Introduction
The Galapagos Petrel Pterodroma phaeopygia is endemic to the Galapagos Archipelago, where it is known to breed on only five islands: Floreana, San Cristóbal, Santa Cruz, Isabela and Santiago. The first four are inhabited, while Santiago has no resident human population. Nesting occurs only in the humid highlands, where vegetation cover is dense (Harris Reference Harris1970, Coulter Reference Coulter, Croxall, Evans and Schreiber1984, Coulter et al. Reference Coulter, Cruz, Cruz and Moors1985, Griffin et al. Reference Griffin, King, Savidge, Cruz and Cruz1988). All breeding populations have been seriously reduced through predation by introduced mammalian predators and destruction of nesting habitat (Coulter et al. Reference Coulter, Cruz, Cruz and Moors1985, Tomkins Reference Tomkins and Moors1985, Cruz and Cruz Reference Cruz and Cruz1987a). Given the reduced nesting success and limited distribution, the Galapagos Petrel has been listed as ‘Critically Endangered’ in the IUCN Red List (Hilton-Taylor Reference Hilton-Taylor2000, Granizo et al. Reference Granizo, Pacheco, Rivadeneira, Guerrero and Suárez2002, BirdLife International 2009).
Inter-island differences have been found in timing of breeding, morphology, egg size, and vocalisations, suggesting the possibility that island populations of the species might be distinct (Cruz and Cruz Reference Cruz and Cruz1990, Tomkins and Milne Reference Tomkins and Milne1991). Tomkins and Milne (Reference Tomkins and Milne1991) also suggested that seasonally segregated breeding populations may exist on San Cristóbal, raising the possibility that two genetically differentiated populations could exist on that island. Recent genetic analyses revealed the existence of at least three, and possibly five distinct populations existing in the archipelago (Friesen et al. Reference Friesen, González and Cruz-Delgado2006).
Although some in-depth studies on the breeding biology of the Galapagos Petrel have been conducted on the islands of Santa Cruz, Santiago and Floreana (Harris Reference Harris1970, Cruz and Cruz Reference Cruz and Cruz1990, Tomkins and Milne Reference Tomkins and Milne1991), no complete study has been conducted to date on San Cristóbal, where information on petrels is restricted to a limited number of observations on phenology and ethology conducted by Tomkins and Milne (Reference Tomkins and Milne1991). The humid highlands of San Cristóbal are among the areas of the archipelago being more seriously affected by invasive species and human occupation. Almost 93% of the humid highland ecosystems of the island have been transformed and occupied for agriculture (Snell et al. Reference Snell, Tye, Causton, Bensted-Smith and Bensted-Smith2002). Thus, a thorough knowledge of the breeding ecology and factors affecting nesting success of the local petrel population is critical to development of sound management strategies for its conservation.
In this paper we present the results of a study conducted during 2002–2003 to describe several parameters related to the breeding biology of the species on San Cristóbal, with special emphasis on nesting habitat characterisation, phenology, nesting success, and causes of egg and chick mortality. Finally, we discuss the conservation implications of our results, and conclude with some insights and actions that could be adopted in the short term to improve current management practices and ensure the long-term survival of the San Cristóbal Galapagos Petrel population.
Methods
Study area
The Galapagos archipelago straddles the equator approximately 960 km west of mainland Ecuador and 1,100 km south of Costa Rica, and is comprised of seven major islands (> 100 km2), 11 smaller islands (> 1 km2) and more than 120 islets and rocks. The total land area of the archipelago is approximately 7,995 km2, most of which (97%) is protected as a National Park. Additionally, the Galapagos Marine Reserve protects the waters within 40 nautical miles of the island group (about 133,000 km2).
San Cristóbal is the fifth largest island of the archipelago, covering 55,709 ha (Figure 1). Geologically, the north-eastern half of the island is younger than the south-western half, which contains a large shield volcano (Geist et al. Reference Geist, McBirney and Duncan1986). About 84% of the island is protected as part of the Galapagos National Park, with the rest occupied by agricultural lands and the urban area of Puerto Baquerizo Moreno. The human population of the island is a little over 6,000, with about 10% of these living in the agricultural zone of the highlands, mostly in the town of El Progreso.

Figure 1. Location of the archipelago showing the five islands in which petrels breed, and detailed location of the nests monitored on San Cristóbal.
Climate is controlled by the winds and oceanic currents that bathe the island (Peck Reference Peck2001). During the cool-dry season (locally called garúa season), from June to December, there is prolonged cloud cover in the highlands and no rain in the lowlands, with temperatures in the ranging 19–23°C. During those months, all the nesting areas of the Galapagos Petrel are usually embedded in perpetual drizzle. In contrast, the warm-wet season, from January to March, is usually characterised by sunny skies with occasional heavy rains, and temperatures ranging 24–29°C.
Nest searching
Most nest burrows were located during monitoring activities conducted by the Galapagos National Park Service (GNPS). Other likely breeding sites were also surveyed for additional petrel nests in the 11 most important ravines of San Cristóbal (90 man-hours invested). A total of 199 nest burrows were located in eight breeding colonies (clusters of nests in separate ravines); 91.9% of these nest burrows were on private land. The study period extended from March 2002 through March 2003.
Nesting habitat
To characterise the nesting habitat of petrels, several parameters were evaluated in a subsample of 60 randomly selected nests. Orientation of the nest entrance was estimated using a compass, grouping values into eight categories corresponding to 45° sections of the 360 compass degrees. Slope was measured along a segment from 1 m above to 1 m below the nest entrance. Altitude above sea level, type of substrate (rocky vs. soft-clay), distance to the nearest-neighbour nest, and distance from the nest to the nearest shrub or tree, were also evaluated.
To assess plant composition and vegetation cover in the nesting sites, 1-m radius circular plots were established around 35 of the nests. Trees, shrubs, and herbs were recorded in each of these plots, and data were expressed in terms of frequency (the probability of finding at least one individual of the species in a sample plot). Vegetation canopy cover was estimated with a densiometer and expressed as a percentage.
Breeding phenology
Periodic visits to all the nest burrows located in the study area were not possible due to time limitations. Access to the nest chamber was also limited in many nest burrows. Therefore, only a subsample consisting of 53 accessible nests were periodically monitored; 47 of these nests were found before egg-laying or when they had eggs, while six additional nests were found when they already contained chicks. Nests were visited at least once a week and nest contents checked using a torch and a mirror, with more frequent visits (every 1–2 days) during the laying and hatching periods. When it was necessary for taking measurements, birds were captured either by hand, or using a device developed by Cruz-Delgado (unpubl. data) if the nest chamber was hard to reach by hand.
Data on laying, hatching and fledging dates were grouped into 15-day intervals for the analyses. Accurate egg-laying dates were obtained directly for 23 nests. In another 20 nests, laying dates were estimated by subtracting the average incubation period from the known hatching date. Precise hatching dates could be determined for 32 nests, and were estimated in another eight nests for which the laying date was known by adding the average incubation period to the hatch date. We obtained 11 precise records of fledging date and estimated another 20 using the average duration of the nestling stage.
Fourteen chicks whose exact age was known were periodically measured and weighed. A Mitutoyo caliper (± 0.01 mm) was used for measuring bill and tarsus. Bill length was measured from the tip of the upper mandible to the edge of the forehead feathers. Tarsus was measured from the middle of the mid-tarsal joint to the distal end of the tarso-metatarsus. A metal rule (± 0.1 mm) was used to measure wing length from the wrist joint to the tip of the longest primary or feather sheath if primaries had not erupted. Birds were weighed with a 1,000 g (± 10 g) Pesola spring scale. Means are followed by one standard deviation (SD), and ranges are given in parentheses. Growth values were adjusted to logistic (bill, tarsus, wing) and polynomial (weight) regression curves.
Nest success and mortality
Because not all nests were located prior to egg laying, nest success data were analysed using the Mayfield method (Mayfield Reference Mayfield1961, Reference Mayfield1975) to estimate the daily survival rate for petrel nests. Nest success and mortality were calculated separately for the incubation stage (the period from laying to hatching) and for the chick-rearing stage (the period from hatching to fledging). The product of the two rates gave the overall nest survival rate.
Causes of nest failure were assessed by careful observation of nest contents and analysis of the shells or body remains found, following Tomkins (Reference Tomkins and Moors1985), Major (Reference Major1991), and Booth et al. (Reference Booth, Minot, Fordham and Innes1996). In most cases, carcasses or egg-shells provided tell-tale signs of the identity of the predator. According to Tomkins (Reference Tomkins and Moors1985) nestlings that disappeared from their nests leaving no remains were assigned to rat predation when rat droppings or other signs were found, although other causes of mortality and further scavenging by rats cannot be dismissed. Three excrement sets of feral cats and two owl pellets found in the area surrounding the nest burrows were also analysed to determine the potential predatory activities of these species.
If precautions are not taken, researcher disturbance during and before egg-laying may strongly affect reproductive parameters in seabirds (Brown and Morris Reference Brown and Morris1994, Carney and Sydeman Reference Carney and Sydeman1999). All visits were made when adult birds were not in the nest, so it is unlikely that they were affected. In addition, the number of visits to the nests was minimised to reduce disturbance, and checking of nest contents did not require any manipulation. Only 14 chicks were periodically taken from their nests for measurement; in these cases the manipulation time was minimised and a hood was placed on the chicks to reduce stress.
Results
Nesting habitat
All the nests were located in ravines descending to the south-south-east coast of the island, in areas with dense vegetation cover. Altitude varied between 400 m and 690 m, with an average of 547 ± 63 m. Slope averaged 51° ± 12° (range: 31–79°; n = 60) and most of the nests had their entrance oriented to the north and west (83% oriented between 225° and 45°). A total of 96.7% of the nest burrows were built on soft-clay substrate, with only 3.3% on rocky areas (Figure 2).

Figure 2. Summary of major characteristics of the sites where nest burrows were located on San Cristóbal (n = 60 for all the parameters except for vegetation cover where n = 35).
Vegetation located in the immediate surroundings of the nests included native ferns of 11 different families. Flowering plants were represented by seven families. The dominant plant in the shrub layer at the nesting sites was the endemic shrub Miconia robinsoniana, present at 85.7% of the sampled plots; followed by the woody fern Cyathea weatherbyana, present at 60% of the plots (Table 1). Less frequent were the introduced blackberry species Rubus niveus, and the introduced tree Syzygium jambos. Two herbaceous ferns, Blechnum polypodioides and Asplenium feei, were also present in high frequencies around the nests (Table 1). Overall vegetation cover in the nesting areas was usually very high, averaging 68.2% (range: 12.2–95.7%; Figure 2).
Table 1. Ranked list of the most important plant species in 1-m radius sample plots located around petrel nests (n = 35 plots). Frequency is percent of plots where a given species is present (only those species with frequencies over 5% are shown).

* (i) invasive alien species; (n) native species; (e) endemic species.
Breeding phenology
Petrels visited the nests for several weeks to renew and dig out nest burrows. The birds were then absent from their nests for a long period before egg-laying. This pre-laying exodus period on San Cristóbal averaged 35.4 ± 8.9 days (range: 24–46; n = 7) and was measured as the interval between the date an adult was detected for the last time in the nest and the date of egg-laying.
Eggs were laid continuously from mid-May to late-October, with a peak during the first two weeks of August. However, 11.6% of the eggs were laid dispersed over the period from November to January and March. A large majority of the egg laying (76.7% of the clutches) was concentrated in a period of just four months, between the second half of May and the first half of September (Figure 3).

Figure 3. Phenology of the Galapagos Petrel on San Cristóbal Island, showing the percent of eggs laid, eggs hatched, and chicks fledged in nests with accurate information, by 15-day intervals.
Birds laid only one, white ovate egg. The colour of the egg became darker with the passage of time. Egg size averaged 59.6 ± 3.1 mm length (range: 52.3–63.4) and 43.7 ± 1.9 mm width (range: 42.0–49.5 mm; n = 13). Three eggs weighed in an early stage of incubation averaged 58.17 g (range: 52–61 g), which represents 15% of the average weight of an adult bird (386.8 g, n = 41).
The incubation period was 50.8 ± 1.9 days (range: 46–53; n = 15). Two infertile eggs were still incubated until 61 and 70 days after laying date. The hatching period extended from July to late-December, with 77.5% of the chicks hatching between July and October (Figure 3).
The duration of the nestling stage averaged 103.7 ± 3.6 days (range: 98–108; n = 6). Most fledglings left their nests from mid-October to late-March, with 61.3% of the nestlings successfully flying from December to mid-February (Figure 3).
Chicks experienced very rapid growth during the first eight weeks of age. Bill length maintained a continuous growing pattern. Tarsus length reached its maximum growth (40.6 mm) at 78 days of age. Wing remiges appeared between 40 and 50 days of age, and wing length reached a length similar to that of the adults by the time of fledging. Chick weight reached a maximum (563.1 g) greater than the average adult at 72 days of age, and decreased slightly thereafter until fledging (Figure 4).

Figure 4. Growth pattern of petrel chicks (n = 14) on San Cristóbal as a function of age; logistic (bill, tarsus, wing) and polynomial (weight) regression curves are shown for each variable measured.
Nest success and causes of mortality
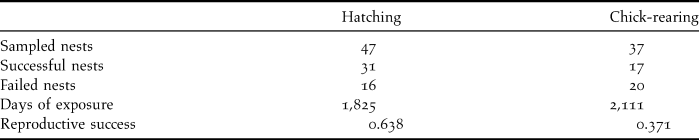
Overall reproductive success in 53 monitored nests, estimated using the Mayfield method, was 23.6%, being 63.8% for eggs and 37.1% for chicks (Table 2). Rat predation caused the loss of a total of 10 eggs and 16 chicks, and was the main cause of reproductive failure (72.2%). All the chicks predated by rats were less than 21 days old, indicating that the first three weeks after hatching are probably when they are most vulnerable. No losses could be attributed to rat predation after that age. Two additional nestlings that were close to fledging were killed by feral dogs (5.6% of failures). Infertile eggs were recorded in three nests (no embryo was found after checking egg content), accounting for 8.3% of nesting failure. Nest abandonment by adults explained another 13.9% of nest failures. Abandonment was observed in three nests during the incubation period and two nests during the nestling stage (with nestlings subsequently dying of starvation).
Table 2. Numbers of nests sampled and their outcomes, along with hatching and chick-rearing success, estimated using the Mayfield method.
Discussion
Nesting habitat
Our study shows that the nesting habitat on San Cristóbal is restricted to preserved ravines at altitudes over 400 m in good condition with native vegetation. The presence of nest burrows in those areas is dependent on two major factors: (1) the existence of dense vegetation cover formed by native shrubs and ferns, with dominance of Miconia robinsoniana, and (2) the availability of soft-clay soils in ravines with steep sides. Previous studies have provided general descriptions of the breeding habitat of petrels on other islands of the archipelago (Harris Reference Harris1970, Cruz and Cruz Reference Cruz and Cruz1987a,Reference Cruz and Cruzb), reporting that the nesting habitat on Santa Cruz is mostly located in Miconia forests, similar to where nests are found on San Cristóbal, while on Floreana, nests are mostly located in Scalesia forests. The nesting habitat of petrels on Santiago is quite different as nest burrows are located in more open areas and rocky substrate. Isabela is the least explored island, with only nine petrel nests located, all in rocky areas surrounded by Guava Psidium guajava plants.
One important characteristic of the petrel nesting areas on San Cristóbal is that over 90% of the nests are located outside the limits of the Galapagos National Park, on privately-owned agricultural land. This is a unique feature that makes the San Cristóbal petrel population much more vulnerable and threatened than others in the archipelago. In contrast, all the petrel nests on Floreana, Santiago and Isabela are located in the National Park; on Santa Cruz only about 20% of the petrel nests are located outside the protected area (Jiménez-Uzcátegui and Wiedenfeld Reference Jiménez-Uzcátegui, Wiedenfeld, Danulat and Edgar2002, Valarezo Reference Valarezo2006, Valarezo-Ortega and Wiedenfeld unpubl. data).
Slope and vegetation cover are two major factors linked to nest site selection on San Cristóbal. Simons (Reference Simons1985) and Brandt et al. (Reference Brandt, Parrish and Hodges1995) reported that Hawaiian Petrels Pterodroma sandwichensis also prefer to build their nests in areas of steep slope. A steep slope facilitates the excavation of the burrow, offers a better panoramic view from the nest entrance for the bird, and contributes to the take off and landing. In addition, dense shrub vegetation makes the nest burrow more resistant to collapse as the woody roots strengthen the walls of the nest chamber (Brandt et al. Reference Brandt, Parrish and Hodges1995). Consistent with this statement is our observation that the only two nests located in areas with less vegetation cover collapsed partially during a period of heavy rainfall.
The rapid expansion of the introduced blackberry is one of the most important drivers of degradation of petrel nesting habitat and constitutes a critical problem for the survival of the San Cristóbal petrel population. The growth of this invasive plant can rapidly block the entry to the nest burrow and hinder access by the birds. The majority of the nests monitored on San Cristóbal were located in areas with absence of blackberries. No petrel nests were found in surveys conducted in ravines that had been heavily invaded by blackberries. However, during the study period two active nests that were being monitored became completely blocked by blackberries and their entry had to be cleared by the researchers. Blackberries now occupy a wide area of the agricultural zone of San Cristóbal (TNC-CLIRSEN 2006), and the plant is considered to be one of the most invasive species, causing dramatic effects on Galapagos ecosystems, especially in the humid highlands of the inhabited islands (Tye et al. Reference Tye, Soria, Gardener, Veitch and Clout2002).
Breeding phenology
Breeding traits of the Galapagos Petrel are similar to those of other species of procellarids, with nestling attendance and incubation shared by both members of the pair, long incubation bouts, and a pre-laying exodus from the nest (Warham Reference Warham1990, Gardner Reference Gardner1999, Booth et al. Reference Booth, Minot, Imber and Fordham2000, Imber et al. Reference Imber, West and Cooper2003, Priddel et al. Reference Priddel, Hutton, Carlile and Bester2003).
The breeding cycle of petrels nesting on San Cristóbal is similar to those described previously for other islands of the archipelago (Harris Reference Harris1970, Cruz and Cruz Reference Cruz and Cruz1990), although remarkable differences have been found in timing of breeding among the different islands. According to Tomkins and Milne (Reference Tomkins and Milne1991), laying on Santa Cruz is highly clumped around June-August, while on Santiago it is less synchronised, extending from March to May, and on Floreana eggs were laid continually from January to June; the laying dates on San Cristóbal extended through most of the year, from November to August. Our data are consistent with these observations, although the peak of laying in our sample was in August which differs notably from that found by Tomkins and Milne (Reference Tomkins and Milne1991). However, it is clear that the petrel population of San Cristóbal has the most prolonged breeding period among the islands.
Tomkins and Milne (Reference Tomkins and Milne1991) reported a bimodal pattern in egg-laying dates (with a marked discontinuity in March-April), suggesting that two distinct populations might coexist on San Cristóbal. Our data may be concordant with this finding although not conclusive, as the number of nests located between November and March was too small to derive comparisons. More studies are probably needed to determine whether two distinct phenologically separated populations nest on San Cristóbal.
Nesting success and causes of mortality
Alien invasive species are currently the main single threat to Galapagos biodiversity (Bensted-Smith Reference Bensted-Smith2002). Invasive predators in particular are having dramatic effects on several bird species in the Galapagos (Griffin et al. Reference Griffin, King, Savidge, Cruz and Cruz1988). Dogs and pigs predate on nestlings and adult birds, rats eat eggs and chicks, and ungulates (goats, donkeys, cattle) may destroy nests with their trampling (Coulter Reference Coulter, Croxall, Evans and Schreiber1984, Coulter et al. Reference Coulter, Cruz, Cruz and Moors1985, Cruz and Cruz Reference Cruz and Cruz1987b). The sum of all these effects has caused a sharp decline in the past in reproductive success of several species of seabirds, particularly the Galapagos Petrel (Harris Reference Harris1970, Tomkins Reference Tomkins and Moors1985).
There is a wide range of values for nesting success in the genus Pterodroma reported in the literature (Table 3), including previous estimates of nesting success for the Galapagos Petrel. Harris (Reference Harris1970) estimated nest success of < 10% on Santa Cruz. Tomkins (Reference Tomkins and Moors1985) estimated a 0% and 5.1% success in two consecutive years on Santa Cruz, while a 39.5% and 48.9% success were reported for Floreana and Santiago respectively. The populations of San Cristóbal had a lower nesting success of only 11.4% (Tomkins Reference Tomkins and Moors1985). Despite the different methods used to assess nesting success, our data show that the petrel population of San Cristóbal may be in only a slightly better position than that encountered 20 years ago.
Table 3. Reproductive success of species of the genus Pterodroma on some oceanic islands.

* Breeding success was estimated after a control programme of invasive species.
** Rats were not the main cause of nesting failure.
Tomkins (Reference Tomkins and Moors1985) suggested that the most important factor behind nesting failure was predation by introduced rats, and that this effect would be particularly important a few days before and after hatching. Cats and dogs may also predate adult birds, but probably have a minor effect on eggs and chicks. Our data lead to similar conclusions regarding the dramatic effects of introduced rats on the mortality of petrel eggs and chicks, particularly during the pre-hatching stage and during the first three weeks of life. Little evidence of predation by dogs was observed in our study, and feral cat excrement and owl pellets located in the nesting areas did not indicate that these animals had been preying on petrels. No evidence of predation by feral pigs was observed during our study, although they have been indicated by other authors as important threats to petrels on San Cristóbal and, particularly, on Santiago (Coulter et al. Reference Coulter, Cruz, Cruz and Moors1985, Tomkins Reference Tomkins and Moors1985).
The large number of abandoned eggs recorded by Tomkins (Reference Tomkins and Moors1985) on San Cristóbal has been attributed to intraspecific competition for nest burrows, and was considered a major component of non-predatory losses. In our study, we found only 13.9% of failures that could be directly attributed to nest abandonment. Although lack of experience or scarcity of food might also be causes of desertion, in one of the monitored nests the egg was ejected from the burrow by a new adult bird occupying it, which is consistent with previous observations. Intraspecific competition for nest burrows can be a real problem for the future of petrels at San Cristóbal, given the scarcity of nesting habitat caused by invasive plant species expansion and agriculture.
Some authors have also suggested that exotic ungulates might contribute to nesting habitat destruction by changing the vegetation structure, compacting the clay soil, or eventually destroying nest burrows by trampling (Coulter Reference Coulter, Croxall, Evans and Schreiber1984, Coulter et al. Reference Coulter, Cruz, Cruz and Moors1985, Cruz and Cruz Reference Cruz and Cruz1987b). Horses and cattle have been observed frequently wandering in search of water in and around ravines with petrel nests on San Cristóbal. However, it is not likely that they are a direct cause of nest destruction on the island, because the nest burrows are usually located in areas with very difficult access. Our observations do suggest that horses and cattle may be indirectly involved in nesting habitat degradation, as they tend to open walking paths and establish new clearings in areas of dense native vegetation cover, all of which facilitates the expansion of invasive exotic plants such as blackberry and Guava, both of which are rapidly colonising petrel nesting sites on San Cristóbal.
Conservation and management implications
Several management strategies have been applied for the conservation of the Galapagos Petrel, after evidence of its decline (Coulter Reference Coulter, Croxall, Evans and Schreiber1984). Most efforts have concentrated on the islands of Santiago, Floreana, and Santa Cruz, where nests are located in the protected area under the jurisdiction of the GNPS. Management activities in those islands have included yearly nest monitoring, bird ringing, and predator control in areas with a high density of petrel nests (Cruz and Cruz Reference Cruz and Cruz1996, Oldfield and Vargas Reference Oldfield and Vargas1999).
Based on genetic evidence, Friesen et al. (Reference Friesen, González and Cruz-Delgado2006) concluded that actions should be directed at maintaining viable breeding populations on all islands where the species occurs, so that existing genetic diversity is not lost and evolutionary processes are not affected. In this sense, it was recommended that the San Cristóbal population should be managed as a separate conservation unit (sensu Crandall et al. Reference Crandall, Bininda-Emonds, Mace and Wayne2000), as there was strong evidence for both genetic isolation and adaptive distinctiveness (Friesen et al. Reference Friesen, González and Cruz-Delgado2006).
The petrel population of San Cristóbal is probably the most threatened of the archipelago. Two factors contribute to this situation and should be addressed in the near future to ensure petrel conservation: (1) habitat reduction and degradation as a consequence of agricultural activities and blackberry expansion; and (2) high losses of eggs and nestlings caused by rat predation.
Managing invasive plants in nesting sites is critical. The blackberry should be continuously removed to avoid the obstruction of nest entrances, particularly during the peak of the nesting season. Restoration of nesting habitat should also include the removal of other invasive plants such as Guava, which prevents the regrowth of native shrubs (Rentería et al. Reference Rentería, Atkinson, Guerrero and Mader2006). Open spaces left by removed blackberries and Guavas should then be reforested with native plants of Miconia robinsoniana. Reforestation efforts should be expanded to the outer parts of the nesting sites to establish a natural barrier against invasive plants. This could also help to establish connection corridors among different patches of native forests and increase available nesting habitat.
Rodent removal campaigns in the surroundings of the major nesting colonies are also essential to control rat predation and increase nesting success, following previous successful experiences from other islands (Cruz and Cruz Reference Cruz and Cruz1987a, Imber et al. Reference Imber, Harrison and Harrison2000). Poisoning campaigns should be conducted during discrete periods of time and not throughout the whole year, and different rodenticides should be used alternately to avoid the development of resistance in the rodents (Ishizuka et al. Reference Ishizuka, Okajima, Tanikawa, Min, Tanaka, Sakamoto and Fujita2007).
Finally, as most petrel nests of San Cristóbal are located on privately owned agricultural land, it is clear that no strategy for the conservation of petrels can achieve appropriate results if land owners are not involved (González et al. Reference González, Montes, Rodríguez and Tapia2008, Cruz-Delgado et al. Reference Cruz-Delgado, Wiedenfeld and González2009). We suggest that land use agreements, stewardship contracts, conservation easements, or other innovative approaches should be adopted in the short term, so that the owners can have incentives to preserve or restore those ravines that host petrel nests, under the advice and supervision of the GNPS.
Acknowledgements
We thank all the Galapagos National Park Service staff, and particularly W. Tapia, E. Naula, and N. García for providing the facilities to conduct this study. Special thanks to D. Rodríguez and R. Aguas for their continuous support during the field work. We are also grateful to M. Coulter, A. Tye, H. Jäguer, C. Lougheed, and F. Cruz for their suggestions on the methodological design of the research. V. L. Friesen and two anonymous reviewers made very helpful comments on a previous version of the manuscript. The Charles Darwin Foundation provided technical support during the first stages of the research. Financial support was provided by the Araucaria Program of the Spanish Agency for International Cooperation (AECI). The authors declare that the experiments conducted comply with the legal regulations of the Galapagos National Park and with current laws of Ecuador.











