine the association between WCRF/AICR recommendations and biomarkers of inflammation and antioxidant status. Previous studies investigated adherence to WCRF/AICR recommendations with morbidity and mortality in women. For example, a 22 to 24 % lower cancer-specific mortality for meeting one to two recommendations and 61 % lower for meeting five to six recommendations were reported( Reference Hastert, Beresford and Sheppard 3 ). A larger cohort observed similar results with a 34 % lower mortality for individuals who met six to seven recommendations( Reference Vergnaud, Romaguera and Peeters 2 ). The association between excess body weight and elevated CRP levels is well known and has been described for many populations( Reference Das 33 , Reference Fogarty, Glancy and Jones 34 ).
The association of overweight with the biomarkers may be due to cytokines produced in adipose tissue or due to direct effects of dietary components associated with a high BMI. For example, obese women consumed more red/processed meat, fat and salt, food components that were related to higher γ-tocopherol in a previous report( Reference Fung, Rimm and Spiegelman 35 ). While dietary intake of γ-tocopherol and blood levels are poorly correlated( 36 – Reference Herrera and Barbas 39 ), red/processed meat consumption is thought to be related to inflammatory responses during digestion, which may lead to an increase in circulating γ-tocopherols( Reference Montonen, Boeing and Fritsche 40 ). The stimulation of inflammatory cytokines by excess adiposity may also influence γ-tocopherol levels( Reference Cooney, Franke and Wilkens 20 , Reference Allin and Nordestgaard 41 ). Among antioxidants, γ-tocopherol appears to be unique, in that higher circulating levels are directly associated with oxidative stress, whereas most antioxidants are reduced under conditions of oxidative stress( Reference Jiang, Lykkesfeldt and Shigenaga 18 , Reference Tanaka, Wood and Cooney 19 ). The wide range of adverse conditions associated with elevated γ-tocopherol, and the general correlation with risk factors in the present study indicate it may be a useful integrative marker for assessing future disease risk. The occurrence of hyper γ-tocopherolaemia was weakly related to lower overall adherence scores without reaching statistical significance, suggesting a higher cutoff for defining this state might be indicated. Since very few supplements contain γ-tocopherol, it is unlikely that high circulating γ-tocopherol levels are due to supplement intake. While γ-tocopherol accounts for 80 % of tocopherols in the diet, most is excreted or metabolised in healthy people( 36 , Reference Herrera and Barbas 39 ).
Strengths of the present study include the ethnic diversity of the women, as well as the use of a previously validated FFQ( Reference Stram, Hankin and Wilkens 26 ). We analysed baseline nutritional data prior to implementation of the study interventions, thereby capturing usual dietary and lifestyle habits from the year before dietary changes were initiated. Given the generally good health status of the participants, it is unlikely that unknown, underlying conditions affected the biomarker levels. By contrast, given the healthy status of the women, their high adherence to lifestyle recommendations, and the strict eligibility criteria, the participants probably represented a relatively narrow range in biomarker levels. Therefore, the results may not be generalisable to a population with a higher prevalence of chronic conditions. The study was also limited by the small sample size and the low statistical power, the lack of a general energy-density variable, and the absence of information on dietary supplement intake.
Diet–disease associations are difficult to determine due to the length of time required for quantifiable symptoms to be expressed and chronic diseases to develop. Elevated levels of biomarkers in healthy people may help to identify persons at a higher risk for chronic disease development later in life. The fact that WCRF/AICR adherence scores and BMI status showed similar associations with the three biomarkers suggests that the type of foods consumed may be less important in determining future disease risk than the excess body weight resulting from poor dietary patterns. The current findings suggest that adherence to the WCRF/AICR guidelines is associated with lower levels of biomarkers that indicate oxidative stress and inflammatory status, in particular, serum CRP and γ-tocopherol, and may lower future disease risk.
Acknowledgements
We are very grateful to the dedicated study participants.
This work was supported by grants R01CA80843 (principal investigator: G. M.) and P30CA71789 (principal investigator: M. Carbone) from the National Cancer Institute. The funding agency had no role in the design, analysis or writing of this article.
None of the authors has conflict of interest.
The authors' contributions are as follows: G. M. conceived the original studies, obtained funding, supervised the data analysis and finalised the manuscript. R. V. C. and A. A. F. provided laboratory results and interpreted the results. Y. M. and F. B. collated the statistical information and drafted the manuscript. All authors read and approved the findings of the study.











