Carotenoids are natural pigments, synthesised by plants and micro-organisms, but not by animals or humans. These pigments are found in food, especially in fruits and vegetables. Large epidemiological studies suggest a protective effect of a high intake of fruits and vegetables on all-cause mortality(Reference Agudo, Cabrera and Amiano1–Reference Steffen, Jacobs, Stevens, Shahar, Carithers and Folsom5). Consumption of fruits and vegetables could have a protective effect on stroke and CHD(Reference Dauchet, Amouyel and Dallongeville6–Reference Joshipura, Hu and Manson10). Concerning cancer, the benefits of fruit and vegetable intake are more controversial and the potential protective effect seems to depend on the type of cancer. Some studies did not show evidence of a strong association with ovarian cancer(Reference Koushik, Hunter and Spiegelman11), breast cancer(Reference van Gils, Peeters and Bueno-de-Mesquita12), overall colon rectal cancer(Reference Koushik, Hunter and Spiegelman13, Reference Lin, Zhang, Cook, Rexrode, Liu, Manson, Lee and Buring14) and renal cell carcinoma(Reference Weikert, Boeing and Pischon15). However, some studies suggest potential benefits of fruit and vegetable consumption for some other cancers such as cancer of the upper aero-digestive tract(Reference Boeing, Dietrich and Hoffmann16) and lung cancer(Reference Neuhouser, Patterson, Thornquist, Omenn, King and Goodman17, Reference Smith-Warner, Spiegelman and Yaun18).
More information is needed to ascertain the association between the intake of single nutrients, such as carotenoids, and the risk of all-cause mortality.
The hypothetical protective role of carotenoids could come from their antioxidant properties(Reference Paiva and Russell19). Literature on the implication of free radicals in the ageing process is well documented(Reference Finkel and Holbrook20, Reference Harman21) but the relationship between total plasma carotenoids and mortality in free-living elderly populations via their antioxidant roles has not been previously studied. Other underlying mechanisms such as inflammation mechanisms or immunomodulatory mechanisms have also been mentioned(Reference Paiva and Russell19).
Our objective is to explore the relationships between total plasma carotenoids at baseline and 9-year mortality risk in a healthy elderly population.
Experimental methods
Study population
The ‘Epidemiology of Vascular Ageing’ (EVA) study is a 9-year longitudinal study with six follow-up periods(Reference Akbaraly, Arnaud, Hininger-Favier, Gourlet, Roussel and Berr22, Reference Berr, Coudray, Bonithon-Kopp, Roussel, Mainard and Alperovitch23). During the first 2 years, 1991–1993 (EVA0), 1389 volunteers (575 men and 814 women, age range 59–71 years) residing in the town of Nantes (Western France) were recruited from electoral rolls, and to a lesser extent, via information campaigns. All subjects were community residents and underwent a complete examination in the EVA study centre where they spent half a day. The last follow-up of the EVA study (EVA6) was conducted between June 2000 and December 2001. The study protocol was approved by the Ethical Committee of the University Centre Hospital of Kremlin-Bicêtre (Paris). Signed informed consent was obtained from all participants at enrolment.
Data collection
Vital statistics and date and cause of death were collected throughout the 9 years of follow-up. At each of the EVA steps, and at the end of the last year of study, the vital status of individuals for whom we had no feedback was collected from town hall civil registries. The cause of death was determined with the help of both the subject's general practitioner and family.
At baseline, the general questionnaire allowed us to obtain information on sociodemographic factors such as sex, age, educational level ( ≤ primary school or ≥ high school), plus lifestyle habits such as smoking habit (current and ex-smokers or non-smokers) and alcohol intake ( ≥ 20 ml or < 20 ml per d). In addition, height and weight were measured. Two independent measures of systolic and diastolic blood pressure were taken with a digital electronic tensiometer (SP9 Spengler) after a 10 min rest. Cognitive performances were assessed using the Mini Mental Status Examination(Reference Folstein, Folstein and McHugh24). Blood samples were collected between 08.30 and 09.30 hours after a 12 h fast. Total plasma cholesterol and plasma glucose levels were measured using standard methods.
Health characteristics considered in this analysis were Mini Mental Status Examination score, BMI, diabetes status (plasma glucose level ≥ 7·80 mmol/l, use of anti-diabetic drugs or diabetes medical history), dyslipidaemia (total cholesterol ≥ 6·20 mmol/l, use of lipid-lowering drugs or dyslipidaemia medical history), hypertension (systolic or diastolic blood pressure ≥ 140 or ≥ 90 mmHg respectively, or use of hypertensive drugs or hypertension medical history), history of vascular diseases (self-reported history of myocardial infarction, angina pectoris, stroke).
Laboratory procedures
Spectrophotometric assay of plasma carotenoids
After precipitation of plasma proteins with ethanol, carotenoids were extracted with hexane and measurements of absorbance on the hexane phase at 350, 450 and 550 nm were performed (spectrophotometer Uvikon 860; Kontron, Rotkreuz, Switzerland). Concentrations were calculated on the basis of a molecular extinction factor at 450 nm of 134 000 litres/mol per cm. Absorbance values at 350 and 550 nm were used to correct the absorbance obtained at 450 nm by applying an adequate equation. Coefficients of intra- and inter-assay variations were 5·4 and 4·9 %, respectively.
Thiobarbituric acid-reactive substances and plasma selenium determination
Plasma levels of thiobarbituric acid-reactive substances (TBARS) were determined by a fluorometry method as described by Richard et al. (Reference Richard, Portal, Meo, Coudray, Hadjian and Favier25) and described previously(Reference Berr, Richard, Roussel and Bonithon-Kopp26). Se was determined in serum using electrothermal atomic absorption spectrometry (Perkin Elmer 5100 ZT; Norwalk, CT, USA) according to Arnaud et al. (Reference Arnaud, Prual, Preziosi, Favier and Hercberg27) and described previously(Reference Akbaraly, Arnaud, Hininger-Favier, Gourlet, Roussel and Berr22).
Statistics
Survival was analysed with actuarial methods, and Wilcoxon tests were used to compare survival between total plasma carotenoid quintile groups. Associations between total plasma carotenoids and mortality were determined by Cox proportional hazards regression models in which year of age during the study was used as the time axis, with left truncation at the age of study entry. Multivariate analyses were adjusted for potential confounding variables and similar analyses were repeated after additionally taking into account TBARS levels and plasma Se levels (analysed as continuous variables). The proportional hazards assumption was verified by adding a time-dependent variable to the model(Reference Collett28). In these analyses, total plasma carotenoid level was considered by quintiles defined in each sex and was also considered as a continuous variable when the strength of analyses was too small to allow a categorical treatment. Results of Cox multivariate regressions were expressed by hazard ratios (HR) with their 95 % CI. All interactions between total plasma carotenoids and other variables were tested. Statistical analyses were performed using SAS software version 9.1 (SAS Institute, Inc., Cary, NC, USA).
Results
Of the 1389 study participants included in the analyses, 1283 had measurements of total plasma carotenoids and complete information on covariables. Characteristics according to sex are shown in Table 1. During the 9-year follow-up, ninety-three deaths occurred, with a higher rate in men than in women (sixty-one men, thirty-two women; P ≤ 10− 4). A higher mortality rate was observed in current and former smokers, in regular alcohol consumers, in participants with low concentration of plasma Se and with high BMI and participants with diabetes, hypertension and CVD (results not shown).
Table 1 Characteristics of 1283 participants included in the analyses according to sex
(Mean values and standard deviations)

MMSE, Mini Mental State Examination; TBARS, thiobarbituric acid-reactive substances.
* Measurements available for 484 men and 687 women.
Total plasma carotenoid level was significantly higher in women (3·08 (sd 1·33) μmol/l) than in men (2·19 (sd 0·99) μmol/l) (Table 1) and a discrepancy in the distribution was observed between men and women (Fig. 1).
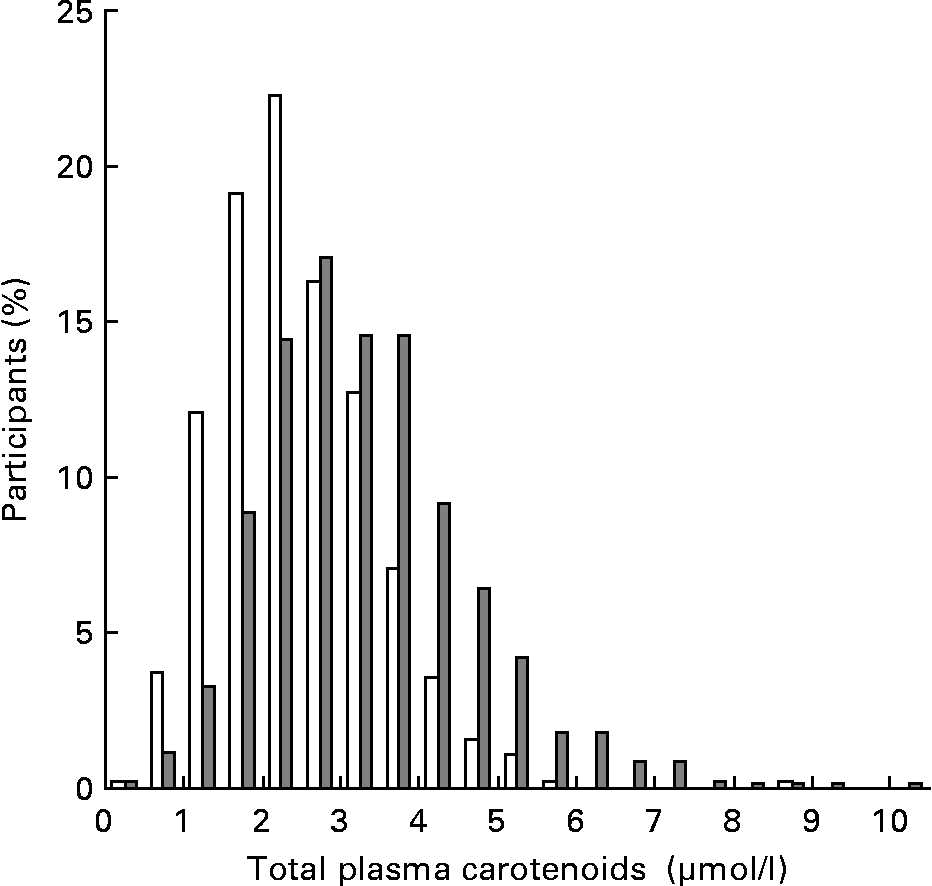
Fig. 1 Distribution of total plasma carotenoid levels in men (□) and women (![]() ).
).
Means of total plasma carotenoids were significantly higher in surviving individuals (2·75 (sd 1·27) μmol/l) than in those who died (2·12 (sd 1·12) μmol/l) (P < 10− 4). This association was found to be sex-dependent. The relationship was found to be significant for men (2·24 (sd 0·97) v. 1·76 (sd 0·94) μmol/l; P = 0·0002) but not for women (3·09 (sd 1·34) v. 2·83 (sd 1·11) μmol/l; P = 0·27). Comparison of survival distributions among total plasma carotenoid quintiles shows that mortality increased in subgroups with the lowest percentile groups of total plasma carotenoids in men but not in women (Fig. 2).

Fig. 2 (a) Survival distributions for each total plasma carotenoid quintile group exclusively in men: quintile (Q) 1, < 1·36 μmol/l (♦); Q2, 1·36–1·86 μmol/l (![]() ); Q3, 1·86–2·3 μmol/l (+); Q4, 2·3–2·9 μmol/l (
); Q3, 1·86–2·3 μmol/l (+); Q4, 2·3–2·9 μmol/l (![]() ); Q5, ≥ 2·9 μmol/l (▲). (b) Survival distributions for each total plasma carotenoid quintile group exclusively in women: Q1, < 2·0 μmol/l (♦); Q2, 2·0–2·60 μmol/l (
); Q5, ≥ 2·9 μmol/l (▲). (b) Survival distributions for each total plasma carotenoid quintile group exclusively in women: Q1, < 2·0 μmol/l (♦); Q2, 2·0–2·60 μmol/l (![]() ); Q3, 2·60–3·25 μmol/l (+); Q4, 3·25–4·04 μmol/l (
); Q3, 2·60–3·25 μmol/l (+); Q4, 3·25–4·04 μmol/l (![]() ); Q5, ≥ 4·04 μmol/l (▲).
); Q5, ≥ 4·04 μmol/l (▲).
The bivariate Cox proportional hazard regression (Table 2) model showed that men in the lowest quintile (Q) of total plasma carotenoids had a significantly higher risk of mortality than men in the highest (HRQ1v.Q5 4·08 (95 % CI 1·77, 9·45)). No significant association was found in men who had a plasma carotenoid level within Q2, Q3 or Q4 compared with subjects in Q5 (Q2 v. Q5, HR 1·69 (95 % CI 0·65, 4·36); Q3 v. Q5, HR 1·07 (95 % CI 0·38, 3·05); Q4 v. Q5, HR 1·24 (95 % CI 0·46, 3·34)). The global P value in men was P = 0·0003. No significant association (P = 0·20) was found in women (Q1 v. Q5, HR 0·61 (95 % CI 0·18, 2·11); Q2 v. Q5, HR 1·73 (95 % CI 0·68, 4·39); Q3 v. Q5, HR 0·59 (95 % CI 0·17, 2·03); Q4 v. Q5, HR 0·74 (95 % CI 0·23, 2·33)).
Table 2 Association between total plasma carotenoid level by quintile (Q) and all-cause mortality: results of Cox proportional hazards regression analysis
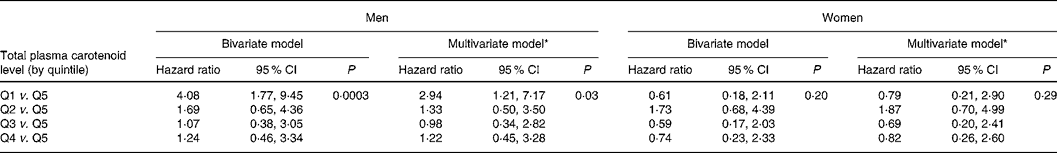
* Model adjusted for sociodemographic factors (age, education level, marital status), lifestyle habits (smoking habits and alcohol intake) and health factors (diabetes, hypertension, cardiovascular antecedents, dyslipidaemia and BMI).
At baseline, a significant association was observed between concentration of plasma carotenoids and education level in women (lower concentrations observed in women with low education level) and marital status in men (higher concentrations in married men). In both sexes, a lower total plasma carotenoid concentration was also observed in participants who were regular alcohol consumers, in participants with diabetes, hypertension, CVD history, and a higher concentration was observed in dyslipidaemic participants. Plasma carotenoid concentrations were also negatively correlated with BMI and positively correlated with plasma Se in both sexes and with TBARS levels in women.
The association between total plasma carotenoid levels and 9-year risk mortality was analysed after adjustment for all factors associated with mortality and/or with total plasma carotenoids in each sex separately; results are presented in Table 2. The multivariate Cox hazard proportions regression models showed that low levels of plasma carotenoids were associated with higher mortality risk in men but not in women after controlling for age, education level, marital status, smoking habits, alcohol intake and health factors (diabetes, hypertension, cardiovascular antecedents, dyslipidaemia, BMI). The HR of 9-year mortality in men with plasma carotenoid levels in the lowest quintile compared with men with plasma carotenoid levels in the highest quintile was 2·94 (95 % CI 1·21, 7·17). No significant association was found for men who had a plasma carotenoid level within Q2, Q3 or Q4 compared with subjects in Q5 (Q2 v. Q5, HR 1·33 (95 % CI 0·50, 3·50); Q3 v. Q5, HR 0·98 (95 % CI 0·34, 2·82); Q4 v. Q5, HR 1·22 (95 % CI 0·45, 3·28)), suggesting a threshold effect.
Similar analyses were performed after adjusting for (1) TBARS levels, which could be considered as biological markers of oxidative stress; (2) plasma Se level, which was found to be associated with all-cause mortality in both sexes. After these supplemental adjustments, total plasma carotenoid levels still remained associated with 9-year mortality risk in men: global P value = 0·04 after adjustment for TBARS (Q1 v. Q5, HR 2·67 (95 % CI 1·08, 6·61)) and global P value = 0·04 after adjustment for plasma Se (Q1 v. Q5, HR 2·52 (95 % CI 1·03, 6·21)). Total plasma carotenoids remained unrelated to mortality in women.
The cause of death was determined by the subject's general practitioner for 88·1 % of subjects. Cancer was the first leading cause of death (n 45; 44·5 %). Men who died of cancer had a significantly lower total plasma carotenoid mean compared with those in surviving individuals (2·34 (sd 0·97) v. 3·09 (sd 1·34) μmol/l; P = 0·0002). Results of Cox models showed a significant association between total plasma carotenoid level analysed as continuous variables and cancer mortality risk in men (unit = 1 μmol/l; HR 1·85 (95 % CI 1·14, 3·03); P = 0·01) but not in women (HR 1·07 (95 % CI 0·75, 1·56); P = 0·67). After taking into account the sociodemographic, life habits and health variables, this association in men remained significant (HR 1·72 (95 % CI 1·02, 2·86); P = 0·04).
Discussion
The present study shows that low total plasma carotenoid level was significantly associated with all-cause mortality and mortality by cancer, in men but not in women, after controlling for the main potential confounding factors. We also highlighted that this association was independent of plasma Se level, which was found to be significantly associated with all-cause mortality in this population(Reference Akbaraly, Arnaud, Hininger-Favier, Gourlet, Roussel and Berr22).
The EVA study included a large number of volunteers, whose educational status and cognitive function levels are known to be linked with mortality risks, and this proportion is higher in the EVA cohort than in the average French elderly population. Despite this selection, total plasma carotenoid concentrations were within the same ranges as those of different European populations.
The lower total plasma carotenoid concentrations in men compared with women in our cohort has been described in several epidemiological studies(Reference De Waart, Schouten, Stalenhoef and Kok29, Reference Olmedilla, Granado and Southon30), especially for β-carotene(Reference Hercberg, Preziosi, Galan, Devanlay, Keller, Bourgeois, Potier de Courcy and Cherouvrier31, Reference Wallstrom, Wirfalt, Lahmann, Gullberg, Janzon and Berglund32). Regarding the large discrepancy in the distribution of total plasma carotenoid concentration between the two sexes, as the threshold of the lowest quintile in women ( < 2·0 μmol/l) was about the median in men, the lack of evidence of association between total plasma carotenoid concentration and mortality in women could be explained by the fact that it is only participants with very low levels who have a higher risk of mortality. Additionally, the higher number of deaths observed in men than in women (61 v. 32) could also participate to the lack of evidence of an association in women. This difference of concentrations of plasma carotenoids between men and women could result from a higher fruit and vegetable intake in women than in men explainable by exogenous factors such as socio-cultural factors leading to better dietary habits in women – a gender difference – but we can not exclude the effect of endogenous factors in the hormonal differences or lipid and nutrient transport differences(Reference Hercberg, Galan, Preziosi, Bertrais, Mennen, Malvy, Roussel, Favier and Briançon33) – a sex difference. Anyway, the differences between men and women for carotenoid distributions and mortality rate led us to conclude that only stratified analyses on sex should be undertaken to investigate and better understand relationships between plasma carotenoid level and mortality risk.
Our finding was supported by results from the MacArthur studies on Successful Aging(Reference Hu, Reuben, Crimmins, Harris, Huang and Seeman34), in which low levels of serum β-carotene (median value ≤ 0·17 μmol/l) were significantly associated with 7-year all-cause mortality in men (OR 2·30 (95 % CI 1·23, 4·31)) but not in women (OR 0·85 (95 % CI 0·42, 1·75)). In the Women's Health and Aging studies (n 632; 70–79 years)(Reference Ray, Semba, Walston, Ferrucci, Cappola, Ricks, Xue and Fried35) a significant link was found in women between higher total serum carotenoids and a lower risk of mortality (for 1 sd increase of log total carotenoids, relative risk 0·77 (95 % CI 0·64, 0·84)). However, we noted in this study that women's geometric means of total serum carotenoids were very low (mean 1·63 μmol/l). In non-stratified analyses, two other studies showed an association between high levels of carotenoids compounds and lower mortality risk. First, in the European study ‘Survey in Europe on Nutrition and the Elderly, a Concerted Action’ (SENECA) (n 1168; 70–75 years)(Reference Buijsse, Feskens, Schlettwein-Gsell, Ferry, Kok, Kromhout and de Groot36), plasma carotene concentrations were significantly associated with a lower mortality risk (for an increment of 0·39 μmol/l, relative risk 0·79 (95 % CI 0·70, 0·89)). Second, in a study on 638 independently living elderly subjects aged 65–85 years(Reference De Waart, Schouten, Stalenhoef and Kok29), analyses of tertiles of carotenoids showed a significant link between all-cause mortality and xanthophyll carotenoids, but not with total serum carotenoids even if tests for trends were significant (P = 0·02). Discordance of the results according to the carotenoid compounds studied could come from the fact that all carotenoid compounds have not the same biological properties. Finally, in another study led by Fletcher et al. on 1214 subjects (75–84 years)(Reference Fletcher, Breeze and Shetty37), the relationship between plasma β-carotene and all-cause mortality during the 4·4-year follow-up did not remain statistically significant after adjustment for potential confounding factors. The absence of a significant link could be explained by a sex effect, which was not reported, or by the advanced age of the population or more probably by relatively higher baseline levels of plasma β-carotene in this population.
Concerning the randomised trials, two randomised controlled trials led in the general population have investigated the effects of supplementation on the incidence of cancer and all-cause mortality(Reference Hercberg, Galan, Preziosi, Bertrais, Mennen, Malvy, Roussel, Favier and Briançon33, Reference Blot, Li and Taylor38). In the Linxian trial conducted on 29 584 adult subjects(Reference Blot, Li and Taylor38) a significantly lower 5-year total mortality risk occurred among those receiving supplementation with β-carotene, vitamin E and Se. In the primary prevention trial SU.VI.MAX including 13 017 French adults(Reference Hercberg, Galan, Preziosi, Bertrais, Mennen, Malvy, Roussel, Favier and Briançon33) a significant protective effect of 7·5 years' combined antioxidant including β-carotene supplementation on all-cause mortality was observed in men but not in women. In this trial, the effect of supplementation was also studied after stratification on initial antioxidant plasma levels. A net benefit was observed only in men with a low status of β-carotene or ascorbate but not in women(Reference Galan, Briançon and Favier39). However, in these combined multi-antioxidant supplementation studies, it is impossible to isolate the proper effect of carotenoids on mortality. However, the recent meta-analyses led by Bjelakovic et al. (Reference Bjelakovic, Nikolova, Gluud, Simonetti and Gluud40), carried out on sixty-eight randomised trials with 232 606 participants, showed that supplementation of β-carotene singly or combined significantly increased mortality. One explanation could be that instead of having a role in the pathogenesis of many chronic diseases, oxidative stress may be a consequence of pathological conditions. By eliminating free radicals from our organism, we interfere with some essential defensive mechanisms(Reference Bjelakovic, Nikolova, Gluud, Simonetti and Gluud40). In this meta-analysis the authors did not take into account sex as a covariable which could influence the intervention effect across the trials and constitutes a limitation for interpreting their results.
When considering the underlying causes of death, we found a significant association between low total plasma carotenoids and higher cancer mortality in men. The present results should be viewed with some caution, given that only forty-five cancer deaths occurred. While low intake or having a low serum concentration of β-carotene was suggested to be associated with an elevated risk of cancer by epidemiological studies(Reference Peto, Doll, Buckley and Sporn41) and by one large randomised trial conducted in China, in the early 1980s(Reference Blot, Li and Taylor38), the results of recent trials recommend caution concerning the potential benefit of supplementation of carotenes, by showing a higher rate of lung cancer in smoker participants who received a supplement containing β-carotene compared with those receiving placebo in the ATBC Study(42–Reference Virtamo, Pietinen, Huttunen, Korhonen, Malila, Virtanen, Albanes, Taylor and Albert44). Then, the association between carotenoids and cancer seems to be specific to the cancer site. Our data did not allow investigating associations between total plasma carotenoids on specific cancer sites.
In a previous study, we showed a significant association between low plasma Se levels and all-cause mortality risk and cancer mortality in both sexes(Reference Akbaraly, Arnaud, Hininger-Favier, Gourlet, Roussel and Berr22). In the present analyses, after adjustment on plasma Se levels, associations between total plasma carotenoids and mortality risk remained statistically significant, suggesting that plasma Se and plasma carotenoids have each of them a proper protective effect on mortality risk. This result was supported by the Women's Health and Aging Studies results(Reference Ray, Semba, Walston, Ferrucci, Cappola, Ricks, Xue and Fried35).
Currently, the mechanism of this potential relationship is still under debate and, as it has been described by Paiva & Russell, several hypotheses can explain this observation(Reference Paiva and Russell19). One of them involves the antioxidant properties. In the present study, analyses were repeated after controlling for TBARS levels, a lipidoperoxidation marker; the present results remained unchanged, suggesting that the association between total plasma carotenoids and mortality observed in our cohort did not arise from an antioxidant protection. However, the oxidative stress marker role of TBARS seems controversial and we have to remain cautious with such a conclusion even if, in a placebo-controlled single-blind study, Hininger et al. showed that carotenoid supplementation (lutein, lycopene, β-carotene) did not lead to a significantly measurable improvement in antioxidant defence in apparently healthy subjects (n 175; 25–45 years)(Reference Hininger, Meyer-Wenger and Moser45). The other underlying mechanism by which low levels of carotenoids could contribute to an increased risk of mortality may be related to inflammation. In the MacArthur Studies of Successful Aging, Hu et al. showed that serum β-carotene concentrations were inversely associated with C-reactive protein and IL-6 levels, and they showed an independent and synergic effect between low β-carotene concentrations and high inflammation burden on mortality risk(Reference Hu, Reuben, Crimmins, Harris, Huang and Seeman34). Unfortunately, inflammation markers were not available in the present study. Finally, two other mechanisms have also been mentioned; on one hand, a possible pro-inflammatory and immunomodulatory mechanism is hypothesised by the carotenoid's activation of lipoxygenase activities(Reference Paiva and Russell19). On the other hand, it has been suggested that carotenoids may also be involved in the activation of gene expression, which encodes the message for an element of gap junction (connexin 43) required for cell-to-cell communication(Reference Paiva and Russell19). To our knowledge, neither the activity of lipoxygenases nor measurements of connexin 43 have ever been taken into account in epidemiological studies interested in the relationship between carotenoids and chronic diseases or mortality in general populations. So, at this point, it seems difficult to be more precise on the mechanism by which carotenoids could act. Finally, we cannot exclude that carotenoids in the present study might have been serving as markers for other protective factors present in fruits and vegetables, but that are not acting as effective agents themselves. Further biological research is necessary to confirm the association between carotenoids and mortality, particularly in elderly subjects, to better understand the mechanism of action, and so to be able to determine if the protective association of carotenoids on mortality found in men but not in women is only a random effect, a sex or a gender difference.
By showing, prospectively, in a general healthy elderly population, that total plasma carotenoid levels were an independent associated marker of mortality in men after taking into account potential confounding factors, the present study suggests that total plasma carotenoid levels could be a ‘healthy diet’ indicator in elderly populations. Further studies are necessary to explore the mechanism which could explain the relationship.
Acknowledgements
We report no conflict of interest. T. N. A. was supported by a grant from the ‘Société Française de Nutrition’. The EVA study was carried out under an agreement between INSERM and the Merck, Sharp & Dohme-Chibret Laboratories (West Point, PA, USA) and was supported by the EISAI laboratory (France). C. B. designed the study and contributed to the paper. A. F. designed the biological measurements. T. N. A. carried out the analysis and wrote the paper. The authors thank Sarah Jane Flaherty for her attentive English corrections of the manuscript.








