The production of seeds is critical for survival of plants, especially annuals and biennials, and is an essential link from one generation to the next (Fenner and Thompson Reference Fenner and Thompson2005; Harper Reference Harper1981). Soil seedbanks assure the survival of a plant species in highly variable and unpredictable environments (Baskin and Baskin Reference Baskin and Baskin2014; Cavers Reference Cavers1983). Many species in highly disturbed habitats such as annual cropping systems produce copious quantities of seeds that often exhibit strong dormancy (Baskin and Baskin Reference Baskin and Baskin2014). Population growth in perennial plant species may also depend on their ability to produce large quantities of seeds, especially in simple herbaceous perennials that rely little on vegetative reproduction [e.g., curly dock (Rumex crispus L.), spotted knapweed (Centaurea stoebe L.), and broadleaf plantain (Plantago major L.)] (Harper Reference Harper1981). Management of weedy or invasive plant species that depend on annual seed production is best achieved by preventing or substantially limiting this critical life stage (DiTomaso Reference DiTomaso2000). Limiting seed production and seed rain of invasive plant species is especially important for restoration programs where the successful establishment of native or more favorable naturalized plant species is a major goal (Godefroid et al. Reference Godefroid, Piazza, Rossi, Buord, Stevens, Aguraiuja, Cowell, Weekley, Vogg, Iriondo, Johnson, Dixon, Gordon, Magnanon, Valentin, Bjureke, Koopman, Vicens, Virevaire and Vanderborgh2011).
Equally important is knowledge about the probability of seeds in the soil seedbank to germinate and emerge from various soil depths in a given year and the number of years that seeds can remain viable in the seedbank (Benvenuti et al. Reference Benvenuti, Macchia and Miele2001; Roberts and Feast Reference Roberts and Feast1973). The emergence of seedlings from soil is negatively correlated with soil depth, with maximum emergence levels occurring at depths of 2 cm for most species (Benvenuti et al. Reference Benvenuti, Macchia and Miele2001; Yenish et al. Reference Yenish, Doll and Buhler1992). Larger-sized seeds are generally able to emerge from greater soil depths than smaller seeds (Baskin and Baskin Reference Baskin and Baskin2014; Benvenuti et al. Reference Benvenuti, Macchia and Miele2001). Plant species whose seeds have physical (hard seed coats) and/or physiological dormancy (Baskin and Baskin Reference Baskin and Baskin2014) are most long-lived in soil and include many troublesome agronomic weeds [e.g., velvetleaf (Abutilon theophrasti Medik.), common ragweed (Ambrosia artemisiifolia L.)]. Seeds of grasses and members of some dicot families such as the dogbanes (Apocynaceae) are typically shorter lived in soil, as they lack thick protective structures (Baskin and Baskin Reference Baskin and Baskin2014; Burnside et al. Reference Burnside, Wilson, Weisberg and Hubbard1996; Lewis Reference Lewis1973).
Box 1 Management Implications
Two invasive species that continue to be troublesome in many natural and seminatural habitats in the northeastern United States and southeastern Canada, and increasingly in the midwestern United States, are the perennial vines black swallowwort and pale swallowwort. Although perennials, these vines reproduce primarily by seeds attached to a tuft of soft hairs that facilitate dispersal by wind and animals. Two poorly understood but important features of their biology are the length of time seeds remain viable in the soil and the ability of seedlings to emerge from different soil depths. In this 4-yr study in central New York State, most swallowwort seedling emergence occurred during the first year (92% in 2012), and no new seedlings of either species emerged in the third (2014) or fourth (2015) years. Pale swallowwort had relatively poor emergence at sowing depths of 0 cm (11%), 5 cm (6%), and 10 cm (0.05%—only one seedling), while 37% of pale swallowwort seeds emerged at 1 cm. Emergence of the larger-seeded black swallowwort was generally greater: 0 cm, 16%; 1 cm, 71%; 5 cm, 6%; and 10 cm, 26%. Importantly, a large portion of the seeds that germinated at the 5- and 10-cm depths died before reaching the soil surface. A management implication of this seedbank study is that emergence of pale swallowwort seedlings from soil is prevented if seeds are buried in soil to a depth of at least 10 cm. Burial at greater depths is recommended for black swallowwort. However, burial of seeds through soil disturbance may not be practical in natural areas, especially if swallowwort densities are relatively low, as this may stimulate the emergence of other undesirable species. In areas where swallowwort densities are relatively high, soil disturbance and burial of seeds may be a practical option as a first step in the restoration of such heavily infested sites. Since all seedlings of the two swallowwort species that emerged did so during the first 2 yr of the study, control efforts should focus on limiting seedling establishment during this critical period. The lack of seedling emergence for both species in years 3 and 4 of the study is promising and suggests a relatively transient seedbank. Therefore, if additional seed production is prevented or limited in these habitats for at least 3 yr, restoration of the affected area has a high likelihood of success
Pale swallowwort (Vincetoxicum rossicum) and black swallowwort (Vincetoxicum nigrum) are perennial European herbaceous vines in the dogbane family (Apocynaceae: subfamily Asclepiadoideae) that have invaded many habitats (e.g., old fields, forest edges and understories, pastures) in the northeastern United States and southeastern Canada (DiTommaso et al. Reference DiTommaso, Lawlor and Darbyshire2005; Sheeley and Raynal Reference Sheeley and Raynal1996). The two species were introduced into North America in the late 1800s, likely as ornamentals, and have escaped cultivation (DiTommaso et al. Reference DiTommaso, Lawlor and Darbyshire2005). Although they are perennials, reproduction occurs mostly by the production of wind-dispersed comose seeds borne in follicles in late summer and autumn. Seeds of both species can be polyembryonic, giving rise to two or more seedlings, but this feature is more common in pale swallowwort seeds than black swallowwort seeds (Cappuccino et al. Reference Cappuccino, Mackay and Eisner2002; DiTommaso et al. Reference DiTommaso, Lawlor and Darbyshire2005; Sheeley Reference Sheeley1992). The vines often form dense stands of 130 stems m−2 or more, with individual stems growing 1 to 2 m in length and producing 100 to 400seedsstem−1 (DiTommaso et al. Reference DiTommaso, Lawlor and Darbyshire2005; Sheeley Reference Sheeley1992; Smith et al. Reference Smith, DiTommaso, Lehmann and Greipsson2006).
Control of the swallowworts is challenging, with short-term efforts to date focused on herbicidal control and physical control strategies such as mowing (Averill et al. Reference Averill, DiTommaso and Morris2008; DiTommaso et al. Reference DiTommaso, Milbrath, Bittner and Wesley2013; Lawlor and Raynal Reference Lawlor and Raynal2002; McKague and Cappuccino Reference McKague and Cappuccino2005; Mervosh and Gumbart Reference Mervosh and Gumbart2015). Longer-term research is assessing the potential of several biological control agents to manage these two invasive perennial vines (Berner et al. Reference Berner, Cavin, Mukhina and Kassanelly2011; Dolgovskaya et al. Reference Dolgovskaya, Volkovitsh, Reznik, Moseyko and Milbrath2016; Gibson et al. Reference Gibson, Vaughan, Biazzo and Milbrath2014; Hazlehurst et al. Reference Hazlehurst, Weed, Tewksbury and Casagrande2012; Leroux Reference Leroux2014; Maguire et al. Reference Maguire, Sforza and Smith2011; Milbrath and Biazzo Reference Milbrath and Biazzo2016; Weed and Casagrande Reference Weed and Casagrande2010, Reference Weed and Casagrande2011; Weed et al. Reference Weed, Gassmann and Casagrande2011a, Reference Weed, Gassmann, Leroux and Casagrande2011b). Given the challenge to date of effectively managing seed production in these invasive vines, a better understanding of the emergence patterns of swallowwort seeds in the soil seedbank and their longevity in soil will assist in developing effective management strategies. To date, no studies have been performed to determine seedling emergence patterns and seed longevity for either swallowwort species beyond three growing seasons or at soil depths greater than 1 cm (Averill et al. Reference Averill, DiTommaso and Morris2008; Ladd and Cappuccino Reference Ladd and Cappuccino2005; Magidow et al. Reference Magidow, DiTommaso, Ketterings, Mohler and Milbrath2013; Milbrath et al. Reference Milbrath, Davis and Biazzo2017).
The objectives of this 4-yr seedbank study were to determine (1) the emergence pattern of seeds of black swallowwort and pale swallowwort sown at four soil depths for four growing seasons and (2) the fate of nongerminated seeds at the four soil depths during this same period.
Materials and Methods
Seed Collection
Black swallowwort seeds used in this study were collected in August 2011 from the grounds of the Cornell University Cooperative Extension Office, Millbrook, Dutchess County, NY (41°46′N, 73°44′W). Pale swallowwort seeds were collected at the same time from the Great Gully Preserve, Cayuga County, NY (42°48′N, 76°40′W). Mature follicles were collected from at least 100 plants per species, all growing in the open field. Seeds were cleaned, and filled seeds (i.e., likely containing a viable embryo) were placed in paper envelopes in a refrigerator at 4 C until the start of the trial in October 2011.
To estimate initial viability of seeds, three lots of 100 seeds of each species were moist stratified at 4 C for 3 mo. Stratified seeds were germinated in an incubator at 25:20 C and a photoperiod of 14:10 h (L:D), and remaining ungerminated seeds were tested for viability with a 1% solution of tetrazolium chloride. Seed viability was 97% for black swallowwort and 95% for pale swallowwort.
Experimental Design
This common garden study was conducted in an open (full sun) old field in Ithaca, NY (42°26′36″N, 76°30′00″W) from 2011 to 2015. The experiment used a three-way factorial treatment structure in a completely randomized design with two swallowwort species (pale and black swallowwort), four seed-burial depths (0, 1, 5, and 10 cm), four harvest years (1, 2, 3, or 4 yr since sowing), and five replicates for a total of 160 microplots. The experiment was conducted in microplots consisting of plastic pots (32-cm diameter, 35-cm tall) buried with the rims nearly flush with the surrounding soil. Pots were in rows at 0.5-m intervals within the rows. The within-row area was covered with black fabric mulch to suppress weeds, and the between-row area was periodically mowed. Soil collected from a local forest site with no history of swallowwort was screened through 1.2-cm hardware cloth to remove stones and large rhizomes. Forest soil was used to initiate the experiment with a relatively weed seed–free soil so as not to interfere with the emergence and removal of swallowwort seedlings. One hundred swallowwort seeds were sown in each microplot in fall 2011; black and pale swallowwort were sown in separate pots. Seeds were placed in custom-built 2-cm-deep seed pans composed of a PVC ring (23-cm diameter) with a 0.5-mm mesh bottom. For the 1-, 5-, and 10-cm soil-depth treatments, 1 cm of soil was first placed on the screened pan, seeds were scattered on the soil surface, and another 1 cm of soil was used to cover the seeds. The pan and seeds were then transported to the field and placed at the appropriate depth in the pots and buried with more of the forest soil. For the 0-cm depth treatment, the screened pans contained 1 cm of soil and were placed in the soil-filled pots. Seeds were scattered on the soil surface within the pans. This approach has several advantages over using mesh bags: (1) seeds are in a more natural environment; (2) emergence of seedlings is not restricted by the mesh bag; and (3) seeds cannot move downward more than 1 cm, thereby making the recovery of seeds by wet sieving more efficient. During the winter months, pots were covered with 1.2-cm hardware cloth to prevent seed predation by small mammals. Weeds in the microplots were cut to prevent disturbance of buried seeds and primarily consisted of dandelion (Taraxacum officinale G. H. Weber ex Wiggers), red sorrel (Rumex acetosella L.), common chickweed [Stellaria media (L.) Vill.], and broadleaf plantain.
Emergence rates were estimated the following years by monitoring seedling emergence twice weekly beginning in early May and weekly from July through September. Seedlings were counted and removed at the root crown with blunt forceps. To aid in removing seedlings in the 5- and 10-cm treatments, a 20-cm blunt-tipped microspatula was used to expose the root before extracting a seedling with forceps. Seedlings arising from the same seed (polyembryony) were scored as a single establishment event. Beginning in 2012, a subset of seed pans was retrieved annually in October. An additional 5 cm of soil from the surface of each pot was collected from the 0-cm treatments of 2012 and 2013 to capture seed that likely had been flushed from the seed pans. Seed pans were stored at 4 C for no longer than 14 d, after which the samples were spread out and dried at 30 C for 48 h. Dried soil samples were screened; recovered seeds, seed coats, and seed coat fragments were counted; and any signs of seed predation were noted. Intact seeds were assessed for viability with a 1% solution of tetrazolium chloride.
Data Analysis
The data were analyzed using a generalized linear model with a Poisson distribution and log-link function (PROC GENMOD, SAS v. 9.4, SAS Institute, Cary, NC). The independent, fixed variables of swallowwort species, burial depth, and harvest year were used in analyses of the following dependent variables: cumulative seedling emergence, number of nongerminated seeds damaged by unknown organisms, and number of unaccounted seeds. Stepwise removal of nonsignificant (P>0.05) interaction terms was used to determine the best model for each parameter. For the dependent variables of number of fatally germinated seedlings and dead seeds (nonviable or rotted), several treatment combinations had to be excluded due to lack of variability (all zeros). Thus, a one-way analysis on remaining treatment combinations was performed. All four harvest years were included in analysis of cumulative seedling emergence, whereas only the first two harvest years were included in analyses for the remaining variables due to deterioration of the seeds. Because viable seeds were recovered from only one treatment group, no analysis could be performed on that variable. Standard errors for back-transformed data were approximated using the methods of Deming (Reference Deming1964). For significant effects, multiple comparisons among means used a Bonferroni correction.
Results and Discussion
The majority of seedlings that successfully emerged did so during the first year of the experiment (92% in 2012, based on initial number of seeds sown). No new seedlings emerged in the third (2014) or fourth (2015) years. Short-lived seedbanks are known for related species in the Apocynaceae (Burnside et al. Reference Burnside, Wilson, Weisberg and Hubbard1996). Minor differences in cumulative seedling emergence between the fourth harvest year and other years was due to nonexperimental variability in emergence among pots (species by year interaction and depth by year interaction; Table 1; data not shown). We considered the seed the basic unit of this study, and thus did not include in our counts extra seedlings that originated from the same seed (polyembryony). Although we were careful to note whether multiple seedlings emerged from the same polyembryonic seed, emergence counts could have been slightly overestimated if several shoots arose from the same seed, especially since approximately 64% and 22% of pale swallowwort and black swallowwort seeds, respectively, were polyembryonic in preliminary tests. Seedling emergence in the second year (2013) occurred primarily in the 0-cm treatment (94% of 2013 emergence), largely from seeds that had previously been washed out of the seed pans. Such seedlings typically were found around the inside edges of the pots. The remaining seedlings (17 total) were recorded only from the 1- and 5-cm treatments. In field studies near Ottawa, Canada (Ladd and Cappuccino Reference Ladd and Cappuccino2005) and at multiple sites in New York State (Averill et al. Reference Averill, DiTommaso, Mohler and Milbrath2010; Milbrath et al. Reference Milbrath, Davis and Biazzo2017), ranges of first-, second-, and third-year emergence rates for surface-sown seeds of both species were reported to be 4% to 39%, 0% to 25%, and 0% to 3%, respectively. In a microplot study in Ithaca, NY, Magidow et al. (Reference Magidow, DiTommaso, Ketterings, Mohler and Milbrath2013) reported first-year emergence percentages of surface-sown pale swallowwort seeds in two separate years across two soil types and three pH treatments of 50% and 32%, respectively; these levels were greater than for black swallowwort (40% and 9%, respectively). Seedling emergence for surface-sown seeds in our study was relatively low (first year: 5% black swallowwort, 9% pale swallowwort; second year: 13% black swallowwort, 2% for pale swallowwort), especially compared with the findings of Ladd and Cappuccino (Reference Ladd and Cappuccino2005) and Magidow et al. (Reference Magidow, DiTommaso, Ketterings, Mohler and Milbrath2013). It is unclear why emergence rates for surface-sown seeds in our study were low. One possible reason may be that desiccation of seeds in our screened pan setup may have been severe. The seed pans may also have allowed a large number of seeds on the soil surface to be washed or blown out of the pans during heavy rainfall or strong winds. In fact, we were unable to recover a large proportion of the seeds initially sown on the soil surface of pots (Table 2).
Table 1 Results from the generalized linear model analysis for the effect of different burial depths and years of burial on black and pale swallowwort seeds in a common garden experiment.Footnote a
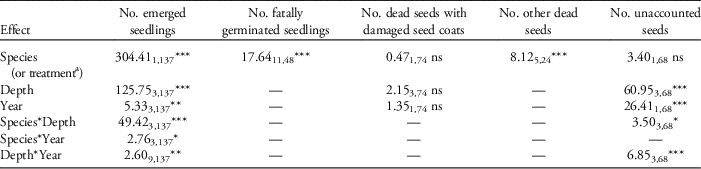
a Values given under each variable are: F-value, numerator and denominator degrees of freedom (subscript), and level of significance. All four harvest groups (years) were included for cumulative seedling emergence, whereas only the first two harvest groups were included for the remaining variables due to deterioration of seeds. Nonsignificant interaction terms (P>0.05) were iteratively removed beginning with the full (three-factor) model, except for fatally germinated seedlings and other dead seeds, for which a one-factor model (treatment) was used (see “Materials and Methods”).
*P<0.05; **P<0.01; ***P<0.001, ns=not significant.
Table 2 Unaccounted seeds (mean±SE, back-transformed) of swallowwort that were buried at different depths for the first 2 yr of a common garden experiment.Footnote a
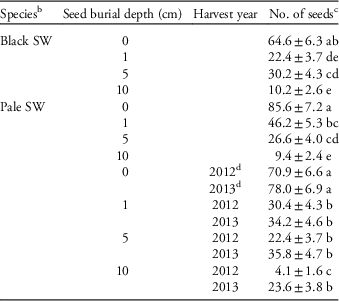
a Within each interaction, means followed by the same letter are not significantly different (multiple comparisons among means using the Bonferroni correction, P>0.05, n=10).
b SW, swallowwort.
c Because initial seed numbers were 100, counts are equivalent to percentages.
d 2012=year 1; 2013=year 2.
Seedling emergence varied for the two swallowwort species according to the depth of seed burial (species by depth interaction; Table 1; Figure 1). Pale swallowwort had relatively poor cumulative emergence at sowing depths of 0 cm (11%), 5 cm (6%), and 10 cm (0.05%—only one seedling), while a significantly greater percentage (37%) of pale swallowwort seedlings emerged at 1 cm. Ladd and Cappuccino (Reference Ladd and Cappuccino2005) reported emergence rates for pale swallowwort seeds sown at 1-cm depth of 71%, 0.4%, and 0.4% in the 3 yr of their study, respectively, for a total emergence of 72%. Black swallowwort had greater emergence than pale swallowwort at most burial depths (Figure 1). At least two-thirds of all sown seeds of black swallowwort emerged at depths of 1 and 5 cm, and 26% emerged at 10 cm. To our knowledge, these are the first data on seedling emergence patterns across various soil depths for black swallowwort. Only 16% of the surface-sown black swallowwort emerged, which was not different from pale swallowwort (Figure 1). Black swallowwort seed is larger than pale swallowwort seed (up to three times greater mass), and early seedlings of black swallowwort are thus typically larger and longer (Milbrath Reference Milbrath2008; Milbrath and Biazzo Reference Milbrath and Biazzo2016; Milbrath et al. Reference Milbrath, DiTommaso, Biazzo and Morris2015). Maximum emergence of buried seeds therefore occurred at 1 cm for pale swallowwort and at 1 to 5 cm for black swallowwort, with decreasing emergence as burial depth increased, which is typically observed for many species (Benvenuti et al. Reference Benvenuti, Macchia and Miele2001; Yenish et al. Reference Yenish, Doll and Buhler1992).
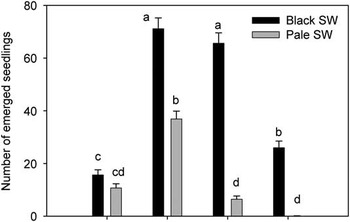
Figure 1 Number of emerged black and pale swallowwort (SW) seedlings (mean+SE, back-transformed) from an originally sown 100 seeds buried at different soil depths, averaged over the 4 yr of the common garden experiment. Multiple seedlings originating from a single seed (polyembryony) were treated as a single seedling. Bars denoted by the same letter are not significantly different (multiple comparisons among means using the Bonferroni correction, P>0.05, n=20).
Filled seeds were only recovered in fall 2012 from pots of black swallowwort at the 0-cm depth; the seeds were 66% viable. An average of 24 viable black swallowwort seeds per pot were recovered. It is unclear why viable seeds were not recovered from other treatments. No viable seeds were recovered after the second growing season.
A significantly large portion of the seedlings that germinated at the 5-cm depth (pale swallowwort) or the 10-cm depth (both species) died before reaching the soil surface, i.e., fatal germination, based on the presence of extra germinated seed coats that were otherwise undamaged (Tables 1 and 3). More pale swallowwort seeds showed fatal germination than black swallowwort at the 5-cm depth (Table 3). This finding is not surprising, given the larger seed size of, and hence more resources available to, black swallowwort seeds relative to pale swallowwort seeds to allow emergence from greater soil depths (Baskin and Baskin Reference Baskin and Baskin2014; DiTommaso et al. Reference DiTommaso, Lawlor and Darbyshire2005; Milbrath Reference Milbrath2008).
Table 3 Fatal germination and other dead seeds (mean±SE, back-transformed) of buried swallowwort seeds for the first 2 yr of a common garden experiment.Footnote a
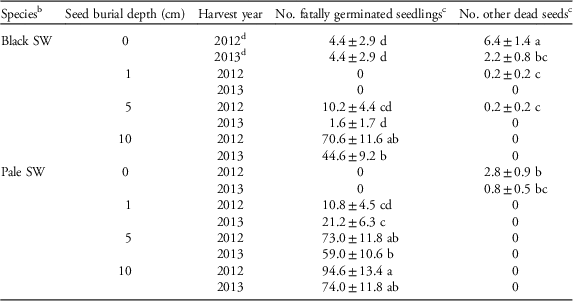
a Means within each column followed by the same letter are not significantly different (multiple comparisons among means using the Bonferroni correction, P>0.05, n=5). Zero values for various treatment combinations are indicated but could not be included in analyses.
b SW, swallowwort.
c Because initial seed numbers were 100, counts are equivalent to percentages.
d 2012=year 1; 2013=year 2.
A small proportion of seeds (2.8±3.2 seeds pot−1, n=80, range: 0 to12) showed signs of predation, which did not vary by treatment combination (Table 1). Although we did not directly assess seed predation or other causes of seed mortality in the two swallowwort species in our study, removal or consumption of weed seeds on or near the soil surface by various vertebrate (e.g., birds, mice) and invertebrate (e.g., carabid beetles, crickets) predators is well documented (e.g., Kulkarni et al. Reference Kulkarni, Dosdall and Willenborg2015; Westerman et al. Reference Westerman, Hofman, Vet and van der Werf2003). A similar number of seeds died for other reasons, particularly black swallowwort at the 0-cm depth in year 1 (Tables 1 and 3). Many seeds could not be accounted for, especially for pale swallowwort compared with black swallowwort at the 0- and 1-cm seed depths (Tables 1 and 2). Given the relatively smaller size of pale swallowwort seeds relative to black swallowwort seeds, it is possible that the smaller seeds were more easily dislodged by rain or wind, especially those sown on the soil surface. Invertebrate seed predators such as ground-dwelling carabid beetles and crickets may have also preferentially removed or consumed the smaller pale swallowwort seeds, as they may have been easier to consume or transport (Honek et al. Reference Honek, Martinkova and Jarosik2003). A trend for reductions in fatal germination and dead seeds and increases in unaccounted seeds from year 1 to year 2 at some burial depths are likely due to deterioration of the seeds and seed coats (Tables 2 and 3). Seeds recovered following the third and fourth growing seasons had become too deteriorated to assess accurately.
From a management perspective, the findings from this 4-yr soil seedbank study suggest that where tillage or other soil disturbance is possible (e.g., plowing of an old field, pasture), burial of seeds to a depth of least 10 cm is an effective means of preventing emergence of pale swallowwort seedlings. Burial at this soil depth will severely limit but not prevent emergence of black swallowwort seedlings. Therefore, burial of larger-sized black swallowwort seeds at greater depths is recommended. In natural sites where soil disturbance may be less practical, this strategy should likely only be considered if swallowwort densities are especially high. Since all seedlings of the two swallowwort species that emerged did so during the first 2 yr of the study, control efforts should focus on limiting seedling establishment during this critical period. The lack of seedling emergence for both species in years 3 and 4 of the study is encouraging and suggests a relatively transient seedbank. Therefore, if additional seed production is prevented or limited in these habitats, perhaps for a period of at least 3 yr, restoration of the affected area has a high likelihood of success.
Acknowledgments
We thank Françoise Vermeylen (Cornell University) for statistical advice. Financial support was provided by the U.S. Department of Agriculture (USDA), Agricultural Research Service project numbers 1907-22620-003-01S and 8062-22620-004-21S. Mention of trade names or commercial products in this article is solely for the purpose of providing specific information and does not imply recommendation or endorsement by the USDA. The USDA is an equal opportunity provider and employer. This material is also based upon work that is supported by the National Institute of Food and Agriculture, USDA, Hatch under #223820.








