Metabolic syndrome (MetS) is a complex of interrelated risk factors for CVD and type 2 diabetes (T2DM), including impaired glucose metabolism, dyslipidaemia, hypertension and abdominal adiposity(Reference Alberti, Eckel and Grundy1). The prevalence of MetS varies from 20–40 % worldwide depending upon the chosen MetS diagnostic criteria, as well as regional, lifestyle and ethnic variations, has been rapidly increasing over the past decades and is predicted to continue to increase(Reference Ranasinghe, Mathangasinghe and Jayawardena2,Reference Al-Oubaidy, Awadh and Lafta3) . Insulin resistance (IR) and compensatory hyperinsulinaemia characterised by an increased amount of circulating insulin are pivotal pathophysiological mechanisms in the development of MetS, which can be aggravated by obesity(Reference Avramoglu, Basciano and Adeli4–Reference Sowndarya, Joseph and Shenoy6). Identification of a favourable diet that can mediate glucoregulatory status has become increasingly relevant due to the staggering healthcare and economic burden of associated cardiometabolic comorbidities and the biological plausibility of the relationship between diet, as a modifiable risk factor and glycaemic control.
Pistachio (Pistacia vera L.) is a nutrient-dense nut with a cardioprotecitve dietary composition, including a favourable fatty acid profile rich in MUFA and PUFA, as well as vegetable proteins, dietary fibre, potassium, Mg and vitamin K(Reference Dreher7). These dietary factors have been shown to improve the glycaemic status, as evidenced by decreased IR and blood glucose concentrations(Reference Risérus8,Reference Azemati, Rajaram and Jaceldo-Siegl9) . Pistachio nuts also contain high phenolic compounds, such as anthocyanins, chlorophylls, carotenoids, phytosterols and γ-tocopherol(Reference Tomaino, Martorana and Arcoraci10) with strong antioxidant properties. These compounds have been shown to reduce oxidative stress and protection against the risk of chronic diseases(Reference Ghasemynasabparizi, Ahmadi and Mazloomi11). Beneficial effects of pistachio consumption on CVD risk factors such as lipid profile(Reference Hadi, Asbaghi and Kazemi12) and blood pressure(Reference Asbaghi, Hadi and Campbell13) have been reported in previous studies.
Some studies reported the effects of pistachio consumption on reducing fasting blood sugar (FBS), insulin concentrations, homeostasis model of insulin resistance (HOMA-IR)(Reference Hernández-Alonso, Salas-Salvadó and Baldrich-Mora14) and glycated Hb) levels(Reference Parham, Heidari and Khorramirad15). By contrast, others reported no changes in glycated Hb(Reference Hernández-Alonso, Salas-Salvadó and Baldrich-Mora14) FBS, insulin and HOMA-IR(Reference Sauder, McCrea and Ulbrecht16) levels following pistachio consumption. Inconsistent results from trials might be explained by different study designs, dose and duration of intervention, variety of age groups and gender.
In 2019, Ribeiro et al.(Reference Ribeiro, Silva and Almeida17) published a systematic review on this topic that included only four clinical trials representing 177 participants. The results indicated that pistachio consumptions significantly improved glycaemic control by reducing the FBS and HOMA-IR in T2DM patients. However, this study has only been reported qualitatively. In addition, several new trials are available and used different doses and duration to find the pistachio effect on glycaemic markers. Whether new RCT changed the result of the previous meta-analysis is unknown.
To address this gap in research, we conducted a systematic review and meta-analysis of randomised controlled trials to synthesise and quantify the effects of pistachio consumption on the markers of glycaemic control in individuals who present with an increased risk of CVD including MetS, dyslipidaemia, dysglycaemia, obesity and T2DM.
Methods
Ethical considerations
We adhered to the Preferred Reporting Items for Systematic Reviews and Meta-analysis guidelines guidelines for designing, conducting and reporting the present work(Reference Moher, Liberati and Tetzlaff18). No ethical committee approval was required or obtained due to the nature of this study.
Literature search strategy
Online databases (PubMed, Scopus, ISI Web of Science and Cochrane Library) were searched systematically from inception until June 2019 using the following keywords: (‘pistachio’ OR ‘pistachios’ OR ‘pistacia’) AND (‘intervention’ OR ‘intervention study’ OR ‘intervention studies’ OR ‘controlled trial’ OR ‘randomized’ OR ‘randomized’ OR ‘random’ OR ‘randomly’ OR ‘placebo’ OR ‘assignment’ OR ‘clinical trial’ OR ‘assignment’ OR ‘randomized controlled trial’ OR ‘randomized clinical trial’ OR ‘RCT’ OR ‘blinded’ OR ‘double blind’ OR ‘double blinded’ OR ‘trial’ OR ‘clinical trial’ OR ‘trials’ OR ‘pragmatic clinical trial’ OR ‘cross-over studies’ OR ‘cross-over’ OR ‘cross-over study’ OR ‘parallel’ OR ‘parallel study’ OR ‘parallel trial’). No language restriction was considered while searching the mentioned databases. Also, a reference list of all included relevant original research articles and review publications were manually screened to ensure the screening of any additional studies that were not identified in our online search results and to minimise any potential risk of publication bias.
Study selection
The reference manager software Endnote, version X8 (Thomson Reuters, NY, USA) was used to exclude duplicated publications and carry out the screening processes. The screening processes is summarised below.
First, two investigators (OA and EGh) independently scanned the titles and abstracts of the retrieved articles to exclude the ineligible studies. Any conference proceedings, protocols of RCT, letters to editors, commentaries, studies with insufficient data or duplicate publications of identical studies were excluded. The full texts of the remaining articles were reviewed by the same two researchers to attest the suitability for inclusion in the present study. In case of contradiction, a consensus was made through discussion with a third author (AH). Studies were included in the present analysis if: (1) they had an RCT design irrespective of a parallel or cross-over design and examined the effects of pistachio consumption (without attention to the pistachio varieties) on the biomarkers of glycaemic control; (2) the study population were adults (aged 18–60 years) with an increased risk of CVD including MetS, dyslipidaemia, dysglycaemia, obesity and T2DM and (3) the duration of the intervention was a minimum of 4 weeks to ensure sufficient time to observe clinically meaningful changes in the biomarkers glycaemic markers post-intervention.
Data extraction
Two independent researchers (E.Gh and O.A.) extracted the following information for each included studies using a standardised protocol as follows: (1) surname of the first author; (2) geographical location of the study; (3) publication year of the study; (4) study design; (5) study sample size; (6) basic characteristics of the study participants, including gender, age, BMI and health history; (7) dosage of pistachio consumption in the intervention; (8) duration of the intervention and (9) the means and sd for FBS concentrations, fasting serum insulin concentrations and HOMA-IR levels in each study group. We contacted the corresponding authors of the trials via email to obtain the required endpoints that were not reported in the full text of their study. Any disagreement or indistinct issues were resolved by consensus or consultation with a third reviewer (AH).
Quality assessments
Seven items were used to assess the methodological quality of the enrolled studies based on the Cochrane Risk of Bias Assessment tool(Reference Higgins and Green19): (1) random sequence generation; (2) allocation concealment; (3) blinding of participants and researchers; (4) blinding of the outcome evaluators; (5) incomplete outcome data; (6) selective reporting and (7) other sources of bias as described(Reference Higgins and Green19). We then evaluated each trial to ascertain whether there was a low, unclear or high risk of bias. Quality assessment was performed by two authors (OA and EGh) independently and their judgements were compared.
Statistical analyses
Statistical analyses were performed using STATA version 11.2 (Stata Corp.). To calculate effect sizes, mean changes and sd of fasted insulin and glucose concentrations and HOMA-IR levels were calculated by subtracting the post-intervention from the baseline concentration values in each of the intervention groups. In the event of sd of the mean difference was not stated, we imputed it based on Cochrane guidance(Reference Higgins, Thomas and Chandler20), using a correlation coefficient of 0·5. Effect sizes were expressed as the weighted mean difference between the groups who consumed pistachio and controls, with a 95 % CI. If only SE were reported, SD were calculated using the formula: (sd = se × square root (n)), where n was the number of subjects in each group. We used the I 2 statistics to test statistical heterogeneity among studies. An I 2 value > 50 % indicates substantial heterogeneity(Reference Higgins, Thompson and Deeks21). If significant heterogeneity between studies was observed, a random effects model was applied for all analyses. Also, subgroup analyses were performed to account for the impacts of certain factors including (1) the BMI of study participants (dichotomous: overweight (25–29·9 kg/m2) or obese (≥ 30 kg/m2)); (2) the duration of the intervention (dichotomous: ≥ 12 or < 12 weeks) and (3) the type of disease (polytomous: T2DM, MetS or other diseases). To detect the influence of a single study on the overall estimate, we conducted a sensitivity analysis by removing one study each time and re-calculating the analysis. Publication bias was assessed using a funnel plot and statistical analysis of Begg’s test. Results were considered significant at P < 0·05.
Results
Study selection
Fig. 1 summarises the flow of literature during the search and study selection protocol. A total of 1457 publications were identified in our initial online search, of which 476 duplicate records were excluded. The titles and abstracts of the 981 remaining articles were assessed, and 963 items were eliminated due to non-human RCT designs. Eighteen remaining items were then evaluated using by the review of the full text. Of the eighteen evaluated items, eleven records were excluded due to the lack of information about the glucoregulatory status(Reference Hernández-Alonso, Salas-Salvadó and Baldrich-Mora14,Reference Edwards, Kwaw and Matud22,Reference Kocyigit, Koylu and Keles23,Reference Sheridan, Cooper and Erario24,Reference Gebauer, West and Kay25,Reference Kay, Gebauer and West26,Reference Aldemir, Okulu and Neşelioğlu27,Reference West, Gebauer and Kay28,Reference Nieman, Scherr and Luo29,Reference Carughi, Bellisle and Dougkas30) and randomisation(Reference Sari, Baltaci and Bagri31). After all the exclusion criteria were applied, seven trials were remained(Reference Parham, Heidari and Khorramirad15,Reference Sauder, McCrea and Ulbrecht16,Reference Li, Song and Nguyen32,Reference Wang, Li and Liu33,Reference Gulati, Misra and Pandey34,Reference Hernández-Alonso, Salas-Salvadó and Baldrich-Mora35,Reference Kasliwal, Bansal and Mehrotra36) using the online search. Also, one trial(Reference Wilson37) was included in the final analyses via a manual search. The final analyses of the present meta-analysis included eight trials(Reference Parham, Heidari and Khorramirad15,Reference Sauder, McCrea and Ulbrecht16,Reference Li, Song and Nguyen32,Reference Wang, Li and Liu33,Reference Gulati, Misra and Pandey34,Reference Hernández-Alonso, Salas-Salvadó and Baldrich-Mora35,Reference Kasliwal, Bansal and Mehrotra36,Reference Wilson37) . All included trials(Reference Parham, Heidari and Khorramirad15,Reference Sauder, McCrea and Ulbrecht16,Reference Li, Song and Nguyen32,Reference Wang, Li and Liu33,Reference Gulati, Misra and Pandey34,Reference Hernández-Alonso, Salas-Salvadó and Baldrich-Mora35,Reference Kasliwal, Bansal and Mehrotra36,Reference Wilson37) reported the effects of pistachios on FBS concentrations, six(Reference Sauder, McCrea and Ulbrecht16,Reference Li, Song and Nguyen32,Reference Wang, Li and Liu33,Reference Gulati, Misra and Pandey34,Reference Hernández-Alonso, Salas-Salvadó and Baldrich-Mora35,Reference Wilson37) on insulin concentrations and three(Reference Parham, Heidari and Khorramirad15,Reference Sauder, McCrea and Ulbrecht16,Reference Hernández-Alonso, Salas-Salvadó and Baldrich-Mora35) on HOMA-IR levels.

Fig. 1. PRISMA flow diagram of study selection process.
Study characteristics
Characteristics of analysed trials are shown in Table 1. The eight trials(Reference Parham, Heidari and Khorramirad15,Reference Sauder, McCrea and Ulbrecht16,Reference Li, Song and Nguyen32,Reference Wang, Li and Liu33,Reference Gulati, Misra and Pandey34,Reference Hernández-Alonso, Salas-Salvadó and Baldrich-Mora35,Reference Kasliwal, Bansal and Mehrotra36,Reference Wilson37) included an overall 535 participants, of which 282 participants were allocated to pistachios consumption groups and 253 to control groups. The sample size of the included trials ranged from 22(Reference Wilson37) to 108(Reference Hernández-Alonso, Salas-Salvadó and Baldrich-Mora35) participants individually. Studies were published between 2009 and 2015 and were conducted in the USA(Reference Sauder, McCrea and Ulbrecht16,Reference Li, Song and Nguyen32,Reference Wilson37) , China(Reference Wang, Li and Liu33), India(Reference Gulati, Misra and Pandey34,Reference Kasliwal, Bansal and Mehrotra36) , Spain(Reference Hernández-Alonso, Salas-Salvadó and Baldrich-Mora35) and Iran(Reference Parham, Heidari and Khorramirad15). The duration of the intervention varied between 4(Reference Sauder, McCrea and Ulbrecht16) and 24(Reference Gulati, Misra and Pandey34) weeks across the included trials. The daily recommended dosage of pistachio consumption varied between 25 and 70 g or 20 % and 35 % of the total energy expenditure of study participants. Five(Reference Li, Song and Nguyen32,Reference Wang, Li and Liu33,Reference Gulati, Misra and Pandey34,Reference Kasliwal, Bansal and Mehrotra36,Reference Wilson37) and three(Reference Parham, Heidari and Khorramirad15,Reference Sauder, McCrea and Ulbrecht16,Reference Hernández-Alonso, Salas-Salvadó and Baldrich-Mora35) trials had parallel or cross-over designs, respectively. All trials were carried out on both females and males(Reference Parham, Heidari and Khorramirad15,Reference Sauder, McCrea and Ulbrecht16,Reference Li, Song and Nguyen32,Reference Wang, Li and Liu33,Reference Gulati, Misra and Pandey34,Reference Hernández-Alonso, Salas-Salvadó and Baldrich-Mora35,Reference Kasliwal, Bansal and Mehrotra36,Reference Wilson37) . The mean age of the participants ranged from 37·7(Reference Kasliwal, Bansal and Mehrotra36) to 57·1(Reference Wilson37) years and mean baseline BMI varied from 26·1(Reference Kasliwal, Bansal and Mehrotra36) to 32·0(Reference Parham, Heidari and Khorramirad15) kg/m2. Participants in the trials presented with cardiometabolic abnormalities including dysglycaemia(Reference Hernández-Alonso, Salas-Salvadó and Baldrich-Mora35), overweight(Reference Wilson37), obesity(Reference Li, Song and Nguyen32), MetS(Reference Wang, Li and Liu33,Reference Gulati, Misra and Pandey34) , dyslipidaemia(Reference Kasliwal, Bansal and Mehrotra36) and T2DM(Reference Parham, Heidari and Khorramirad15,Reference Sauder, McCrea and Ulbrecht16) .
Table 1. Characteristics of the included studies in the meta-analysis

DB, double-blinded; CG, control-group; CO, cross-over; PA, parallel; NR, not reported; F, female; M, male; TEE, total energy expenditure.
Quality assessments
The random allocation of participants was described in all included trials(Reference Parham, Heidari and Khorramirad15,Reference Sauder, McCrea and Ulbrecht16,Reference Li, Song and Nguyen32,Reference Wang, Li and Liu33,Reference Gulati, Misra and Pandey34,Reference Hernández-Alonso, Salas-Salvadó and Baldrich-Mora35,Reference Kasliwal, Bansal and Mehrotra36,Reference Wilson37) . Methods of random sequence generation were described in three of the trials(Reference Parham, Heidari and Khorramirad15,Reference Li, Song and Nguyen32,Reference Hernández-Alonso, Salas-Salvadó and Baldrich-Mora35) which had a low risk of bias, whereas the other five(Reference Sauder, McCrea and Ulbrecht16,Reference Wang, Li and Liu33,Reference Gulati, Misra and Pandey34,Reference Kasliwal, Bansal and Mehrotra36,Reference Wilson37) had a high risk of bias. Five trials(Reference Parham, Heidari and Khorramirad15,Reference Sauder, McCrea and Ulbrecht16,Reference Li, Song and Nguyen32,Reference Wang, Li and Liu33,Reference Hernández-Alonso, Salas-Salvadó and Baldrich-Mora35) exhibited a low risk of bias, and three(Reference Gulati, Misra and Pandey34,Reference Kasliwal, Bansal and Mehrotra36,Reference Wilson37) had an unclear risk when considering allocation concealment. The risk of bias was high in all of the evaluated studies with regard to the blinding of participants and researchers(Reference Parham, Heidari and Khorramirad15,Reference Sauder, McCrea and Ulbrecht16,Reference Li, Song and Nguyen32,Reference Wang, Li and Liu33,Reference Gulati, Misra and Pandey34,Reference Hernández-Alonso, Salas-Salvadó and Baldrich-Mora35,Reference Kasliwal, Bansal and Mehrotra36,Reference Wilson37) . Five trials(Reference Li, Song and Nguyen32,Reference Wang, Li and Liu33,Reference Hernández-Alonso, Salas-Salvadó and Baldrich-Mora35,Reference Kasliwal, Bansal and Mehrotra36,Reference Wilson37) had an unclear risk of bias when considering blinding of outcome assessors and three(Reference Parham, Heidari and Khorramirad15,Reference Sauder, McCrea and Ulbrecht16,Reference Gulati, Misra and Pandey34) had a low risk. Two trials(Reference Li, Song and Nguyen32,Reference Wang, Li and Liu33) had an unclear risk of bias when considering completing the outcome data and the other six(Reference Parham, Heidari and Khorramirad15,Reference Sauder, McCrea and Ulbrecht16,Reference Wang, Li and Liu33,Reference Gulati, Misra and Pandey34,Reference Hernández-Alonso, Salas-Salvadó and Baldrich-Mora35,Reference Kasliwal, Bansal and Mehrotra36) showed a low risk of bias. Concerning selective outcome reporting, five trials(Reference Sauder, McCrea and Ulbrecht16,Reference Gulati, Misra and Pandey34,Reference Hernández-Alonso, Salas-Salvadó and Baldrich-Mora35,Reference Kasliwal, Bansal and Mehrotra36,Reference Wilson37) had a low risk of bias (Table 2).
Table 2. Quality assessment of included studies based on Cochrane guidelines

L, low; H, high, U, unclear.
Meta-analysis
Effects of pistachio consumption on fasting blood sugar concentrations
Eight eligible studies with nine effect sizes, including a total of 535 participants, examined the effect of pistachio consumption on FBS. Combined results from the random-effects model indicated that FBS concentration significantly reduced following pistachio consumption (weighted mean difference: −522 mg/dl, 95% CI: (−7·80, −2·64), P < 0·001). There was a significant between-study heterogeneity (I 2 = 68·5 %, P = 0·001; Fig. 2). Results of subgroup analyses showed the benefits of pistachio consumption on decreasing FBS concentrations in all the evaluated subgroups independent of the duration of the trial and type of cardiometabolic abnormities; however, we observed these benefits only in participants with obesity, unlike their counterparts who presented with comorbid overweight (Table 3). Findings from sensitivity analysis showed that none of the studies significantly influenced the overall effect. Also, between-study heterogeneity was not affected by the omission of any of the studies.
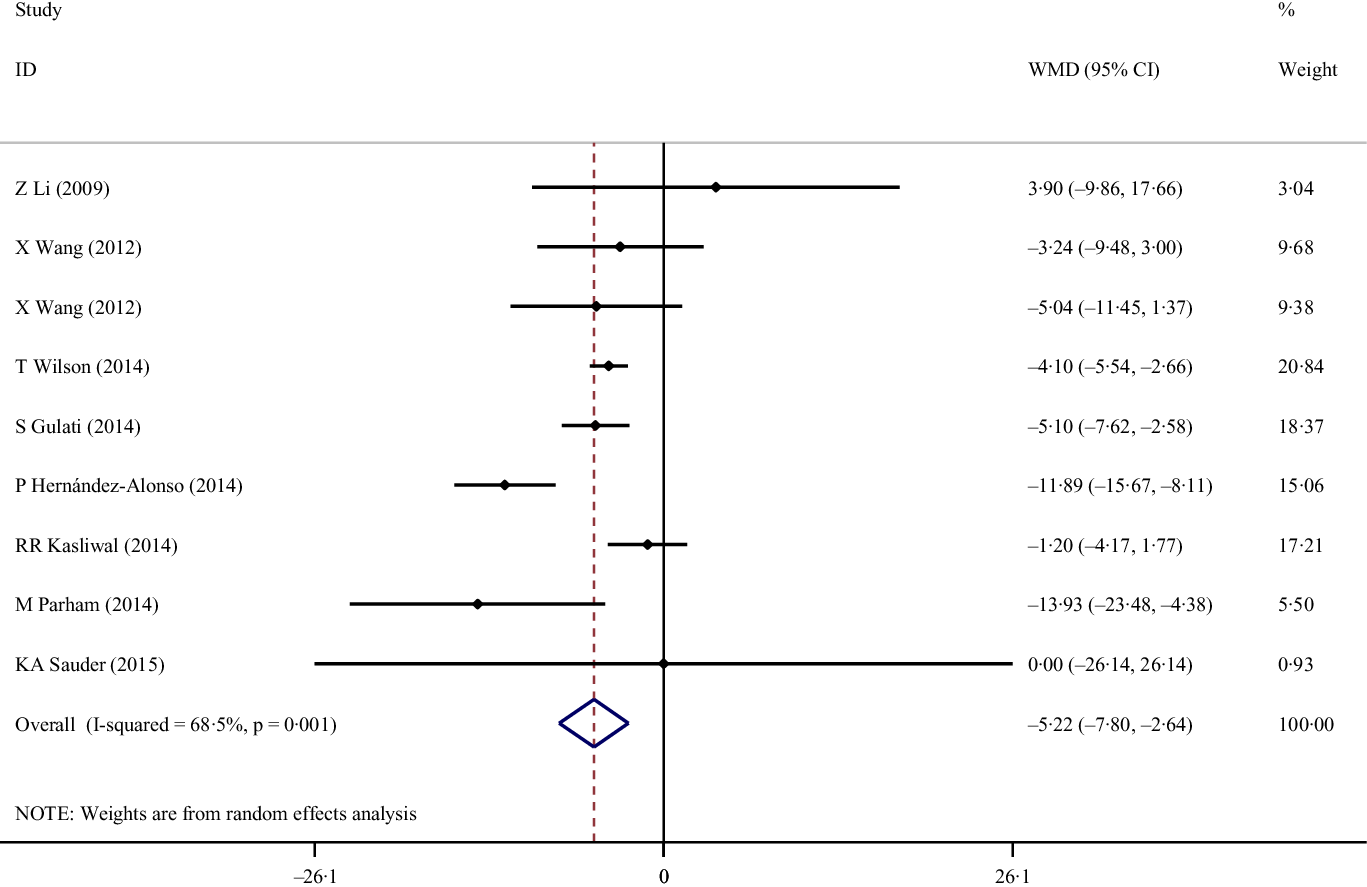
Fig. 2. Forest plot of the effects of pistachios on fasting blood sugar concentrations.
Table 3. Subgroup analyses of pistachios consumption on glycaemic profile
(Coefficient values and 95 % confidence intervals)

FBS, fasting blood sugar; T2DM, type 2 diabetes; MetS, metabolic syndrome; WMD, weighted mean differences.
Effects of pistachio consumption on insulin concentrations
A total of six trials with seven treatment arms, including 405 participants, reported the effects of pistachio consumption on fasting serum insulin concentrations. Pooled findings from the random-effects model showed the significant effects of pistachio consumption on serum insulin concentrations (weighted mean difference: –1·86 µIU/ml, 95 % CI: (–3·13, –0·59), P < 0·01), despite a significant heterogeneity across the evaluated trials (I 2 = 71·3 %, P < 0·01; Fig. 3). Results of subgroup analyses revealed the positive effects of pistachio consumption on reducing serum insulin concentrations only in overweight participants who did not present with T2DM and MetS following an intervention period of ≥ 12 weeks as shown in Table 3. By removing Wilson’s study(Reference Wilson37), the overall estimated effect of pistachio consumption on insulin concentrations changed to a non-significant value (–2·11 µIU/ml, 95 % CI: (–4·39, 0·16)). However, the omission of any of the studies could not reduce the heterogeneity.

Fig. 3. Forest plot of the effects of pistachios on insulin concentrations.
Effects of pistachio consumption on homeostasis model assessment of insulin resistance R
After pooling effect sizes from three studies with a total of 256 participants, we observed that pistachio consumption did not affect HOMA-IR levels in the group who consumed pistachio compared with controls (weighted mean difference: −0·66, 95 % CI (−1·89, 0·58), P = 0·30). We also observed significant heterogeneity across the evaluated trials (I 2 = 85·4 %, P < 0·01; Fig. 4). However, subgroup analysis was not conducted because of the limited number of studies. In addition, the overall meta-analysis of HOMA-IR was not sensitive to individual studies. However, between-study heterogeneity disappeared after excluding the Hernández-Alonso et al.(Reference Sheridan, Cooper and Erario24) study from the analysis (I 2 = 0·0 %, P = 0·89).

Fig. 4. Forest plot of the effects of pistachios on homeostasis model assessment of insulin resistance.
Publication bias
Visual inspection of the funnel plots showed no evidence of asymmetry in the effects of pistachio consumption on the glycaemic indices. We observed no publication bias for FBS (P = 0·83), insulin (P = 0·88) and HOMA-IR (P = 0·60) levels. It should be noted that, given the small number of studies included in the present analysis (< 10 studies), the funnel plot and statistical analysis of Begg’s test should be interpreted with caution.
Discussion
The present study is the first systematic review and meta-analysis of RCT that evaluated the effects of pistachio consumption on glucoregularoy status in individuals at risk for CVD.
Our results showed that pistachio consumption can effectively reduce FBS and insulin concentrations compared with control. However, the results of this study should be interpreted with caution due to the high heterogeneity.
Results of subgroup analyses reiterated the benefits of pistachio consumption on decreasing FBS in cohorts with obesity independent of the duration of the trial and type of cardiometabolic abnormities compared with those with comorbid overweight. By contrast, pistachios consumption decreased serum insulin concentrations in overweight participants who did not present with T2DM and MetS and not in longer intervention periods (< 12 weeks).
The positive effects of pistachio consumption on glucoregulatory status could be attributed, in part, to the low glycaemic index pistachio nuts(Reference Kendall, Josse and Esfahani38). Consumption of pistachio nuts with high carbohydrate foods with a high glycaemic index, including parboiled rice, pasta and mashed potatoes, has been shown to slow the intestinal absorption of carbohydrates and reduce total postprandial glycaemic response by 20–30 %(Reference Parham, Heidari and Khorramirad15,Reference Kendall, Josse and Esfahani38) . Consumption of pistachio nuts has been also associated with reduced rates of dietary fat digestion, slow energy release and increased digestibility of dietary fibre(Reference Baer, Gebauer and Novotny39). The favourable nutritional composition of pistachio nuts, including high MUFA and PUFA and low SFA content, may also contribute to these positive effects through mechanisms described(Reference Siri-Tarino, Sun and Hu40).Replacement of SFA by MUFA and PUFA has been shown to improve glycaemic control and IR(Reference Paniagua, de la Sacristana and Sánchez41,Reference Gillingham, Harris-Janz and Jones42,Reference Moon, Lee and Kang43) . Pistachio nuts are rich in phenolic compounds and hypocholestrolemic agents including anthocyanins, chlorophylls, catechins, carotenoids, phytosterols and tocopherol with antioxidant properties. These biological compounds elicit anti-glycaemic effects(Reference Liu, Blumberg and Chen44,Reference Bellomo and Fallico45,Reference Tomaino, Martorana and Arcoraci10) and were shown to reduce the risk of T2DM(Reference Montonen, Knekt and Järvinen46). Quercetin and catechin compounds have been reported to modulate the activity of intestinal α-glucosidase and pancreatic α-amylase and regulate intestinal glucose absorption(Reference Akkarachiyasit, Charoenlertkul and Yibchok-Anun47,Reference Wilson, Luebke and Morcomb48) . Pistachio nuts have been shown to inhibit the oxidation of aldohexose(Reference Wang, Li and Liu33). These mechanisms may explain the improved glycaemic response following the consumption of pistachio nuts. Pistachio nuts also have other favourable dietary factors, including a high Mg(Reference Hata, Doi and Ninomiya49) and phosphorus content, with implications in the metabolism of B group vitamins, regulation of endocrine hormones and modulation of glucose response(Reference Ghasemynasabparizi, Ahmadi and Mazloomi11). Long-term consumption of pistachio nuts has been shown to induce glucagon-like peptide-1 release and insulin-sparing effects in individuals with prediabetes(Reference Hernández-Alonso, Salas-Salvadó and Baldrich-Mora14). Similarly, the intake of pistachio nuts has been shown to upregulate the secretion of glucagon-like peptide-1 in individuals with MetS(Reference Kendall, West and Augustin50). The beneficial effects of pistachio nuts on insulin metabolism could be attributed, in part, to increased glucagon-like peptide-1 levels. Glucagon-like peptide-1 and gastric inhibitory polypeptide are gastric hormones which can stimulate pancreatic insulin secretion and suppress glucagon secretion in a glucose-dependent manner(Reference Yabe and Seino51). Other mechanisms have been also proposed to explain the positive effects of pistachio nuts intake on glycaemic control and insulin sensitivity, including the modulation of miRNA(Reference Ortega, Mercader and Moreno-Navarrete52,Reference Hernández-Alonso, Giardina and Salas-Salvadó53) although the exact molecular mechanism remain to be elucidated.
Implications for practice and safety
Severe adverse effects were not reported following the consumption pistachio nuts. However, gastrointestinal symptoms, including bloating, diarrhoea, constipation, flatulence and abdominal pain, have been reported in some individuals attributed to the high fructan content of pistachio nuts(Reference Fedewa and Rao54). Further, the high energy content of pistachio nuts(Reference Dreher7) could result in exceeding the daily total energy requirements and obesity; however, a previous trial showed that the daily consumption of either a high or recommended dose of pistachio nuts for 12 weeks in individuals did not change BMI or waist-to-hip ratio in individuals with MetS compared with controls(Reference Wang, Li and Liu33) – a finding that has been corroborated in other populations(Reference Edwards, Kwaw and Matud22,Reference Sheridan, Cooper and Erario24) . Current evidence does not support a relationship between nut consumption and weight gain, albeit nuts are energy-dense food(Reference Flores-Mateo, Rojas-Rueda and Basora55). Indeed, the consumption of nuts has been associated with reduced risk of obesity, due to the inhibition of enzymatic activity of amylase and α-glucosidase(Reference Parham, Heidari and Khorramirad15,Reference Coates and Howe56) , reduced rate of carbohydrate and fat digestion and absorption and inducing satiety, which decrease the consumption of unhealthy foods(Reference Bes-Rastrollo, Wedick and Martinez-Gonzalez57,Reference Freisling, Noh and Slimani58) .
Strength and limitations
The strengths of the present study were the completion of the analyses based on mean changes between intervention and control groups that is more accurate than changes within groups and yielded greater effect sizes. In addition, the study complied with the Preferred Reporting Items for Systematic Reviews and Meta-analysis guidelines guidelines and comprehensive search. Our observations may be interpreted with cautioning due to some limitations, including the potential influence of confounding factors such as racial and lifestyle factors across the -lkjstudied cohorts and types of pistachio on the efficacy of pistachio consumption on outcomes which is not uncommon in studies of this type. Also, the pistachio varieties affect the clinical results. As degree of mastication can also influence the glycaemic and insulinemic response to nuts(Reference Cassady, Hollis and Fulford59). Further, the evaluated trials had a small sample size with shorter intervention periods.
Conclusions
Pistachio consumption may improve glucoregulatory status in individuals at risk for CVD, as evidenced by decreasing fasting glucose and insulin concentrations. Future long-term large-scale trials are needed to confirm our observations.
Acknowledgements
None.
The present work did not receive any specific grant from funding agencies in the public, commercial or not-for-profit sectors.
O. A. and E. Gh. designed and conceived the study, searched databases, screened articles and extracted data. E. Gh. performed the statistical analyses. O. A. and E. Gh. interpreted the results and drafted the manuscript with contributions from A. H. and M. K. All authors reviewed and commented on subsequent drafts of the manuscript. O. A. and E. Gh. have the primary responsibility for the final content.
The authors declare that they have no competing interests.












