Fish is a key food in a well-balanced diet. It is the best dietary source of two long-chain n-3 PUFA, namely DHA and EPA, and is rich in high-quality proteins, essential metals such as Se, Mn and Cu, and vitamins( Reference Gil and Gil 1 ). Intakes of DHA and EPA n-3 PUFA have been linked to low rates of death from CHD( Reference Bang and Dyerberg 2 ). DHA and EPA intakes have also been found to be important for neurodevelopment during pregnancy and childhood( Reference Simmer 3 , Reference Lewin, Schachter and Yuen 4 ) as well as related to plasma biomarkers that reflect lower levels of inflammation and endothelial activation in CVD and other chronic and acute diseases, including chronic disease kidney disease, sepsis and acute pancreatitis( Reference Rangel-Huerta, Aguilera and Mesa 5 ).
On the other hand, there has been concern about the potential harm from certain contaminants found in fish, such as polychlorinated biphenyls (PCB) not similar to dioxins (NDL-PCB), which are synthetic organochlorine compounds previously used in industrial and commercial processes( 6 ). They are considered persistent organic pollutants due to properties such as their high chemical and biological stability and great lipophilicity( Reference Jones and de Voogt 7 ).
The European Food Safety Authority assesses the contamination of NDL-PCB by analysing PCB congeners 28, 52, 101, 138, 153 and 180, which account for 50 % of the 209 total congeners. Normally, for the assessment of total NDL-PCB, the six previously indicated congeners are used as indicators( 8 ).
As for the health effects of these pollutants, few studies are currently available. The International Agency for Research on Cancer classified PCB in Group 2A (probably carcinogenic to humans), based on limited evidence in human subjects and sufficient evidence from animal studies( Reference Lauby-Secretan, Loomis and Grosse 9 ). This carcinogenicity may possibly be related to the effects on the aryl hydrocarbon receptor, a transcription factor that affects gene expression( 10 , 11 ). In addition, prenatal (but not postnatal) exposure to PCB and dioxins has been associated with deficits in childhood neurological development( Reference Ribas-Fitó, Sala and Kogevinas 12 – Reference Nakajima, Saijo and Kato 14 ). There are some studies that relate PCB exposure to some other effects like skin abnormalities, deficiencies of the immune system in breast-fed infants, thyroid disorders and others( Reference Smith 15 – Reference Emmett, Maroni and Jefferys 17 ).
It is very difficult to isolate the effects produced only by NDL-PCB, because there is an overlap of exposures with PCB similar to dioxins (DL-PCB) and polychlorinated dibenzo-p-dioxins/polychlorinated dibenzofurans (PCDD/PCDF) whose effects are difficult to separate, so it is not known what effect corresponds to each of the compounds independently. This makes the toxicological database too limited to allow the establishment of a health-based reference value for NDL-PCB( 8 ).
Although a Tolerable Daily Intake (TDI) for NDL-PCB from fish is still not defined, the TDI for PCB that is used internationally, and which is proposed in the studies by Tryphonas et al. ( Reference Tryphonas, Hayward and O’Grady 18 , Reference Tryphonas, Luster and Schiffman 19 ) and Arnold et al. ( Reference Arnold, Bryce and Stapley 20 , Reference Arnold, Bryce and Karpinski 21 ), is 20 ng/kg per d for the 209 congeners of NDL-PCB, as confirmed by the Agency for Toxic Substances and Disease Registry in 2000.
The aim of the present study was to assess the daily intake of NDL-PCB from the consumption of fish in Spain. For this purpose, the intake of fish in the Spanish population was stratified at the 5th percentile (P5), 50th percentile (P50) and 95th percentile (P95) for different population groups: toddlers, other children, adolescents and adults. The concentration of NDL-PCB in samples of fish taken between 2013 and 2016 in the Comunitat Valenciana was also analysed and stratified at P5, P50 and P95. Based on the concentration of NDL-PCB identified in the fish analysed and the fish consumption of each population group, the intake of these contaminants was estimated in two different scenarios: upper bound (UB) and lower bound (LB). Finally, these estimates were compared with the tolerance limits so that a safe threshold for consumption of fish may be established and the nutritional benefits of this can be obtained in the diet.
Material and methods
Dietary intake
Fish consumption data come from a 2005 population-based nutrition survey in the case of adolescents and other children; the Spanish Agency for Food Safety’s FIAB Survey in the case of adults; and the Food Patterns of Spanish Schoolchildren and Adolescents in the case of the toddlers. The sample of adults includes people over 18 years old, the adolescent sample those from 11 to 18 years of age, the sample of other children includes children from 4 to 10 years of age, and the sample of toddlers includes children aged 1 to 3 years. The data pertaining to the P5, P50 and P95 levels of fish consumption were taken to calculate the intake of NDL-PCB in different types of fish-consuming individuals. All data were obtained from the Comprehensive Food Consumption Database of the European Food Safety Authority( 22 ).
Food sampling
The study took place in Valencian Community region, located in the east and south-east of Spain with about 5 million inhabitants. The average consumption of fish was evaluated in the different regions of the Spanish territory, specifically for the years of the study (2013 to 2016) and these data have been subjected to a means comparison test (ANOVA) between the different regions. A total of eighty-five fish samples were analysed, twenty-two in 2013, twenty-one in 2014, twenty-one in 2015 and twenty-two in 2016. Sampling was carried out by inspectors of the Department of Public Health as dictated by European legislation in ANNEX II of Regulation (EU) No. 252/2012( 23 ), where random sampling methods for the official control of dioxin (PCDD/PCDF), DL-PCB and NDL-PCB levels are established in certain food products. Samples were taken at randomly selected different local markets of the Valencian Community. The fish species sampled were also selected at random. Samples were packaged in glass, aluminium, polypropylene or polyethylene containers and transported in an ice box. Once in the laboratory they were stored at –80°C until the date of analysis.
Chemical analysis
Only the edible part of fish was used for the chemical analysis; the entire sample was crushed, homogenized and subsequently frozen at –17°C until analysis, taking account of the specific provisions for fish in Regulation (EU) No. 252/2012( 23 ). Sample analysis was performed by the Public Health Laboratory of Valencia using the in-house method described below.
The extraction solvent was hexane and three successive extraction cycles were carried out. The extract was evaporated to near dryness. The extracts were dissolved in hexane for sample cleaning. Purification was carried out in two stages, using first an acid treatment and subsequently silica columns. The fat was removed by performing an extraction with 96 % (w/w) sulfuric acid, from which the supernatant was taken and dissolved in 10 ml hexane. After the acid treatment, 1 ml of of the supernatant/hexane sample was loaded into the 500 mg silica column previously conditioned with hexane. The final extract of the sample was evaporated to dryness under a stream of nitrogen, reconstituted by the addition of 0·5 ml hexane and then transferred to two vials with a 250 μl insert for determination by GC–MS/MS. The detection by GC–MS/MS was performed in a gas chromatograph coupled to a triple-quadrupole mass detector (TSQ Quantum™, ThermoFisher Scientific) equipped with a DB-XLB column (30 m length, 0·250 mm diameter, 0·50 μm film thickness)( Reference Cocco, Guignard and Hoffmann 24 , Reference Nikonova and Gorshkov 25 ).
The quantification was done by a calibration curve with internal standard in each sequence of samples, with the results calculated using the formula:
where
C=concentration in the sample (ng/g fresh weight);
C c=concentration in the chromatogram (ng/ml);
V f=final volume (0·5 ml);
V i=initial volume;
V SPE=volume of the aliquot taken for the extraction of the solid phase (2 ml); and
P m=weight of the sample taken to perform the analysis.
The concentration of NDL-PCB was expressed in two different scenarios: the LB scenario, which implies that the unquantified results are considered zero; and the UB scenario in which the unquantified results are established as the limit of quantification( 26 ).
Model of exposure estimation
The dietary intake of NDL-PCB was expressed in nanograms per kilogram of body weight per day. The dietary exposure was estimated with the deterministic method taking into account two different scenarios, UB and LB, within each population group, combining the consumption of fish in grams per kilogram of body weight per day at P5, P50 and P95, thus considering the percentile stratified in different population groups (adults, adolescents, children and toddlers), with the P5, P50 and P95 that are calculated according to the concentration of the sum of the NDL-PCB indicators in the fish samples analysed. In this way we are able to estimate the level of contaminants to which the population may be exposed according to their level of fish consumption, high (P95), medium (P50) or low (P5), also taking account of the contamination of the fish as high (P95), medium (P50) or low (P5).
The estimation of the daily intake of the contaminant was made using the formula:
where
Ei=estimated intake of NDL-PCB;
ΣTp=sum of the concentration of NDL-PCB indicators in fish in each scenario; and
Cp=consumption of fish in each scenario.
It was calculated whether there are significant differences between the two possible scenarios for all population groups and percentiles. They were compared using ANOVA with 95 % confidence level (P<0·05).
Risk assessment
Once the dietary intake of NDL-PCB was estimated, the risk that the estimated intake exceeded the tolerance limits was calculated. Taking into account that studies by Tryphonas et al. ( Reference Tryphonas, Hayward and O’Grady 18 , Reference Tryphonas, Luster and Schiffman 19 ) and Arnold et al. ( Reference Arnold, Bryce and Stapley 20 , Reference Arnold, Bryce and Karpinski 21 ) stipulate the limit at 20 ng/kg per d for the 209 congeners, and that the congeners included in the present study represent 50 %( 8 ), the value obtained should not exceed 10 ng/kg per d. The probability of not exceeding this value with a 95 % CI was calculated using the formulas:
and
where
p=probability of the event studied;
q=(1−p); and
n=sample size.
As in the exposure model, it was calculated whether there are significant differences between the two possible scenarios in the risk analysis. They were compared with the χ 2 test with 95 % confidence level (P<0·05). The statistical software package IBM SPSS Statistics version 22 was used for the analysis.
Results
When comparing the fish consumption means for the years 2013 to 2016 in the different regions of the Spanish territory, we found that there were no statistically significant differences in the total fish consumed (P=0·777).
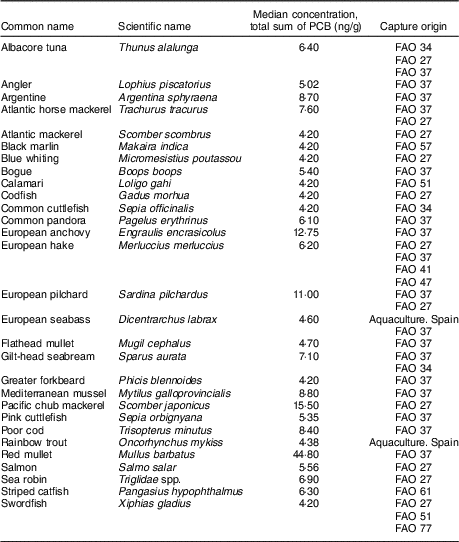
Table 1 shows all the different fish species sampled, the median concentration of PCB (ng/g) for each species and the extractive fishing zone from which each species was obtained.
Table 1 Analysed fish and capture origins
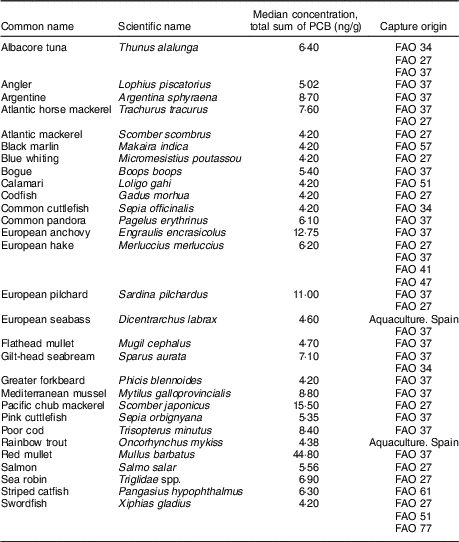
PCB, polychlorinated biphenyl.
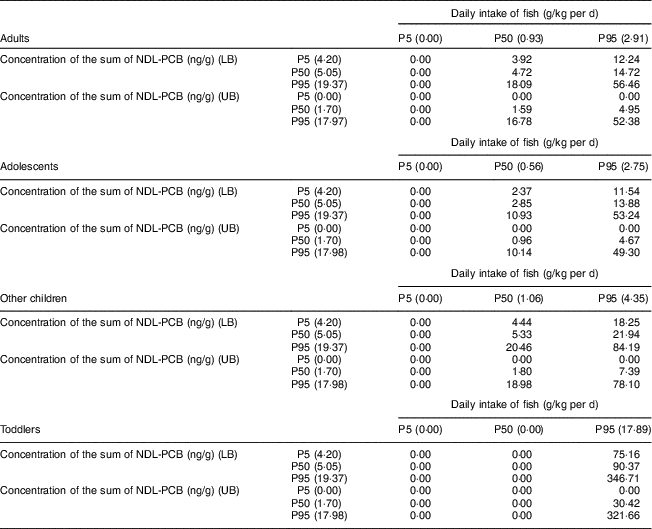
Table 2 shows the daily intake of NDL-PCB classified according to the P5, P50 and P95 of fish intake combined with the P5, P50 and P95 of the concentration of the sum of NDL-PCB indicators. This allows us to estimate nine possible scenarios in UB and another nine in LB, resulting from crossing the percentiles of fish intake with those of NDL-PCB concentration.
Table 2 Estimated daily intake (ng/kg per d) of polychlorinated biphenyls not similar to dioxins (NDL-PCBs) from fish consumption in Spain in different population groups, 2013 to 2016
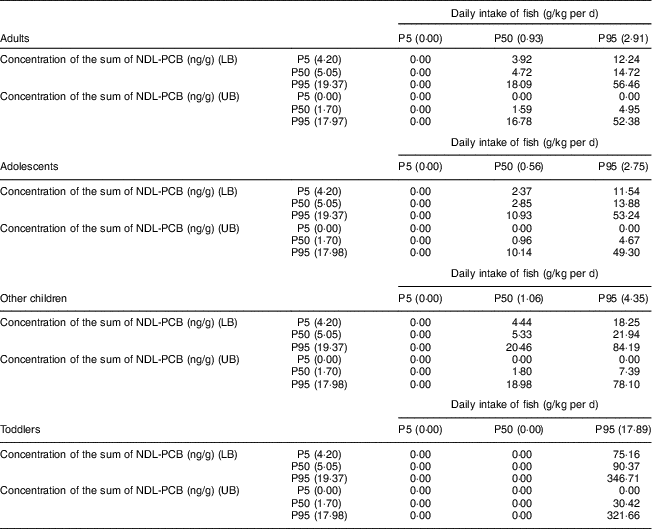
P5, 5th percentile; P50, 50th percentile; P95, 95th percentile; LB, lower bound; UB, upper bound.
It can be observed that those population groups that consume low amounts of fish (P5) are exposed to undetectable amounts of NDL-PCB, but as the intake of fish increases, the intake of NDL-PCB also increases.
Total estimated daily intake of NDL-PCB in P50 of fish consumption in adults ranges between 0·00 and 3·92 ng/kg per d for fish with a low concentration of NDL-PCB and between 1·59 and 4·72 ng/kg/d for fish with medium concentration of NDL-PCB. In these two cases there are significant differences between the UB and LB scenarios (P=0·001 and P=0·010, respectively). For fish with a high concentration of NDL-PCB, the estimated daily intake, between 16·78 and 18·09 ng/kg per d for LB and UB, is not significantly different (P=0·510).
In adolescents, in P50 of fish consumption, the total estimated daily intake of NDL-PCB ranges between 0·00 and 2·37 ng/kg per d for fish with a low concentration of NDL-PCB, between 0·96 and 2·85 ng/kg per d for fish with a medium concentration of NDL-PCB, and between 10·14 and 10·93 ng/kg per d for fish with a high concentration of NDL-PCB. There are significant differences between UB and LB scenarios for consumption of fish with low (P=0·001) and medium concentrations of NDL-PCB (P=0·002), but not for consumption of fish with high NDL-PCB concentration (P=0·555).
For other children, the total estimated daily intake of NDL-PCB in P50 of fish consumption ranges between 0·00 and 4·44 ng/kg per d for fish with a low concentration of NDL-PCB, between 1·80 and 5·33 ng/kg per d for fish with a medium concentration of NDL-PCB, and between 18·98 and 20·46 ng/kg per d for fish with a high concentration of NDL-PCB. In this case, there are significant differences between UB and LB scenarios for consumption of fish with low concentration of NDL-PCB (P=0·001) and medium concentration of NDL-PCB (P=0·007) that do not exist for consumption of fish with high NDL-PCB concentration (P=0·594).
In the toddler group, the total estimated daily intake of NDL-PCB from fish at the P50 consumption level is negligible.
In the case of intake of NDL-PCB from fish consumption at the P95 level, an increase is observed as age of the study subjects decreases, except for the adolescents group. In adults, intake ranges from 0·00 to 12·24 ng/kg per d for fish with a low concentration of NDL-PCB and between 4·95 and 14·72 ng/kg per d for fish with medium NDL-PCB concentration. In these two cases there are significant differences between the UB and LB scenarios, since P=0·001 in both cases. On the contrary, in the case of fish with a high concentration of NDL-PCB, where NDL-PBD intake is 52·98 and 56·46 ng/kg per d, there are no significant differences between LB and UB (P=0·130).
In adolescents, in P95 of fish consumption, the total estimated daily intake of NDL-PCB ranges between 0·00 and 11·54 ng/kg per d for fish with a low concentration of NDL-PCB, between 4·67 and 13·88 ng/kg per d for fish with a medium concentration of NDL-PCB, and between 49·30 and 53·24 ng/kg per d for fish with a high concentration of NDL-PCB. In the same way as in the adults, there are significant differences between UB and LB scenarios for consumption of fish with low (P=0·001) and medium concentrations of NDL-PCB (P=0·001) and no significant difference for consumption of fish with high NDL-PCB concentration (P=0·086).
With respect to the group of other children, the total estimated daily intake of NDL-PCB ranges between 0·00 and 18·25 ng/kg per d for fish with a low concentration of NDL-PCB, between 7·39 and 21·94 ng/kg per d for fish with a medium concentration of NDL-PCB, and between 78·10 and 84·19 ng/kg per d for fish with a high concentration of NDL-PCB, at the P95 level of fish consumption. There are significant differences between UB and LB scenarios for consumption of fish with low (P=0·001), medium (P=0·001) and high concentrations of NDL-PCB (P=0·049).
Finally, in the case of the toddlers with P95 fish consumption level, their total estimated daily intake of NDL-PCB ranges between 0·00 and 75·16 ng/kg per d for fish with a low concentration of NDL-PCB, between 30·42 and 90·37 ng/kg per d for fish with a medium concentration of NDL-PCB, and between 321·66 and 346·71 ng/kg per d for fish with a high concentration of NDL-PCB. As in the previous cases, there are significant differences between UB and LB scenarios for consumption of fish with low (P=0·001) and medium concentrations of NDL-PCB (P=0·001) and no significant difference for consumption of fish with high NDL-PCB concentration (P=0·710).
After this assessment of fish intake based on its potential concentration of NDL-PCB and taking account of the BMI of each group, we identify that the youngest population group, the toddlers, are the ones that present increased exposure to NDL-PCB in the P95 of fish consumption.
Table 3 presents the estimated risk of exceeding the tolerance limits classified according to the P5, P50 and P95 of fish intake combined with the P5, P50 and P95 of the concentration of NDL-PCB indicators. This allows us to estimate nine possible scenarios in UB and another nine in LB, resulting from crossing the percentiles of fish intake with those of NDL-PCB concentration.
Table 3 Risk of intake above the Tolerable Daily Intake (TDI) for polychlorinated biphenyls not similar to dioxins (NDL-PCBs) from fish consumption in Spain, with 95 % CI, in different population groups, 2013 to 2016

P5, 5th percentile; P50, 50th percentile; P95, 95th percentile; LB, lower bound; UB, upper bound.
There is no risk of exceeding the consumption tolerance limits of NDL-PCB for groups whose fish consumption is lower than P5, since they are not exposed to NDL-PCB, but as the consumption of fish increases so too does the intake of NDL-PCB.
At the P50 level of fish consumption in adults, there is a risk that the tolerance limit will be exceeded only when fish with high concentration of NDL-PCB are consumed. The risk is estimated to be between 68 and 81 %. In adolescents, at the P50 level of fish consumption there is also risk only if fish with a high concentration of NDL-PCB are consumed; in this case the risk is 1–5 %. For the other children group, there is once again risk only when fish with high concentration of NDL-PCB are consumed. In the group of toddlers, there is no risk in any of the cases in this percentile of fish consumption.
In P95 of fish consumption, there are significant differences between UB and LB in all population groups in the case that fish with low concentration of NDL-PCB are consumed (P<0·005). In the UB scenario all groups are at risk (adults 22 %, adolescents 15 %, other children 82 %, toddlers 752 %) and in the LB scenario none of them is considered at risk. If the fish consumed have medium and high concentrations of NDL-PCB, all groups are again at risk of exceeding tolerance limits. There are no significant differences between UB and LB scenarios when fish with a high concentration of NDL-PCB are consumed in any of the population groups. In contrast, for fish with medium NDL-PCB concentration, there are significant differences in the adolescents group (P=0·038).
Discussion
The present paper is the first that estimates the intake of NDL-PCB from fish in Spain, in addition to calculating the risk of exceeding tolerance limits. The importance of this is that fish consumption in Spain is one of the main bases of the diet and Spain is one of the countries with the highest fish consumption globally( 27 ). This consumption is widespread throughout the Spanish population; in the Consumer Consumption Database of Households of the Government of Spain( 28 ), it is possible to compare the consumption of fish in the Valencian Community with respect to other communities and the average for Spain, identifying that there are no significant differences. Therefore, using fish consumption data at the national level allows us to know the different scenarios among the Spanish population.
On the other hand, the consumption of fish in Spain is quite homogeneous due to the distribution network, which allows us to consider that the fish analysed are representative of the fish consumed in Spain.
Regarding Table 2, we see that when the age of the population groups decreases the intake of NDL-PCB increases proportionally, less in the case of adolescents that decreases when compared with adults; this is due to the variation in fish intake of each group. This poses a problem, because although the weights of younger populations are lower and therefore the concentration of pollutants consumed is equal to that of the adult population, the effect on these populations will be much greater because their mechanisms of metabolism and elimination of pollutants are less developed; also contributing to a process of future bioaccumulation. This could cause significant health risks for neurological development in this highly vulnerable group; the first years of life being the time where there are more documented problems with neurodevelopment associated with the intake of PCB( Reference Ribas-Fitó, Sala and Kogevinas 12 – Reference Nakajima, Saijo and Kato 14 , Reference Schantz, Widholm and Rice 29 , Reference Winneke, Walkowiak and Lilienthal 30 ).
Taking into account the maximum tolerable intake of 10 ng/kg per d, there are some groups that are at risk due to NDL-PCB, especially the high fish consumers (in all population groups) and the consumers of fish with higher content of NDL-PCB (possibly fish with higher fat concentration, owing to the fact that NDL-PCB, being organic pollutants, tend to accumulate more in fatty tissues( Reference Kodavanti, Ward and Derr-Yellin 31 )) or from places with greater contamination (since in these places the exposure is greater).
Resulting from the present study, it has been identified that fish consumption at a younger age should be restricted to levels below 17·89 g/kg per d and in adults it would not be advisable to exceed 0·93 g/kg per d. While these results may seem strange, in the study, the toddlers who consume fish consume 17·89 g/kg per d and the next step down in consumption estimates that they consume 0 g fish/kg per d. Since these levels are those which suppose a risk, consumption should be lower; but there is no other lower level of intake apart from zero that may be given as safe and recommending a consumption of no fish would not be appropriate. These measures would ensure the positive balance of the nutritional benefits of fish consumption against the health risk posed by the presence of NDL-PCB. Regarding the consumption of 0·93 g fish/kg per d by adults in the case of fish with low and medium concentrations of NDL-PCB, the exposure is below the tolerable level of NDL-PCB intake (10 ng/g per d) and in the case of fish with high concentration is a bit higher, but we must take into account that all the fish that is consumed will not have a high concentration of NDL-PCB.
If the consumption of fish with low or medium concentration of NDL-PCB is average, that is, about 0·93 g/kg per d in adults, 0·56 g/kg per d in adolescents, 0·44 g/kg per d in other children and 0·00 g/kg per d in toddlers, there is no risk of exceeding the tolerance levels, which means that in these cases the population could benefit from the nutritional properties of the fish. If fish with a high concentration of NDL-PCB is consumed, intake should be reduced since there is a considerable increase in the risk of exceeding tolerance levels in adults (68–81 %) and a smaller, although noteworthy, increase in adolescents (1–5 %). It is important to note that when assessing the intake of NDL-PCB only from fish, the models calculated underestimate the true exposure to NDL-PCB and, therefore, the risk of exceeding tolerable values increases.
The results of the estimated intake of NDL-PCB calculated in the present study can be compared with other studies conducted in Europe, but the estimated intakes in different countries are calculated from the consumption of various foods that make up the usual diet, not just fish. In Italy, the study in 2008 by Fattore et al. ( Reference Fattore, Fanelli and Dellatte 32 ) estimated that the intake of the sum of NDL-indicator PCB in children under 6 years of age at the mean and P95 fish consumption levels was 16·1 and 33·8 ng/kg per d, respectively. In children and adolescents under 12 years of age, the NDL-PCB intake at the mean and P95 was 24·6 and 60·0 ng/kg per d, respectively. From this same study, in adolescents (over 12 years old) and adults, the NDL-PCB intake was 10·9 and 23·8 ng/kg per d, respectively, at the mean and P95 levels of fish consumption. Compared with our study, average fish consumers do not exceed these values. The intake data corresponding to other children have a value of 5·33 (LB) and 1·80 (UB) ng/kg per d at P50 and 21·94 (LB) and 7·39 (UB) ng/kg per d at P95. In adults, the estimated values in our study are 4·72 (LB) and 1·59 (UB) ng/kg per d at P50 and 14·72 (LB) and 4·95 (UB) ng/kg per d at P95.
In the case of France, two studies have been carried out. In the study by Arnich et al. ( Reference Arnich, Tard and Leblanc 33 ) conducted in 2009, the sum of NDL-PCB indicators in children at the mean and P95 levels of fish consumption was 12·9 and 27·3 ng/kg per d, respectively. In the case of adults, the corresponding values were 7·7 and 16·0 ng/kg per d. Values in Arnich et al.’s study are slightly higher than those estimated in our study, which makes sense because other components in the diet that can provide NDL-PCB were analysed. In the study by Sirot et al. ( Reference Sirot, Tard and Venisseau 34 ) carried out in 2012, the sum of NDL-indicator PCB in children at the mean and P95 fish consumption levels was 3·77 and 11·7 ng/kg per d, respectively. On the other hand, the corresponding values in adults decreased to 2·71 and 7·90 ng/kg per d. These latter intake values of NDL-PCB are lower than those in Arnich et al. ( Reference Arnich, Tard and Leblanc 33 ) and the UB levels for adults in our study exceed them.
In Germany, in the study by Fromme et al. ( Reference Fromme, Shahin and Boehmer 35 ) conducted in 2009, the average intake of the sum of NDL-PCB indicators in adults was 5·6 ng/kg per d. This value is above those we found at the P50 of adult fish consumers (4·72 and 1·59 ng/kg per d) but lower than those we estimated for the P95 of adult fish consumers (14·72 and 4·95 ng/kg per d).
In the Slovak Republic, Salgovicová and Pavlovicová( Reference Salgovicová and Pavlovicová 36 ) conducted a general population study in 2007 that estimated the intake of the sum of NDL-PCB indicators at the P50 and P95 levels of fish consumption as 17·0 and 45·0 ng/kg per d, respectively. In this case, the values exceed our estimated intakes in all consumers when fish with a medium concentration of NDL-PCB are consumed but are below our estimated levels when fish with a high concentration of NDL-PCB are consumed.
Finally, the European Food Safety Authority( 8 ) conducted a study for the general population in Europe in 2005, which estimated the intake of the sum of indicators of NDL-PCB at 15 ng/kg per d for the average consumer, 20 ng/kg per d for high meat consumers and 35 ng/kg per d for high fish consumers. Comparing the intakes estimated in the present study with the one carried out by the European Food Safety Authority( 8 ), our estimated values are below the latter ones except for high consumers of fish with a high concentration of NDL-PCB (toddlers, 346·71 and 321·66 ng/kg per d; other children, 84·19 and 78·10 ng/kg per d; adolescents, 53·24 and 49·30 ng/kg per d; adults, 56·46 and 52·38 ng/kg per d).
Our study results lead us to some possible indications for policies to improve food safety related to the presence of PCB in the fish consumed in our community. We recommend assiduous monitoring of the presence of PCB and the readjustment of the legislated safety levels since we identified populations at risk of exceeding the TDI, with the risk being between 0 and 34 %, although clearly the most vulnerable groups are the youngest ones in age. Therefore, the concentration values of PCB in fish with zero risk estimated in the results could serve as a guide for the establishment of new legally tolerated levels and other policies to provide greater food security.
We have to take into account that the fish analysed were raw fish. Therefore, if fish are not consumed raw, their content of PCB could vary: part of the fat may be lost and thus the PCB content may be reduced. Future studies on this subject should be carried out to assess if there is a change in the concentration of PCB in fish when cooked.
We have to consider the relative weight of fish consumption (% of grams or % of energy) within the whole diet of each population group, but this aspect has not been possible. We consider that studies on this topic would be of great importance in the assessment of the concentration of PCB from fish consumption in relation to total intake, as well as the proportion of PCB ingested through fish in relation to total PCB from other components of the diet in each population group.
It is expected that our values would be lower than all those estimated in the studies cited above since they studied the intake of NDL-PCB through the total diet and not only fish. It is important to note that despite our levels being inferior in most cases, there are some estimated intakes which are higher in our study, which implies that NDL-PCB have an important presence in the Spanish diet and it is necessary to have a special monitoring of these contaminants.
Acknowledgements
Acknowledgements: The authors wish to thank the University of Valencia and the Master’s Degree in Environmental Pollution, Toxicology and Health. Financial support: This research received no specific grant from any funding agency in the public, commercial or not-for-profit sectors. Conflict of interest: None. Authorship: Authors’ contributions were as follows. M.M.-S.-V.: Study design, data analysis, drafting the article, critical revision of the article, final approval of the version to be published. N.L.S.: Data collection, data analysis, drafting the article, final approval of the version to be published. P.M.R.: Data collection, data analysis, critical revision of the article, final approval of the version to be published. M.I.B.S.: Data collection, data analysis, final approval of the version to be published. I.P.-C.: Drafting the article, critical revision of the article, final approval of the version to be published. A.L.-G.: Study design, data analysis, critical revision of the article, final approval of the version to be published. Ethics of human subject participation: Not applicable.