An evaluation of the nutrient requirements of domestic cats has led to the conclusion that cats are metabolically attuned to carnivorous dietsReference MacDonald, Rogers and Morris1. As such, cats serve as useful models for studies of comparative nutrition and metabolism. Recent epidemiological investigations have yielded evidence which attributes an increased risk for obesity in cats to commercially available, high-carbohydrate, dry-expanded diets as opposed to commercially available, canned, high-fat dietsReference Scarlett, Donoghue, Saidla and Wills2. These observations in cats deviate from experimental finding in the rodent literature, where diets high in fat are found to cause weight gain and induce obesityReference West, Boozer, Moody and Atkinson3, Reference Oscai, Brown and Miller4. Mechanisms by which high-carbohydrate diets might cause obesity in cats are speculative. A high constitutive rate of glucose production from amino acid catabolism and a low capacity for glucose disposal are suggested to direct unutilised carbohydrate toward fatty acid synthesis and storageReference Zoran5. An alternative mechanism is that high dietary carbohydrate exposure extraordinarily prolongs insulin release, resulting in diversion of dietary fat away from oxidation toward storage in adipose. This latter mechanism seems more plausible than the former because feline liver and adipose tissues appear to poorly utilise glucose for fatty acid synthesisReference Richard, Holck and Beitz6.
Ingredients, energy density and palatability of high-carbohydrate, commercial dry-expanded diets are substantively different from those in most commercial canned diets. Because of this, factors other than carbohydrate content might account for an increased obesity risk when dry-expanded diets are fed. Recently, the effect of two concentrations of dietary fat (11 and 21 %; w/w) on body fat mass were evaluated in young cats given commercial dry-type diets soon after gonadectomy (GX; orchiectomy and ovariohysterectomy)Reference Nguyen, Dumon, Siliart, Martin, Sergheraert and Biourge7. Expansion of fat mass occurred with the use of both diets, but the effects on fat mass were greatest with the higher-fat diet. The observations were consistent with obesity risk being less when ‘grocery-store’, dry-expanded diets were used in place of higher-fat, specialty and therapeutic dietsReference Scarlett, Donoghue, Saidla and Wills2. However, findings of other research indicate no significant effect of dietary fat content on body weight when dry-typeReference Kane, Leung, Rogers and Morris8 or cannedReference Lester, Czarnecki-Maulden and Lewis9 diets are used. Cats evaluated in the negative-finding studies were adults (2–4 years of age) and in some cases had gonadectomies long before dietary-fat effects were evaluated.
An understanding of the relationship between diet composition and obesity risk is necessary for healthful management of cats. Over 37 million US households are reported to own cats10. A prevalent healthcare issue among privately owned cats (25–40 %) is overweight to obese body conditionsReference Scarlett, Donoghue, Saidla and Wills2, Reference Lund, Armstrong, Kirk and Klausner11. The overweight to obese cat is at greater risk for lameness, oral disease, dermatopathy, urinary tract disease, neoplasia and diabetes mellitusReference Lund, Armstrong, Kirk and Klausner11, Reference Scarlett and Donoghue12.
In the present study, body condition and endocrine and biochemical factors reputed to affect or associate with body condition were determined in male and female cats fed diets differing in carbohydrate concentration by isoenergetic substitution with dietary fat. Because privately owned cats are commonly gonadectomised and maintained on commercial dry-type diets in the USA, a study objective was evaluation of whether manipulation of dietary carbohydrate content might serve as an effective means of reducing weight gain induced by GX.
Materials and methods
Animals
Twelve male and twelve female purebred domestic shorthair cats, between 4 and 7 months of age, of the specific pathogen-free colony of the Feline Nutrition and Pet Care Center (University of California, Davis, CA, USA) were used. Throughout the study, diet and water were made continuously available, body weights were determined every 7 to 14 d, temperature was maintained between 17 and 26°C, and light and dark periods were 14 and 10 h, respectively. Before the study, the cats were housed in group cages and provided ad libitum access to a nutritionally complete and balanced commercial extruded dry-type diet formulated for growth13. Husbandry and the experimental protocols were reviewed and approved by the University Animal Use and Administrative Advisory Committee. The cats were maintained in accordance with the NRC Guide for the Care and Use of Laboratory Animals14.
Experimental protocol
Cats were individually housed and fed for 3 months a purified adaptation diet that was nutritionally complete and balanced for growth15. Subsequently, cats were evaluated for food intake determinations for 2 months, and then the cats were assigned to four groups of six cats until the end of the study. Each group was balanced for body weight and sex and received a unique purified diet that differed in fat and carbohydrate proportion from the adaptation diet. At 3 months after the dietary group assignments, males were orchiectomised and females were ovariohysterectomised by standard techniquesReference Wilson, Hayes, Bojrab, Crane and Arnoczky16, Reference Crane, Smallwood, Wensing, Bojrab, Crane and Arnoczky17. Jugular venous blood (3 ml) was collected by venepuncture for plasma biochemical analyses 2 weeks before, and every 2 weeks thereafter until 9 weeks after GX. Diet was not withheld before the blood collections. Blood samples were transferred to glass tubes containing 5·5 mg K3EDTA (BD Vacutainer, Franklin Lakes, NJ, USA) and briefly (about 30 min) stored in ice before being centrifuged (about1200 g; 10 min) for plasma extraction. Plasma was stored at − 80°C until later biochemical analyses. At 17 weeks after GX, body fat and lean masses were determined by a previously validated isotopic water-dilution techniqueReference Backus, Havel, Gingerich and Rogers18. For this, jugular venous blood (6 ml) was collected by venepuncture 2–3 h after subcutaneous administration of salinated (9 g sodium chloride/l) 2H-labelled water (99 %; Isotec, Inc., Miamisburg, OH, USA). One male died unexpectedly during the adaptation period without premonitory signs of illness (for example, changed activity, decreased food intake, weight loss). Necropsy findings were consistent with acute ventilatory arrest possibly caused by an idiopathic asthmatic episodeReference Moise and Spaulding19.
Diets
The adaptation and study diets contained similar purified ingredients (Table 1). The principal carbohydrate sources were maize starch and sugar, while the fat sources were chicken fat, maize oil and hydrogenated beef tallow. The sugar:starch weight ratio was the same among the diets, except where sugar was limited to 20 % of DM to minimise metabolisable energy (ME) loss from fructosuriaReference Kienzle20. Maize oil and single-cell oil (ARASCO; Martek Bioscience Corp., Columbia, MD, USA) were added to the lowest-fat diet (diet 2) to meet linoleic and arachidonic acid requirements, respectively15. These oils were added to the other diets so that all study diets contained similar weight proportions of the oils. Protein:ME ratios among the study diets were similar (Table 2). The ME distributions in fat and carbohydrate in the study diets were selected to approximate distributions typically observed in feline commercial canned diets (diet 1) and dry-type diets that are low (diets 3) to moderate (diet 4) in fat. Warm water was variably added to the diets for the purpose of producing similar textured pellets following extrusion of the diets through a meat-grinder die. Water was not added to the lowest-carbohydrate diet (diet 1) because the fat content of the diet was sufficient for forming soft pellets. To firm pellets of diet 1, hydrogenated beef tallow was added 1 : 5 (w/w) with the chicken fat. So that the other diets would be of similar fat composition, the same beef tallow:chicken ratio was used.
Table 1 Ingredient composition of adaptation and study diets

* ARASCO, 33–44 % arachidonic acid (Martek Biosciences, Columbia, MD, USA).
† Composition was (g/kg mixture): CaHPO4, 390; KCl, 200; NaHCO3, 140; NaCl, 122; CaCO3, 110; KHCO3, 100; K2HPO4, 90; MgSO4, 45·0; ferric-citrate·3H2O, 250 mg; ZnSO4·7H2O, 111 mg; MnSO4.H2O, 96 mg; CuSO4·5H2O, 20 mg; NiCl2·6H2O, 7·5 mg; CrCl·6H2O, 6·5 mg; SnCl2·2H2O, 2·5 mg; (NH4)6Mo7O4·4H2O, 1·0 mg; KI, 0·75 mg; Na2SeO3, 0·75 mg; NH4VO3·4H2O, 0·5 mgReference Williams, Morris and Rogers50.
‡ Composition was (g/kg mixture): sucrose, 84·3; ascorbic acid, 20; myo-inositol, 20; dl-α-tocopheryl acetate, 16; nicotinic acid, 10; thiamin·HCl, 2·5; calcium pantothenate, 2·0; menadione, 1·5; retinyl palmitate, 1·1; riboflavin, 1·0; pyridoxine, 1·0; folic acid, 1·0; biotin, 0·1; cholecalciferol, 5·0 mg; cobalamin, 5·0 mgReference Williams, Morris and Rogers50.
Table 2 Calculated weight and metabolisable energy (ME) density and percentage distribution of carbohydrate, fat and protein in purified diets*
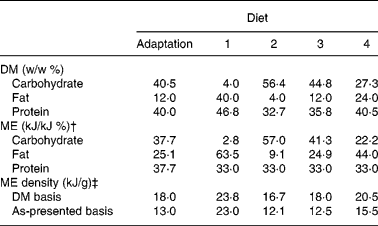
* Maize starch and sugar, casein and soya protein, and chicken fat, tallow and vegetable oils were considered dietary carbohydrate, protein and fat sources, respectively.
† It was assumed that protein, fat and carbohydrate contained 17, 38 and 17 kJ/g, respectivelyReference Atwater and Bryant22.
‡ Calculated using the arithmetic mean of fresh diet moisture content and moisture content in diet remaining in food bowls at the time of replacement with fresh diet.
Food intake determination
Daily DM food intakes were determined until 12 weeks post-GX, when mean body weights of groups plateaued. The intakes were determined by multiplying weight of diet consumed by mean fractional DM content of respective diets. Fractional DM of diets was determined from samples collected for 5 weeks before and 5 weeks after introduction of the study diets. Fractional DM for the adaptation and study diets 1, 2, 3 and 4 were 72 (sd 6), 96 (sd 5), 72 (sd 5), 70 (sd 4) and 74 (sd 3) %, respectively.
Biochemical analyses
Plasma and serum insulin and ghrelin concentrations were assayed in duplicate 50 μl samples using commercial RIA kits (PI-12K and GHRT-89HK, respectively; Linco Research, Inc., St Joseph, MO, USA). Plasma and serum leptin concentrations were determined in duplicate 200 μl samples with a RIA based on polyclonal antiserum with multi-species leptin cross-reactivityReference Delavaud, Bocquier, Chilliard, Keisler, Gertler and Kann21. Each RIA was validated from parallelism responses to increasing dilutions of plasma from three or more cats. Glucose and TAG concentrations in plasma and serum were determined with an automated chemistry analyser (AU440e; Olympus America, Inc., Melville, NY, USA) by the Veterinary Diagnostic Laboratory, College of Veterinary Medicine, University of Missouri, Columbia, MO, USA. Because of cost constraints, analysis of ghrelin was limited to samples collected 2 weeks before and 1, 3, 5, 7, 9 and 17 weeks after GX.
Statistical analysis
The effects of dietary group assignment (diet 1, diet 2, diet 3, diet 4) and sex (male, female) on variable observations were evaluated for each of three experimental periods – the adaptation (pre-GX, common diet), diet-change (pre-GX, group-defining diets) and post-GX (group-defining diets) periods. The variables studied were food intake and body weight for all periods, plasma concentrations of insulin, leptin, ghrelin, glucose and TAG for the diet-change and post-GX periods, and body lean and fat masses for the post-GX period. General linear models ANOVA and post hoc least-squares difference analysis were used to determine the significance of variable differences with diet and sex. A repeated-measures ANOVA model was used when observations on variables were repeated three or more times. Percentage change data were logarithmic transformed before analyses. Paired t tests were used to evaluate effects among variables when only two observations were considered. Regression analysis was used for evaluation of effect of concentration of dietary fat on body lean mass, body fat mass, percentage body fat and percentage change in body weight. Computer software used for the analyses was SAS 9.1 (SAS Institute Inc., Cary, NC, USA). Unless specified, variance estimates are reported as mean values with their standard errors. Differences with P ≤ 0·05 were considered significant; those with P>0·05 < 0·10 were considered a trend.
Results
Food intake
Food intake was evaluated as intake of ME, because the study diets varied in moisture content and energy density. Intakes of ME were calculated as the product of DM intake and DM ME density as estimated from the Atwater values of 17, 38 and 17 kJ/g for dietary protein, fat and carbohydrate, respectivelyReference Atwater and Bryant22. Because within-animal variation in food intake was typically large between days, 7 d mean ME intakes were determined for each cat and used in analyses of effects of diet, sex and GX. Throughout the study, ME intakes by the males were greater (P < 0·05) than those by the females. During the adaptation period, no group differences (P>0·05) were observed in ME intakes (Table 3).
Table 3 Daily dietary mass and energy intakes by eleven male and twelve female cats during the last week of each experimental period for which food intakes were determined (Mean values with their standard errors)

ME, metabolisable energy; GX, gonadectomy.
* Intakes during week 13 after diet change.
† Intakes during post-GX week 12.
Within-group variance in ME intake was large for both sexes (Table 3). Among dietary groups, maximum intakes were between 150 and 270 % of minimum intakes. Because of this, treatment effects on food intake were evaluated against percentage change in ME intake. ME intakes observed in cats assigned to each group during the last week of the adaptation period were used as reference intakes for calculating percentage change in ME intake caused by introduction of the study diets; diets 1, 2, 3 and 4. When the study diets were introduced (Fig. 1; weeks 1 to 13), ME intake changed with time (P < 0·01) and significant time × diet (P < 0·02) and time × sex interactions (P < 0·01) were observed (Table 4). During weeks 1, 2, 3 and 6, percentage change in ME intake by females given the highest-fat diet (64 % ME) was greater (P < 0·05) than that by females given the other study diets. In males, percentage change in ME intake was greater (P < 0·05) in cats given the highest-fat diet than in cats given the lowest-fat diets (9 and 25 % ME) during week 1. The percentage change in ME intake of the highest-fat diet was greater (P < 0·05) than that of the next highest-fat diet (44 % ME) during weeks 4 and 6 by the females and during week 4 by the males. An additional dietary difference was found for females during week 6, when the percentage change in ME intake of the 9 % ME diet was greater (P < 0·05) than that of the percentage change in ME intake of the 44 % diet.

Fig. 1 Percentage changes in metabolisable energy (ME) intake by male (A) and female (B) cats adapted to the same purified diet (weeks − 8 to 0), then given purified diets of varying fat content before (weeks 1 to 13) and after (weeks 14 to 26) being gonadectomised. (Δ), 64 % ME as fat, diet 1; (□), 9 % ME as fat, diet 2; (■), 25 % ME as fat, diet 3; (▲), 44 % ME as fat, diet 4. Values are means of observations for three cats (or two cats in the case of males given the 9 % ME as fat diet). Changes in ME intake before gonadectomy are relative to week 0 ME intakes. Changes in ME intake following gonadectomy are relative to week 13 ME intakes. For clarity, sem estimates are not plotted. Means of sem observed across groups for the adaptation, pre-gonadectomy varying dietary fat, and post-gonadectomy periods are 8·6, 11·1 and 19·4 %, respectively. *Mean value for diet 1 was significantly greater than those for diets 2 and 3 (P < 0·05). †Mean value for diet 1 was significantly greater than that for diet 4 (P < 0·05). ‡Mean value for diet 1 was significantly greater than those for diets 2, 3 and 4 (P < 0·05). §Mean value for diet 2 was significantly greater than that for diet 4 (P < 0·05).
Table 4 Significance of percentage change in metabolisable energy (ME) intake and body weight of eleven male and twelve female cats during 8 weeks of adaptation to a purified diet, 13 weeks after introduction to diets varying in fat content (9, 25, 44 and 64 % ME as fat) and 13 weeks after gonadectomy
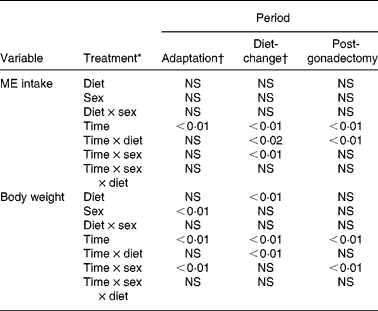
* For the adaptation period, ‘Diet’ represents dietary group to which the cats were later assigned.
† The reference body weights for determination of percentage change were body weights observed during the last week of the adaptation period.
‡ The reference body weights for this period were those observed during the week immediately preceding the period.
ME intakes during the week preceding GX (Fig. 1; week 13) were used as reference intakes for calculating percentage changes in ME intake induced by GX. During the post-GX period (weeks 14 to 26), percentage change in ME intake did not significantly vary with diet or between males and females. However, percentage change in ME intake did vary with time (P < 0·01) and a time × diet interaction (P < 0·01) occurred (Table 4). By the last week of the post-GX period, mean ME intake across the dietary groups was 163 (sem 8) % of reference pre-GX ME intakes.
Body weight
During the adaptation period, body weights among the cats did not vary with dietary group assignment, and the body weights of males were greater (P < 0·01) than those of females. Time (P < 0·01) and time × sex interaction (P < 0·01) effects were observed during this period. Mean body weights of males and females increased (P < 0·02) by 9·6 (sem 1·6) and 3·8 (sem 1·4) %, respectively. The percentage increase in males was greater (P < 0·03) than that in females. During the last week of the adaptation period, mean body weights of males assigned to dietary groups 1, 2, 3 and 4 were 4·41 (sem 0·46), 4·30 (sem 0·55), 4·58 (sem 0·20) and 4·53 (sem 0·04) kg, respectively, while those of females were 2·72 (sem 0·25), 2·70 (sem 0·21), 2·75 (sem 0·17) and 2·72 (sem 0·10) kg, respectively. Although mean body weights among males and females of each group were similar, within-group variances were large in some of the groups; therefore, percentage change in body weight was used in the evaluation of effects of diet and GX. Reference body weights were those observed during the week immediately preceding study diet introduction and body weights observed the week before gonadectomies.
Percentage change in body weight varied (P < 0·01) among the dietary groups by week 13 following introduction of the study diets (Fig. 2). Change in body weight of cats given the highest-fat diet (64 % ME as fat) was greater (P < 0·05) than that in cats given the other diets during the last few weeks of the period. By week 13, body weights of cats given the highest-fat diet increased by a mean of 17·0 (sem 5·0) %, while for cats given the other diets, no significant change in body weight was observed. Body weights of males were consistently greater (P < 0·05) than those of females during this period.

Fig. 2 Percentage changes in body weight of male (A) and female (B) cats adapted to the same purified diet (weeks − 8 to 0), then given purified diets of varying fat content before (weeks 1 to 13) and after (weeks 14 to 29) being gonadectomised. (Δ), 64 % Metabolisable energy (ME) as fat, diet 1; (□), 9 % ME as fat, diet 2; (■), 25 % ME as fat, diet 3; (▲), 44 % ME as fat, diet 4. Values are means of observations of three cats (or two cats in the case of males given the 9 % ME as fat diet). Changes in body weight before gonadectomy are relative to week 0 body weights. Changes in body weight following gonadectomy are relative to week 13 body weights. For clarity, sem estimates are not plotted. Means of sem observed across groups for the adaptation, pre-gonadectomy varying dietary fat, and post-gonadectomy periods are 2·4, 3·1 and 5·9 %, respectively. *Mean value for diet 1 was significantly greater than that for diet 2 (P < 0·05). †Mean value for diet 1 was significantly greater than those for diets 2, 3 and 4 (P < 0·05). ‡Mean value for diet 1 was significantly greater than those for diets 2 and 3 (P < 0·05). §Mean value for diet 1 was significantly greater than those for diets 2 and 4 (P < 0·05).
During the post-GX period, no effect of diet or sex on percentage change in body weight was observed (Table 4), yet time (P < 0·01) and time × sex (P < 0·01) effects were found (Fig. 2). By week 13 of the post-GX period, mean body weight of males increased by 10·4 (sem 3·5) % while that of females increased by 39·4 (sem 5·1) %. The percentage change in body weight at the end of the post-GX period, relative to the body weight at the end of the adaptation period, was determined for each cat. Regression analyses of these observations revealed that percentage change in body weight increased with dietary fat, and that percentage change in body weight was greater at any level of dietary fat in females (weight change = 122+0·50 (% fat ME) %; r 0·59; P < 0·04) than males (weight change = 92+0·61 (% fat ME) %; r 0·80; P < 0·03).
Body composition
During post-GX week 17, when body compositions were determined after withholding of diet, body weights and lean masses of males were greater (P < 0·05) than those of females (Table 5). Body fat mass and body weight percentage as fat of males were not significantly different from those of females. Body fat masses of cats given the 64 % ME as fat diet were greater (P < 0·05) than those in cats given the 9 and 25 % fat diets but not in cats given the 44 % fat diet. Lean mass did not differ with diet. The body fat observations were pooled within treatment, across sex, and regressed against dietary energy as fat using linear and quadratic models. Body fat mass and percentage body fat increased with increasing dietary fat (P < 0·05). Greater correlations were found with quadratic than linear models (Fig. 3). The quadratic relationships indicated that body fat mass would have been minimised when dietary fat was 22 % of ME, while percentage body fat would have been minimised when dietary fat was 19 % of ME.
Table 5 Body composition 17 weeks after gonadectomy of eleven male and twelve female cats given for 32 weeks purified diets containing 9, 25, 44 and 64 % metabolisable energy as fat* (Mean values with their standard errors)
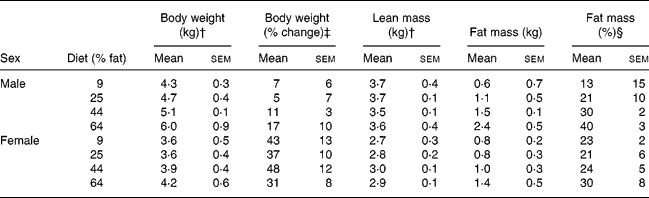
† Mean values for males were greater than those for females (P < 0·05).
‡ Percentage change in body weight from the week preceding gonadectomy. Body weight increased (P < 0·05) in both sexes, but the increase was greater (P < 0·05) in females than males.
§ Body fat mass expressed as a percentage of body weight.

Fig. 3 Body fat mass (●) and body weight percentage (○) as fat in cats as a function of dietary fat content as metabolisable energy (ME). The cats were given diets of varying fat content for 32 weeks and gonadectomised 17 weeks before the body-weight and fat-mass determinations. Values are means (observations for five to six cats), with their standard errors represented by vertical bars. Plotted lines are linear (—,—) and quadratic (…, –-) regression functions derived for body fat mass (fat mass = 0·59+0·018 (% fat ME) kg; r 0·49; P < 0·02 and fat mass = 1·1 − 0·015 (% fat ME) +0·00 044 (% fat ME)2; r 0·53; P < 0·05) and body fat percentage (fat percentage = 18+0·23 (% fat ME) %; r 0·45; P < 0·04 and fat percentage = 26 − 0·33 (% fat ME) +0·0074 (% fat ME)2; r 0·51; P < 0·06). ↑ , Quadratic function minima.
Plasma insulin, glucose and triacylglycerol
At 2 weeks before GX, plasma concentrations of insulin in cats given the highest-fat diet (64 % ME) were greater (P < 0·05) than those of cats given the lower-fat diets (9, 25 and 44 % ME) (Table 6). During the post-GX period, a diet effect on plasma insulin was not observed, but time (P < 0·01) and time × sex (P < 0·05) effects were observed. Plasma concentrations of insulin in females were greater (P < 0·05) and typically more than twice those in the males during post-GX weeks 6, 7 and 8 (Fig. 4). Plasma concentrations of glucose and TAG did not differ with diet or sex before or after GX. Plasma concentrations of glucose differed with time (P < 0·01) following GX, but a consistent trend with time was not observed (Table 7).
Table 6 Plasma biochemical and hormone concentrations in eleven male and twelve female, sexually intact, cats given for 12 weeks purified diets containing 9, 25, 44 and 64 % metabolisable energy as fat* (Mean values with their standard errors)
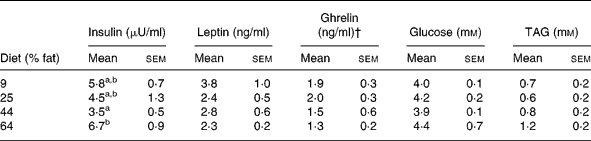

Fig. 4 Plasma insulin concentrations during 9 weeks following gonadectomy of eleven male (●) and twelve female (○) cats given purified diets of varying metabolisable energy as fat (9 to 64 %). Values are means (observations for two to three cats), with their standard errors represented by vertical bars. *Mean value was significantly different from that for females (P < 0·05).
Table 7 Plasma insulin, glucose and triacylglycerol concentrations in eleven male and twelve female cats given diets for ad libitum consumption before and after gonadectomy (GX) (Mean values with their standard errors)

* Mean of concentrations across diet and sex 2 weeks before GX.
† Mean of concentrations across diet and sex observed during 9 weeks after GX.
Plasma leptin and ghrelin
Plasma concentrations of leptin before and after GX did not vary with diet, sex or percentage change in body weight (Table 7). Pre-GX plasma ghrelin concentrations did not vary with dietary fat, but concentrations in females were greater (P < 0·05) than those in males (Table 6). The sex difference was not found during the post-GX period. For weeks 1, 3 and 7 after GX, plasma ghrelin concentrations in cats given the highest-fat diet were significantly lower (P < 0·05) than those in cats given the lowest-fat diets, 25 and 9 % ME as fat (Fig. 5). The mean of plasma ghrelin concentrations determined for the post-GX period decreased with increasing dietary percentage ME as fat (ghrelin concentration = 2·4 − 0·017 (% fat ME) ng/ml; r 0·49; P < 0·02). When food was withheld during week 17 of the post-GX period, plasma ghrelin concentration did not vary among dietary groups or between males and females. Plasma ghrelin concentrations tended to decrease (P < 0·06) as body weight increased (ghrelin concentration = 2·5 − 0·24 (body weightkg) ng/ml; r 0·36; P = 0·09).

Fig. 5 Plasma ghrelin concentrations observed during weeks 1, 3, 5, 7 and 9 following gonadectomy of eleven male and twelve female cats given purified diets of varying metabolisable energy as fat: 9 % (□); 25 % (![]() ); 44 % (
); 44 % (![]() ); 64 % (
); 64 % (![]() ). Values are means (observations for five to six cats), with their standard errors represented by vertical bars. a,b Mean values for a week with unlike letters were significantly different (P < 0·05).
). Values are means (observations for five to six cats), with their standard errors represented by vertical bars. a,b Mean values for a week with unlike letters were significantly different (P < 0·05).
Discussion
In males and females of each dietary group, ME intake and body weight substantially increased following GX (Figs. 1 and 2). These observations are consistent with previous reports on effects of GX on catsReference Nguyen, Dumon, Siliart, Martin, Sergheraert and Biourge7, Reference Crane23–Reference Kanchuk, Backus, Calvert, Morris and Rogers28. An effect of carbohydrate on body weight was found, but it was opposite to that which might be inferred from epidemiological findingsReference Scarlett, Donoghue, Saidla and Wills2. As dietary carbohydrate concentration decreased from 44 to 9 % of ME, body weight increased. It is relevant to note that dietary carbohydrate was varied by isoenergetic substitution of fat for carbohydrate. With respect to dietary fat concentration, the present findings in cats (Fig. 3) are in agreement with previous findings in human subjects and other species; as dietary fat concentration is increased, the risk for weight gain is increasedReference West, Boozer, Moody and Atkinson3, Reference Oscai, Brown and Miller4, Reference Bray and Popkin29.
Although dietary fat is reputed to enhance the palatability of diets made for catsReference Kane, Morris and Rogers30, simple palatability differences do not appear to account for the observed effects of dietary fat on body weight. An initial over-consumption by cats fed the highest-fat diet (64 % of ME as fat) occurred (Fig. 1), and this was associated with a substantive weight gain (+17·0 (sem 5·0) %). While body weight of these cats increased in response (Fig. 2), food intake tended to decrease over time toward amounts observed in cats given the lower-fat diets. In contrast, changes in ME intake and body weight were similar among the other groups despite their consuming diets that ranged widely in fat content (9 to 44 % ME as fat) (Figs. 1 and 2). Together, these findings may indicate a threshold exists at which dietary fat content induces body-weight gain in sexually intact animals. Such a threshold might vary between individuals. For cats given the highest-fat diet, the extent of change in body weight ranged from a slight net loss in one cat ( − 3 %) to gains of 7 to 29 % in the other five cats. The decrease in food intake in the cats fed the highest-fat diet that followed the initial increase may have been a compensatory response by controlling elements of body energy balance. If the response in food intake was compensatory, it was not completely effective. Body weights of cats given the high-fat diet remained greater than those of the other cats. The high-fat diet might have evoked a resetting of the body fat mass to be defended. Such resetting as a result of high dietary fat is suggested to occur in other speciesReference Tremblay31.
Gonadectomy had by far a more potent effect on ME intake and body weight than feeding of the high-fat diet. Body weight increased irrespective of dietary fat content after GX. Mean ME intakes by all dietary groups were increased by more than 25 % by post-GX week 4 (Fig. 1). When body-weight gains among the groups began to level off (Fig. 2), mean ME intakes by each dietary group were increased by more than 50 % of the pre-GX intakes. These results clearly show that manipulation of dietary fat or carbohydrate content does not prevent body-weight gain after GX when food is presented for ad libitum consumption.
An effect of dietary fat on food intake could not be identified during the post-GX period. The power for identifying a food intake effect was reduced by increased, between-individual variation in food intake. Definitive evaluation of an effect of fat following GX will require study of greater numbers of cats than presently used.
Post-GX body-weight gain percentages in females were much greater than those in males (Table 5). The sex difference is consistent with findings of some previous studiesReference Nguyen, Dumon, Siliart, Martin, Sergheraert and Biourge7, Reference Fettman, Stanton, Banks, Hamar, Johnson, Hegstad and Johnston32 but not allReference Martin, Siliart, Dumon, Backus, Biourge and Nguyen33. Observation of a sex difference may depend on when body weights are evaluated relative to GX. Previous comparisons of body-weight gain soon after GX, as in the present study, show greater percentage gains in females than males. Later post-GX comparisons show no significant sex differenceReference Martin, Siliart, Dumon, Backus, Biourge and Nguyen33, Reference Root and Johnston34 or increased incidence of overweight males relative to femalesReference Scarlett, Donoghue, Saidla and Wills2. Two factors might account for study variations. First, within a few weeks of GX, the RMR in females is suggested to decreaseReference Flynn, Hardie and Armstrong24, Reference Hoenig and Ferguson27, whereas in males no such change is reportedReference Kanchuk, Backus, Calvert, Morris and Rogers28. The sex difference may be explained by an initial post-GX weight gain in females that is greater than that in males. Second, males reportedly have lower insulin sensitivity (less by 37 %) than females, and their insulin sensitivity declines with body-weight gainReference Appleton, Rand and Sunvold35. If insulin sensitivity of adipose as opposed to other tissues is not affected during weight gain in cats, then chronically, the lower insulin sensitivity of males relative to females may be manifested as a greater shunting of dietary energy toward adipose.
Declining insulin sensitivity of adipose in man is suggested to underlie the rise in plasma TAG concentration that occurs with weight gainReference Reaven36. Plasma TAG concentration did not significantly change with post-GX body-weight gain in the present study (Fig. 4). A similar constancy of circulating TAG between lean and obese cats has been previously reportedReference Wilkins, Long, Waldron, Ferguson and Hoenig37. Hence, sex differences observed in post-GX weight gain of cats may reflect interactions of several factors, including sex differences in metabolic rate and insulin sensitivity and resilience in insulin sensitivity of adipose in the face of body-weight gain.
The present (Fig. 1) and previous studiesReference Root, Johnston and Olson25, Reference Fettman, Stanton, Banks, Hamar, Johnson, Hegstad and Johnston32 show that food intake by male and female cats is increased soon after GX. Reduction in feedback inhibition of food intake, perhaps mediated by changes in adiposity signals, is suggested to cause the rise in food intake induced by GXReference Kanchuk, Backus, Calvert, Morris and Rogers28. In cats that are sexually intact or that have been gonadectomised long before they are evaluated, plasma leptin concentration is found to increase with body weight and fat massReference Backus, Havel, Gingerich and Rogers18, Reference Appleton, Rand and Sunvold38, Reference Shibata, Sasaki, Honjoh, Ohishi, Takiguchi, Ishioka, Ahmed, Soliman, Kimura and Saito39. This finding is consistent with a suggested adipostat function for leptin in cats. However, our finding of no significant change in plasma leptin concentration within 17 weeks of GX was unexpected because body weight had increased by 10 % in males and 39 % in females. Circulating leptin has been suggested to be a more sensitive indicator of adipose energy deficit rather than abundanceReference Flier40. More substantive increases in body fat may have been needed to significantly raise plasma leptin. Martin et al. Reference Martin, Siliart, Dumon and Nguyen41 and Kanchuk et al. Reference Kanchuk, Backus, Calvert, Morris and Rogers28 report significant rises in plasma leptin of GX cats only after 10 to 16 weeks. GX may cause changes in leptin secretion to lag behind gains in adipose mass. The present leptin observations may have been made during a period of post-GX insensitivity in leptin response to expanding adipose mass.
Like leptin, insulin is an evinced adipostatReference Schwartz, Baskin, Kaiyala and Woods42 and its plasma concentration in cats is increased with increasing body weight and fat massReference Appleton, Rand and Sunvold43. Plasma insulin concentration increased in gonadectomised females of the present study as they gained body weight (Fig. 4). A similar trend was not observed among the males, but their post-GX body-weight gains (10 (sem 4) %) were considerably less than those in the females (39 (sem 5) %). Plasma insulin concentrations in males and females given the highest-fat diet were greater than the concentrations in males and females given the lower-fat diets (Table 5). Because cats given the highest-fat diet had the greatest body weights during the pre-GX period, dietary group differences found in plasma insulin concentration were probably the consequence of adiposity differences. Hence, the results of the present study appear consistent with reports in which plasma insulin concentration in cats is found to rise with increasing body weight.
The feeding of high-carbohydrate diets is suggested to increase the risk for development of diabetes mellitus in otherwise healthy catsReference Zoran5, Reference Rand, Fleeman, Farrow, Appleton and Lederer44. In considering this, it is worthwhile to note that the amount of carbohydrate in the diets studied were widely different. Yet, plasma insulin and glucose concentrations did not vary significantly with diet, unless the diet consumed resulted in body-weight gain. Undesired gain in body weight would appear to more importantly impact need for insulin than dietary carbohydrate content.
Plasma ghrelin concentrations were determined in the present study because of emerging evidence of roles for ghrelin in the control of food intake and regulation of body fat massReference Williams and Cummings45. During the post-GX period, when food intake increased in all dietary groups, plasma ghrelin concentrations were inversely correlated with dietary fat concentration (Fig. 5). Rodent and human studies report similar findings when diets of varying fat content are given in amounts that induce body-weight gainReference Moesgaard, Ahren, Carr, Gram, Brand and Sundler46, Reference Robertson, Henderson, Vist and Rumsey47. Our finding of decreasing plasma ghrelin concentration with increasing dietary fat and body weight seems consistent with the orexigenic function suggested for ghrelin. Our finding of greater plasma ghrelin concentration in females than males (Table 6) is similar to sex-difference observations in human subjectsReference Greenman, Golani, Gilad, Yaron, Limor and Stern48. The cause for a sex difference in plasma ghrelin is unknown, but may be related to gonadal function. Ghrelin–gonadal interactions have been identified in other speciesReference Tena-Sempere49.
In conclusion, the present study shows that high concentrations of dietary carbohydrate, relative to high fat, do not induce body-weight gain or elevation of plasma glucose and insulin concentrations in sexually intact cats when food is made continuously accessible. Sexually intact cats appear to respond as a species to high concentrations of dietary fat with undesired body-weight gain. A threshold level of dietary fat may be required to induce body-weight gain in sexually intact cats. Gonadectomy stimulates food intake to the degree that undesired gains in body weight and fat follow. Gonadectomy reveals a sex-specific difference in body-weight gain and eliminates a sex difference in plasma ghrelin concentration. Substitution of dietary carbohydrate with fat does not appear to prevent weight gain induced by GX, but it appears to lessen the initial expansion of body fat mass. Overall, the study findings indicate that weight gain induced by high dietary fat and GX are probably more important to consider in the long-term health of cats than dietary carbohydrate content.
Acknowledgements
The authors gratefully acknowledge provision of funding for the research through a fellowship awarded to N. J. C. by the Center for Companion Animal Heath, School of Veterinary Medicine, University of California, Davis. The authors thank Ms Debbie Bee, Ms Christine Rieger and staff of the Feline Nutrition and Pet Care Center of the School of Veterinary Medicine for their roles in completing the research.
















