Vitamin D deficiency is a major public health problem in India and has been reported in more than 70–90 % of healthy individuals of all age groups and both sex( Reference Ritu and Gupta 1 ). Vitamin D not only maintains Ca balance and skeletal integrity but also derives many extra-skeletal benefits. In view of poor availability of vitamin D3 from Indian diets, limited sun exposure and absence of mandatory food fortification, vitamin D supplementation assumes an important role in alleviating the burden of vitamin D deficiency in Indians( Reference Ritu and Gupta 1 ). Hence, maintaining sufficient levels of serum 25-hydroxyvitamin D (25(OH)D) through supplementation is of utmost importance.
Vitamin D is a non-polar lipid with poor bioavailability because of its low solubility in aqueous fluids of the gastrointestinal tract( Reference Maurya and Aggarwal 2 ). Micellisation process that disperses fatty molecules into aqueous micellar spheres may enable better absorption( Reference Borel, Caillaud and Cano 3 , Reference Kadappan, Guo and Gamus 4 ). In recent years, nanotechnology-based nanoemulsion formulation of vitamin D3 (d<200 nm) have been shown to be superior to the conventional coarse emulsions (d>200 nm) in terms of bioavailability and homogeneity based on simulated gastrointestinal tract system and in vivo studies in mice( Reference Kadappan, Guo and Gamus 4 ). In a recent open-labelled non-randomised pilot study in children, we observed a significantly greater increase in the serum 25(OH)D levels following supplementation with micellised vitamin D as compared with fat-soluble vitamin D( Reference Marwaha, Yenamandra and Ganie 5 ).
Methods
Study subjects
A total of 250 healthy adults working in three public schools and two government organisations in New Delhi were approached for their willingness to participate in this trial. After being briefed about the study protocol, 200 participants finally volunteered and gave their written consent to be part of the trial. Since the study inclusion criteria were not met, twenty participants were excluded from the study (Fig. 1). A total of 180 vitamin D-deficient subjects were randomly assigned to receive either the micellised or the conventional vitamin D3 formulation. In all, eighty-nine subjects from group A and seventy-seven from group B completed the trial.

Fig. 1 Showing the consort diagram of the trial. mITT, modified intention to treat; PP, per protocol.
The study protocol was approved by the institute’s (All India Institute of Medical Sciences) Ethics Committee. Informed written consent was obtained from all the participants after discussing with them the protocol including the risks and benefits of participating in the study. The study was conducted in accordance with the guidelines of Indian Council of Medical Research. Trial registration identifier: CTRI 2017/10/010187.
Intervention and biochemical analysis
Supplementation was initiated in the month of March/April 2017. Group A received micellised (DePura) and group B received conventional preparation (Calcirol) of vitamin D3 at a dose of 60 000 IU (1500μg) once a month for a period of 6 months. Both the preparations were of the same batch. Fasting samples of blood and urine were collected at baseline and after 6 months of supplementation. Serum 25(OH)D was measured by chemiluminescence assay (Diasorin; intra- and inter-assay CV: 3·5 and 5 %) and parathyroid hormone (PTH) by electrochemiluminiscence assay (Roche Diagnostics; intra- and inter-assay CV: 2·4 and 3·6 %). Serum levels of Ca (intra- and inter-assay CV: 2 and 2·5 %), phosphate (intra- and inter-assay CV: 1·4 and 2·5 %) and alkaline phosphatase (intra- and inter-assay CV: 2 and 2·8 %) were estimated by auto-analyzer (Roche Diagnostics GmbH). Spot urinary analysis for Ca (intra- and inter-assay CV: 3 and 3·1 %):creatinine (intra- and inter-assay CV: 1·6 and 2·1 %) ratio was carried out using Cobas-C 111 (Roche Diagnostics).
The primary outcome measure was serum 25(OH)D and the secondary outcome parameters were serum PTH, Ca, phosphate and alkaline phosphatase and urinary Ca:creatinine ratio. Vitamin D deficiency was graded as severe (25(OH)D <12·5nmol/l) moderate (25(OH)D 12·5–25nmol/l) and mild (25(OH)D 25–<50nmol/l). Serum 25(OH)D ≥50nmol/l was considered as adequate. Hyperparathyroidism was defined as serum PTH >65 pg/ml. Hypercalciuria was diagnosed when urinary Ca:creatinine ratio was >0·2( Reference Pak, Ohata and Lawrence 6 ).
Sample size calculation
The sample size was calculated based on our previous study( Reference Marwaha, Yenamandra and Ganie 5 ) in children, where we observed that the mean serum level of 25(OH)D increased from 15·25 (sd 10·75) to 74·5 (sd 25·5)nmol/l (increased 59·25nmol/l) following supplementation with 60 000 IU/month of conventional vitamin D3 for 6 months. Anticipating an additional increase of at least 17·5 (sd 25·5)nmol/l following supplementation with miscellised vitamin D3 formulation as observed in our earlier study with 5 % α error and 90 % power, we required a sample size of forty-five subjects in each arm. Considering 20 % attrition during follow-up, we needed fifty-seven participants per group. We, however, randomised a total of 180 subjects into two groups as they had given their consent to be part of the clinical trial.
Statistical analysis
All statistical analyses were implemented on Stata 12.0 (Stata Corp LP). Data were presented as number (%) or means and standard deviations/median (minimum–maximum) as appropriate. Baseline categorical and continuous characteristics were compared between the groups using χ 2 test and Student’s t test for independent samples/Wilcoxon rank-sum test (for non-normal data), respectively. We analysed the primary outcome measure (serum 25(OH)D) by both the methods of analyses, that is, modified intention to treat (mITT) and per protocol (PP) analyses. mITT included all individuals who received interventions after randomisation (group A: n 89; group B: n 77) and PP included all individuals who received all six doses of intervention (group A: n 84; group B: n 65). The missing values were imputed using the last observation carried forward technique. Differences in the mean values of 25(OH)D and other biochemical parameters between the two groups were compared using Student’s t test for independent samples. ANCOVA was used to compare the difference in the mean values between the groups adjusting for age and sex. The results were reported as difference in means (95 % CI). Serum PTH and urinary Ca:creatinine ratio were compared using Wilcoxon rank-sum test. The change in all the biochemical parameters at 6 months from baseline was tested using either paired t test or Wilcoxon signed rank test. A P value of <0·05 was considered as significant.
Results
A total of 180 healthy adults aged 21–60 years were randomised into two groups: A and B. Group A received micellised vitamin D3 at a dose of 60 000 IU every month for 6 months, while group B received conventional fat-soluble vitamin D3 in a similar dosing schedule. One subject from group A and thirteen from group B were excluded at the beginning of the trial as they failed to report on the 1st day for supplementation under supervision. Finally, out of eighty-nine subjects from group A and seventy-seven from group B who completed the trial, five from group A and twelve from group B were non-compliant to treatment (Fig. 1).
Baseline demographic and biochemical characteristics of the study patients
A total of eighty-two males (49 %) and eighty-four females (51 %) were included in the study. There were thirty-six males and fifty-three females in group A and forty-six males and thirty-one females in group B, respectively (P=0·013). The mean age of the study subjects in group A was 40·5 (sd 10·3) years, while in group B, it was 37·0 (sd 11·2) years (P=0·036). Both the groups had comparable height, weight and BMI. The mean serum baseline levels of 25(OH)D, Ca, phosphate and alkaline phosphatase and the median values of serum PTH and urinary Ca:creatinine ratio were comparable (Table 1).
Table 1 Baseline characteristics between the groups (Mean values and standard deviations; medians and minimum–maximum values)
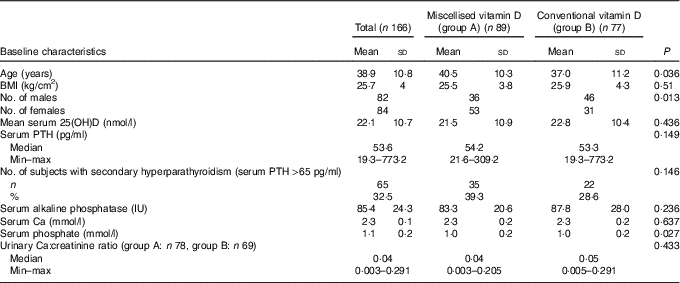
25(OH)D, 25-hydroxyvitamin D; PTH, parathyroid hormone.
mITT analysis showed that following 6 months of vitamin D supplementation, the mean serum 25(OH)D levels in subjects who received micellised vitamin D increased significantly from 21·5 (sd 10·9) to 76·7 (sd 18·8) nmol/l (P<0·001). Similarly, participants who were given conventional vitamin D3 also showed significant increase in serum 25(OH)D from 22·8 (sd 10·4) to 57·8 (sd 16·0) nmol/l (P<0·001). The subjects in the micellised group achieved an additional increase of 20·2 nmol/l (95 % CI 14·0, 26·4, P<0·001) in serum 25(OH)D levels that was statistically highly significant. The difference remained statistically significant even after adjustment for age and sex (17·5 nmol/l; 95 % CI 11·8, 23·1; P<0·001) (Table 2). Out of eighty-nine, eighty-one (91 %) and fifty-two of seventy-seven (67·5 %) subjects in groups A and B, respectively, achieved adequate serum 25(OH)D levels (>50nmol/l) (P<0·001) while 54/89 (60·7 %) and 10/77 (13 %) achieved serum 25(OH)D levels >75 nmol/l (P<0·001).
Table 2 Showing effect of vitamin D supplementation on serum 25-hydroxyvitamin D levels (Mean values and standard deviations; differences and 95 % confidence intervals)
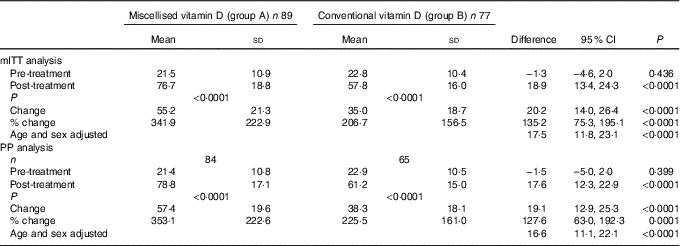
mITT, modified intention to treat; PP, per protocol.
Overall, both males and females showed a significant increase in serum 25(OH)D levels following vitamin D supplementation. The serum levels of 25(OH)D in males from groups A and B increased from 21·5 (sd 8·0) and 24·7 (sd 11·0) nmol/l at baseline (P=0·16) to 76·9 (sd 19·0) and 61·5 (sd 16·7) nmol/l (P<0·001), respectively, at the end of the study. Similarly, serum levels of 25(OH)D among females rose from 21·4 (sd 12·4) and 20·5 (sd 9·6) (P=0·75) at baseline to 80·2 (sd 15·7) and 60·9 (sd 12·8) (P<0·0001) in groups A and B, respectively. The rise was comparable between the two sexes within the groups (Table 2).
PP analysis carried out after excluding subjects who were non-compliant, also revealed similar observations with regard to serum 25(OH)D levels following supplementation as shown in Table 2.
The median baseline serum PTH levels were 54·5 (min–max 21·6–309·2) and 55·2 (min–max 19·8–173·2) pg/ml in groups A and B which decreased to 39·7 (min–max 18·4–106·3) and 47·7 (min–max 15·2–154·1) pg/ml, respectively (P<0·001), following supplementation. The prevalence of secondary hyperparathyroidism in groups A and B was 33 (39·3 %) and 21 (32·3 %) which decreased to 12 (14·3 %) (P<0·001) and 14 (21·5 %) (P=0·052), respectively (Table 3).
Table 3 Showing the effect of vitamin D supplementation on serum parathyroid hormone, calcium, phosphate and alkaline phosphatase levels (Mean values and standard deviations; differences and 95 % confidence intervals)
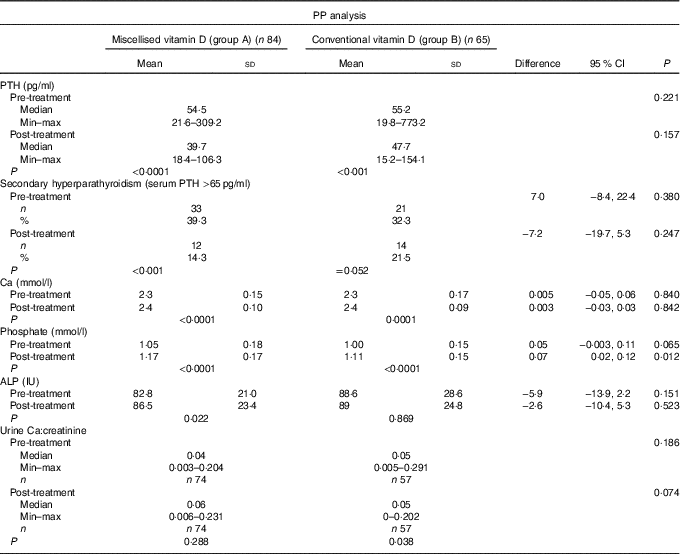
PP, per protocol; PTH, parathyroid hormone; ALP, alkaline phosphatase.
The changes in other biochemical parameters were similar in both the groups except for serum phosphate, which was higher in group A (Table 3).
Discussion
The present study is the first randomised trial that has compared the efficacy of nanoemulsion formulation of vitamin D3 (DePura) with the conventional granular form (Calciferol) in apparently healthy vitamin D-deficient adults based on serum 25(OH)D, PTH and other biochemical parameters. This trial clearly demonstrates significantly greater increase in serum 25(OH)D following supplementation with miscellised vitamin D3 as compared with conventional vitamin D3 preparation. This is consistent with the observations made by Kadappan et al. ( Reference Kadappan, Guo and Gamus 4 ) who in a simulated gastro intestinal tract system and in vivo studies in mice demonstrated that nanoemulsion (d<200 nm)-based delivery system is superior to coarse emulsion in terms of bioavailability and homogeneity of vitamin D absorption.
Studies have also revealed 3·6 times greater absorption of vitamin A in subjects provided with miscellised form than those who received coarse emulsions( Reference Biesalski 7 ). Similarly in a randomised cross-over trial, twelve healthy subjects when administered 500 IU of vitamin E in standard oil, emulsified or miscellised form, showed plasma levels of vitamin E after 4 h of supplementation, which were five times greater in miscellised group than in the oil group( Reference Jacquemin, Hermeziu and Kibleur 8 ).
The bioavailability of nanoemulsion formulations has been reported to increase as the size of the particles encapsulating them decreases. The stearic barrier and small size of the nanopaticle hinder the lipase/colipase adsorption, thereby protecting it from the digestion process. The particle size and entrapment efficiency of nanoparticle is maintained even when it is exposed to various in vivo pH conditions. These nano particles exhibit rapid gastric emptying at an exponential rate. It is because of the above characteristics of miscellised formulations such as DePura that their bioavailability is better than the conventional granular formulation of Calcirol( Reference Pak, Ohata and Lawrence 6 ).
Better bioavailability of nano preparation has further been corroborated, with significantly higher number of our study subjects (91 %) achieving serum 25(OH)D levels of >50nmol/l in the group receiving miscellised vitamin D3 as compared with those who received the conventional formulation of D3 (67·5 %). Furthermore, the percentage of subjects who had achieved serum levels of 25(OH)D >75nmol/l was markedly higher in the miscellised group as against the other group (60·7 v. 12·9 %, P<0·001). These observations are very similar to the findings of our earlier observational study in children comparing the same two preparations( Reference Marwaha, Yenamandra and Ganie 5 ). The results of both our studies comparing DePura with Calciferol in children as well as in adults clearly indicate the superiority of nanotechnology-based preparation over the conventional vitamin D preparations in terms of bioavailability. In view of the above, nanotechnology-based preparations of all fat-soluble vitamins may have a great therapeutic role in patients with malabsorption syndromes (due to inflammatory bowel disease, coeliac disease, short bowel syndrome, hepato-biliary disease, pancreatic insufficiency or bariatric surgery) who suffer from deficiency of essential fatty acids and fat-soluble vitamins including vitamin D( Reference Jacquemin, Hermeziu and Kibleur 8 , Reference Marguilies, Kurian and Elliott 9 ). Conventional fat-soluble vitamin D3 needs to be taken with milk, oil or fat-based products to enhance its absorption, while the micellised form can be taken directly or with water, orange juice etc.
Serum PTH has been proposed as a functional marker for vitamin D deficiency because elevated serum PTH increases the risk for bone loss and fractures in older people. These levels can be potentially modified by vitamin D supplementation( Reference Lotito, Teramoto and Cheung 10 , Reference Sadat-Ali, Al-Omran and Al-Turki 11 ). Significant decrease in serum PTH levels observed following supplementation in both groups is consistent with one of our recent reports( Reference Marwaha, Garg and Gupta 12 ). Despite achieving sufficient levels of serum 25(OH)D following supplementation with micellised or conventional vitamin D3 formulation, 12/84 (14·3 %) subjects in the former and 14/65 (21·5 %) in the latter group continued to have high serum PTH levels. Persistent high levels of PTH in these cases could possibly be due to either (a) continued low dietary intake of Ca that may keep the parathyroid glands active, leading to persistent secondary hyperparathyroidism and (b) chronic deficiency of vitamin D resulting in long-standing parathyroid hyperplasia may take longer time to return to their normal physiological functions( Reference Adam, Viapiana and Gatti 13 ).
Clinical significance of increase in serum Ca, phosphate and alkaline phosphatase levels in both the groups following supplementation is not clear, as the serum values were well within normal limits. The fact that none of the patients developed any major metabolic abnormalities such as hypercalaemia or hypercalciuria even after 6 months of intervention explains the safety of this trial. We did not observe any difference in the serum 25(OH)D levels between men and women in both the groups following supplementation. Dawson-Hughes et al. ( Reference Dawson-Hughes, Harris and Krall 14 ) in their study on the role of dietary supplementation with 500 mg of Ca and 700 IU (17·5μg) vitamin D daily for 3 years found that the serum levels of 25(OH)D were found to be significantly higher in females (males 29·5 (sd 29·0) nmol/l v. females 40·25 (sd 35·75) nmol/l). Sharifi et al. ( Reference Sharifi, Amani and Hajiani 15 ) in their study showed a 2–3-fold increase in serum 25(OH)D levels following supplementation in both males (39·25 v. 75; n 14) and females (25 v. 84; n 13), which was similar to what is observed in the present study.
Limitations
Lack of data on daily dietary intake of Ca was a major limiting factor, as Ca intake is known to influence serum PTH levels independently. Had we evaluated the daily dietary Ca intake of our study individuals and shown lower intake of dietary Ca in those with persistent secondary hyperparathyroidism despite achieving adequate serum 25(OH)D following vitamin D supplementation than those with normal serum PTH levels, the above hypothesis would have been strengthened( Reference Jorde, Sundsfjord and Haug 16 – Reference Aggarwal, Seth and Aneja 18 ). We could also not evaluate the dietary intake of vitamin D as no dietary estimates of vitamin D are available in the published Indian food tables. We, however, had earlier evaluated the daily dietary intake of vitamin D using US Department of Agriculture provisional tables on vitamin D content of foods, which may not be relevant in the Indian context as vitamin D content of most Indian foods recently evaluated by us is negligible (B Sharma and RK Marwaha, unpublished results)( Reference Puri, Marwaha and Agarwal 19 ).
Another limitation of this trial was lack of specific bone formation (N terminal of propeptide of type 1 procollagen; PINP) and resorption (C terminal telopeptide of type 1 collagen-1; CTX) markers. In view of negative association between serum 25(OH)D levels and resorption markers in vitamin D-deficient individuals in cross-sectional studies to maintain Ca homoeostasis, it is conceivable to assume that vitamin D supplementation should affect both bone formation and resorption markers. However, there are limited and conflicting reports in literature. Although most studies report no change in bone resorption/formation markers, some show decrease in either resorption marker alone or both following supplementation. Evaluation of bone markers in the present study may have provided additional information on bone turnover status( Reference Barnes, Robson and Bonham 20 – Reference Genc, Ömer and Aycan-Üstyol 23 ).
In conclusion, supplementation with both micellised and conventional vitamin D3 was safe and resulted in significant rise in serum 25(OH)D levels. Micellised vitamin D3 formulation, however, appeared to be more efficacious. Further studies are needed in patients with malabsorption syndromes (a) to truly elucidate its efficacy over fat-soluble vitamin D3 and (b) to evaluate long-term effects of supplementing micellised vitamin D.
Acknowledgements
The authors gratefully acknowledge the Society for Endocrine Health for Elderly, Adolescents and Children for financial support and manpower.
This research received no specific grant from any funding agency, commercial or not-for-profit sectors.
The contributions of the authors are as follows. R. K. M., A. M. and G. S. designed the study; R. K. M., G. S., A. N., P. A., A. S., A. C., M. G. and T. D. collected data and supervised supplementation; K. M., R. K. M., V. K. S. and G. S. analysed data; N. D. performed laboratory investigations; R. K. M. and G. S. wrote the manuscript.
The authors declare that there are no conflicts of interest.









