Psychosocial stress during childhood and adolescence can have immediate and long-term effects on both mental and physical health, increasing risk for numerous conditions that range from mood and anxiety disorders to upper respiratory infections to premature mortality (Brown et al., Reference Brown, Anda, Tiemeier, Felitti, Edwards, Croft and Giles2009; Cohen, Doyle, Turner, Alper, & Skoner, Reference Cohen, Doyle, Turner, Alper and Skoner2004; Demakakos, Pillas, Marmot, & Steptoe, Reference Demakakos, Pillas, Marmot and Steptoe2016; Miller, Chen, & Parker, Reference Miller, Chen and Parker2011; Repetti, Taylor, & Seeman, Reference Repetti, Taylor and Seeman2002; Syed & Nemeroff, Reference Syed and Nemeroff2017). These associations are thought to be driven in part by gradual changes in the stress response system, specifically in the hypothalamic–pituitary–adrenal (HPA) axis and in inflammatory processes (Ehrlich, Miller, & Chen, Reference Ehrlich, Miller and Chen2016; Heim & Binder, Reference Heim and Binder2012; Slavich & Irwin, Reference Slavich and Irwin2014). Stress activates the HPA axis, resulting in secretion of the hormone cortisol, and increases inflammation. These biological responses to stress promote short-term adaptation to challenging circumstances. However, repeated or chronic activation of these responses can interfere with later HPA axis functioning and inflammatory processes in ways that compromise long-term health (McEwen & Seeman, Reference McEwen and Seeman1999).
A substantial body of work has linked various childhood and adolescent stressors, including socioeconomic disadvantage, maltreatment, harsh parenting, and parental separation or loss, to alterations in the HPA axis and to heightened inflammation. Outcomes of HPA axis functioning include the components of the diurnal rhythm of cortisol (i.e., cortisol awakening response, diurnal slope, and waking and bedtime cortisol levels) and responses to acute psychosocial stress. Previous studies have linked childhood and adolescent stress to both heightened and dampened levels of cortisol, which may be due to differences in stressor characteristics and HPA axis measurement (Chiang, Taylor, & Bower, Reference Chiang, Taylor and Bower2015; Repetti, Robles, & Reynolds, Reference Repetti, Robles and Reynolds2011). Seemingly discrepant findings may also reflect the notion that because excessive exposure to glucocorticoids can be detrimental, hypocortisolism may emerge after sustained periods of hypercortisolism as a counterregulatory mechanism (Fries, Hesse, Hellhammer, & Hellhammer, Reference Fries, Hesse, Hellhammer and Hellhammer2005; Miller, Chen, & Zhou, Reference Miller, Chen and Zhou2007). Indeed, with respect to HPA responses to acute stress, studies have consistently linked chronic or repeated stress to dampened cortisol responses (Bunea, Szentágotai-Tătar, & Miu, Reference Bunea, Szentágotai-Tătar and Miu2017; Cărnuţă, Crişan, Vulturar, Opre, & Miu, Reference Cărnuţă, Crişan, Vulturar, Opre and Miu2015; Carpenter et al., Reference Carpenter, Carvalho, Tyrka, Wier, Mello, Mello and Price2007; Carpenter, Shattuck, Tyrka, Geracioti, & Price, Reference Carpenter, Shattuck, Tyrka, Geracioti and Price2011; Elzinga et al., Reference Elzinga, Roelofs, Tollenaar, Bakvis, van Pelt and Spinhoven2008; Engert et al., Reference Engert, Efanov, Dedovic, Duchesne, Dagher and Pruessner2010; Janusek, Tell, Gaylord-Harden, & Mathews, Reference Janusek, Tell, Gaylord-Harden and Mathews2017; Kraft & Luecken, Reference Kraft and Luecken2009; Lovallo, Reference Lovallo2013; MacMillan et al., Reference MacMillan, Georgiades, Duku, Shea, Steiner, Niec and Vella2009), a pattern that has also been observed in individuals with posttraumatic stress disorder, chronic fatigue syndrome, and fibromyalgia (Heim, Ehlert, & Hellhammer, Reference Heim, Ehlert and Hellhammer2000). Dampened cortisol reactivity to stress has also been associated with poorer lung function, decreased antibody responses to vaccination, and greater adiposity (Carroll et al., Reference Carroll, Bibbey, Roseboom, Phillips, Ginty and Rooij2012; de Rooij, Reference de Rooij2013; Phillips, Carroll, Burns, & Drayson, Reference Phillips, Carroll, Burns and Drayson2005). In addition to its links to HPA axis alterations, childhood and adolescent stress has also been related to heightened levels of circulating inflammatory markers in adulthood (Baumeister, Akhtar, Ciufolini, Pariante, & Mondelli, Reference Baumeister, Akhtar, Ciufolini, Pariante and Mondelli2016; Coelho, Viola, Walss-Bass, Brietzke, & Grassi-Oliveira, Reference Coelho, Viola, Walss-Bass, Brietzke and Grassi-Oliveira2014). Studies of youth have yielded more mixed results, suggesting potential delayed effects on systemic inflammation (Slopen, Koenen, & Kubzansky, Reference Slopen, Koenen and Kubzansky2012; Slopen, Kubzansky, McLaughlin, & Koenen, Reference Slopen, Kubzansky, McLaughlin and Koenen2013). Importantly, heightened inflammation is implicated in numerous age-related chronic conditions, including heart disease and certain cancers (Brydon & Steptoe, Reference Brydon and Steptoe2005; Choy, Reference Choy2012; Elinav et al., Reference Elinav, Nowarski, Thaiss, Hu, Jin and Flavell2013; Libby, Reference Libby2006; Wellen & Hotamisligil, Reference Wellen and Hotamisligil2005).
Although much research has linked childhood and adolescent stress to HPA alterations and heightened inflammation, factors that protect against these are not completely understood. Previous research has pointed to warm and supportive relationships as important protective factors (Brody, Yu, & Beach, Reference Brody, Yu and Beach2016; Carroll et al., Reference Carroll, Gruenewald, Taylor, Janicki-Deverts, Matthews and Seeman2013; Chen, Miller, Kobor, & Cole, Reference Chen, Miller, Kobor and Cole2011), but less is known about individual psychological characteristics that may similarly counteract the biological effects of early stress. Theoretical models purport several individual psychological characteristics, collectively known as psychological resources, that promote resilience in the face of adversity; these include constructs such as mastery or a sense of control over one's life, purpose in life, positive affect, optimism, self-esteem, and the ability to positively reappraise negative emotions and situations (Chen & Miller, Reference Chen and Miller2013; Gallo, de los Monteros, & Shivpuri, Reference Gallo, de los Monteros and Shivpuri2009; Taylor & Seeman, Reference Taylor and Seeman1999). These individual characteristics have independently and together been associated with lower risk for clinical health outcomes such as mortality and cardiovascular disease (Carver, Scheier, & Segerstrom, Reference Carver, Scheier and Segerstrom2010; Lundgren, Garvin, Jonasson, Andersson, & Kristenson, Reference Lundgren, Garvin, Jonasson, Andersson and Kristenson2015; Stamatakis et al., Reference Stamatakis, Lynch, Everson, Raghunathan, Salonen and Kaplan2004; Surtees, Wainwright, Luben, Khaw, & Day, Reference Surtees, Wainwright, Luben, Khaw and Day2006; Taylor & Broffman, Reference Taylor, Broffman, Olson and Zanna2011). They have also been shown to buffer against chronic stress-related increases in inflammation and delayed blood pressure recovery from subsequent acute stress (Boylan, Jennings, & Matthews, Reference Boylan, Jennings and Matthews2016; Elliot & Chapman, Reference Elliot and Chapman2016).
The majority of these studies have focused on adults, raising the question of whether psychological resources can similarly buffer against the adverse effects of chronic or repeated stress during earlier life stages. Adolescence may be an especially important focal point for several reasons. First, the adolescent years are marked by an increase in stress. Demands across domains (school, peers, and family) are known to increase during this time, and adolescents have reported similar or higher levels of psychosocial stress than adults (American Psychological Association, 2014). Second, in addition to facing increasing stress, research suggests that sensitivity to the environment increases during adolescence, potentially rendering adolescents more vulnerable to the effects of stress (Blakemore & Mills, Reference Blakemore and Mills2014; Somerville, Reference Somerville2013; Spear, Reference Spear2009). Evidence for this notion comes from studies showing that adolescents exhibit greater negative affective and neurobiological responses to social threat compared with children and adults (Gunnar, Wewerka, Frenn, Long, & Griggs, Reference Gunnar, Wewerka, Frenn, Long and Griggs2009; Sebastian, Viding, Williams, & Blakemore, Reference Sebastian, Viding, Williams and Blakemore2010; Stroud et al., Reference Stroud, Foster, Papandonatos, Handwerger, Granger, Kivlighan and Niaura2009). Last, the foundations of future adult health may be set during adolescence. The idea here is that with rapid neurobiological and psychosocial development that facilitates the transition into an independent, productive adult, adolescent experiences can not only directly shape biological patterns of functioning, but also modify health trajectories initiated in childhood and contribute to the adoption of long-term health behaviors (Del Giudice, Ellis, & Shirtcliff, Reference Del Giudice, Ellis and Shirtcliff2011; Tottenham & Galván, Reference Tottenham and Galván2016; Viner et al., Reference Viner, Ozer, Denny, Marmot, Resnick, Fatusi and Currie2012).
Studies examining whether psychological resources buffer against the negative health effects of chronic stress during adolescence have found that optimism in combination with meaning in life attenuated the association between socioeconomic disadvantage and obesity, cardiometabolic risk, and inflammatory processes (Chen, Lee, Cavey, & Ho, Reference Chen, Lee, Cavey and Ho2013; Chen, McLean, & Miller, Reference Chen, McLean and Miller2015; Kallem et al., Reference Kallem, Carroll-Scott, Rosenthal, Chen, Peters, McCaslin and Ickovics2013). However, such studies are few, and buffering effects on the HPA axis have not been examined. Yet, as described previously, the HPA axis is thought to be an important pathway linking early stress to poor health. Additionally, studies have focused on only a single form of adversity, namely socioeconomic disadvantage. However, youth encounter a variety of stressors in their lives. Furthermore, more mundane and less severe stressors occurring in everyday life (e.g., conflict, work deadlines), in addition to other various chronic stressors (e.g., childhood maltreatment, major life events), have been linked to poor health outcomes and compromised biological functioning (Chiang, Eisenberger, Seeman, & Taylor, Reference Chiang, Eisenberger, Seeman and Taylor2012; DeLongis, Folkman, & Lazarus, Reference DeLongis, Folkman and Lazarus1988; Dietz et al., Reference Dietz, Stoyak, Melhem, Porta, Matthews, Payne and Brent2013; Luecken & Appelhans, Reference Luecken and Appelhans2006; Miller et al., Reference Miller, Chen and Parker2011; Repetti et al., Reference Repetti, Robles and Reynolds2011; Stawski, Cichy, Piazza, & Almeida, Reference Stawski, Cichy, Piazza and Almeida2013; Stone et al., Reference Stone, Neale, Cox, Napoli, Valdimarsdottir and Kennedy-Moore1994). Whether psychological resources protect against the health-related effects of these other stressors is not entirely clear.
The overall purpose of the present study was to examine whether various stressors were associated with HPA axis alterations and heightened inflammation during late adolescence, and whether psychological resources (mastery, self-esteem, optimism, and the tendency to positively reappraise negative situations) mitigated these associations. We focused on cortisol and interleukin (IL)-6 reactivity to a standard laboratory stress task as indices of HPA axis functioning and inflammatory processes, respectively. We examined several types of stressors, including major life events in the past year, daily negative social interactions across a 15-day period, and the early psychosocial family climate. Given that prior work has tied each of these stressors to health outcomes and biological functioning in both youth and adults (Carroll et al., Reference Carroll, Gruenewald, Taylor, Janicki-Deverts, Matthews and Seeman2013; Chiang et al., Reference Chiang, Tsai, Park, Bower, Almeida, Dahl and Fuligni2016; Fuligni et al., Reference Fuligni, Telzer, Bower, Cole, Kiang and Irwin2009; Kershaw et al., Reference Kershaw, Brenes, Charles, Coday, Daviglus, Denburg and Tindle2014; Loucks, Almeida, Taylor, & Matthews, Reference Loucks, Almeida, Taylor and Matthews2011; Miller & Chen, Reference Miller and Chen2010; Pyykkönen et al., Reference Pyykkönen, Räikkönen, Tuomi, Eriksson, Groop and Isomaa2010; Stroud, Chen, Doane, & Granger, Reference Stroud, Chen, Doane and Granger2016), we did not hypothesize any differential effects according to stressor type. Instead, we hypothesized that all three types of stress would be associated with suboptimal biological profiles, as evidenced by dampened cortisol and heightened IL-6 responses to acute stress. We further hypothesized significant interactions among each of the three types of stress and psychological resources, such that those reporting greater levels of stress and lower levels of psychological resources would exhibit reduced cortisol and elevated IL-6 reactivity. These associations were expected to be attenuated among those who reported stress but had higher levels of psychological resources. Given that our sample included Latino and European-American adolescents, exploratory analyses examined whether there were any ethnic differences in stress associations with cortisol and IL-6 responses and in the potential buffering role of psychological resources. Socioeconomic status, family values, and rates of racial discrimination are known to differ between these two ethnic groups (Fuligni, Tseng, & Lam, Reference Fuligni, Tseng and Lam1999; Greene, Way, & Pahl, Reference Greene, Way and Pahl2006; Sabogal, Marín, Otero-Sabogal, Marín, & Perez-Stable, Reference Sabogal, Marín, Otero-Sabogal, Marín and Perez-Stable1987; Semega, Frontenot, Kollar, & Bureau, Reference Semega, Frontenot, Kollar and Bureau2017), which may translate to differential effects of stress and its interactions with psychological resources on biological responsivity.
Method
Participants
Participants were 18- to 20-year-old adolescents (n = 91; M age = 18.37, SD = .51; 57% female) drawn from a larger sample of 248 adolescents (M age = 18.31, SD = .77) partaking in the second wave of a three-wave longitudinal study on the psychosocial contributions to the development of early health risk from mid-adolescence to early adulthood. During the first wave of the larger parent study, 316 tenth- and eleventh-grade students (M age = 16.40, SD = .74) were recruited, 214 of whom participated in the second wave 2 years later. During the second wave, 34 new participants were added to refresh the sample. Two years after the second wave, 180 participants (M age = 20.29, SD = .74) from Wave 1 and/or Wave 2 provided data during the third and final wave.
Data collection of the larger study involved a home visit during which participants completed a series of questionnaires and a 15-day daily diary protocol. After completing the Wave 2 protocol, participants were prescreened to ensure that they identified as Latino or European-American (other ethnicities were excluded because of insufficient numbers in the larger study) and were at least 18 years of age. Prescreening was based on responses to demographic questionnaires collected in the larger parent study. Eligible individuals were contacted via telephone, and those who responded (n = 169) were given information on the current experimental study and invited to participate. Individuals expressing interest were scheduled for a laboratory visit and instructed to refrain from eating or drinking anything (except water) the hour prior to the visit. Ninety-one participants, 79 of whom were from the original Wave 1 sample and 12 of whom were from the pool of newly added participants at Wave 2, completed the laboratory visit an average of 5.5 (SD = 2.7, range = 1.4–12.2) months after they completed Wave 2 of the parent study. Participants in the present study did not differ from the larger Wave 2 sample on age, gender, parent education, and ethnicity (all ps > .156). They also did not differ on major life events and daily interpersonal stress (all ps > .173), which were assessed as part of the larger parent study.
The subsample in the current study were from European-American (37.36%) and Latino backgrounds (62.64%). Primary caregivers reported on annual household income as well as their own and spouse's highest level of education on an 11-point scale (1 = some elementary school, 11 = graduated from medical law, or graduate school). Education across parents was averaged. Across the study sample, median household income was $79,000 (range = $11,000–$350,000). Approximately 14.44% of participants had parents with less than a high school diploma, 7.78% had at least one parent with a high school diploma, 38.89% had at least one parent who completed vocational trade school or some college, 23.33% had at least one parent with a college degree, and 15.57% had at least one parent who completed some or all of graduate or professional school.
Procedure
Participants arrived at the University of California, Los Angeles (UCLA), Clinical and Translational Research Center and provided informed written consent and anthropometric measures. A nurse then inserted an indwelling intravenous catheter in the antecubital vein of the nondominant arm, after which participants viewed a neutral-content video for 20 min to facilitate acclimation to the testing environment. The first saliva and blood samples were collected after this baseline period. Participants then completed the Trier Social Stress Task (TSST), a well-established acute laboratory-based social stressor that activates the HPA axis and increases inflammation (Kirschbaum, Pirke, & Hellhammer, Reference Kirschbaum, Pirke and Hellhammer1993; Steptoe, Hamer, & Chida, Reference Steptoe, Hamer and Chida2007). Participants delivered a 5-min impromptu speech on why they were the best candidates for their ideal jobs in front of an evaluative panel of expert interviewers consisting of research assistants trained to provide nonverbal negative feedback. Participants were given 5 min to prepare for their speech. Immediately after delivering their speech, participants performed a 5-min mental arithmetic task involving progressively subtracting 13 from 2,935 while the evaluative panel urged them to go more quickly and perform more accurately. After the TSST, participants provided five additional saliva samples (immediately and 30, 45, 60, and 75 min post-TSST onset) and three additional blood samples (30, 60, and 90 min post-TSST onset). After the final blood sample was collected, participants were debriefed and compensated. All visits were conducted between the hours of 12 and 6 pm, and the UCLA institutional review board approved all procedures.
Measures
Major life events
During the second wave visit of the parent study, participants completed a major life event checklist. They reported on whether they had experienced any of 21 events across the domains of family, friends, and school within the past 12 months. Items were adapted from previously used measures of stressful events that have been linked to negative outcomes (Conger et al., Reference Conger, Wallace, Sun, Simons, McLoyd and Brody2002; Hammen, Reference Hammen1991). Example items included parents divorced or separated, family member became seriously ill, a close friend moved quite far away, having a serious falling out or ending a friendship with a close friend, being suspended or expelled in school, and grades in school went down a lot. Affirmative responses were summed across items.
Daily interpersonal stress
Participants also completed a daily diary protocol during the second wave visit of the parent study. Each night for 15 consecutive evenings, participants reported on whether they had experienced any of 8 negative social interactions across the contexts of family, peers, and school. Items were argued with a parent, argued with another family member, argued with a friend, punished by a parent, parents argued, something bad happened to a family member, had an argument or was punished by an adult at school, and was insulted, threatened, or made fun of by someone at school. These items were selected because they have previously been shown to be stressful for adolescents (Chung, Flook, & Fuligni, Reference Chung, Flook and Fuligni2009; Fuligni et al., Reference Fuligni, Telzer, Bower, Cole, Kiang and Irwin2009; Nishina & Juvonen, Reference Nishina and Juvonen2005). Consistent with previous research on daily stress (Chiang et al., Reference Chiang, Tsai, Park, Bower, Almeida, Dahl and Fuligni2016; Grzywacz, Almeida, & McDonald, Reference Grzywacz, Almeida and McDonald2002; Sin, Graham-Engeland, Ong, & Almeida, Reference Sin, Graham-Engeland, Ong and Almeida2015), affirmative responses were summed and recoded as 0 or 1 for each day to indicate whether any 1 of the stressors occurred that day. The average of recoded scores across days was subsequently computed to indicate the proportion of days that at least 1 stressor occurred.
Early adversity
The Risky Families questionnaire (Taylor, Lerner, Sage, Lehman, & Seeman, Reference Taylor, Lerner, Sage, Lehman and Seeman2004) was used to measure early adversity and was administered during the laboratory visit. Specifically, the Risky Families questionnaire assesses the family psychosocial climate in which participants grew up when they were 5 to 15 years old. On a 5-point scale (1 = not at all to 5 = very often), participants indicated how frequently they experienced conflict, violence, corporal punishment, parental affection, neglect, and chaos. The Risky Families questionnaire has been widely used in previous research and aligns with clinical interviews assessing early life stress. Items framed positively were reverse-coded and scores across items were averaged. The scale demonstrated good internal reliability in the present sample (α = .84).
HPA reactivity
Six saliva samples were collected using oral swabs (Salimetrics) throughout the laboratory visit. They were collected immediately after the neutral-content video (baseline) and TSST, and 30, 45, 60, and 75 min after TSST onset. Samples were stored at –80°C until transported on ice to the Laboratory of Biological Psychology at the Technical University of Dresden, Germany, where they were assayed for cortisol in duplicate using high-sensitivity chemiluminescence-immunoassays (IBL, International, Hamburg, Germany). Intra- and interassay coefficients of variations were <10%. Outlier screening revealed a total of 10 samples across time points that had cortisol values greater than 3 standard deviations (SDs) from their respective means. These values were Winsorized and replaced with the value at 3 SDs. Cortisol values were then natural log-transformed to correct for their skewed distributions. We previously reported that the TSST activated the HPA axis, as evidenced by a significant increase in cortisol from baseline to 30 and 45 min after TSST (Chiang, Bower, Irwin, Taylor, & Fuligni, Reference Chiang, Bower, Irwin, Taylor and Fuligni2017).
Inflammatory reactivity
Four blood samples of 6 ml were collected at baseline, and 30, 60, and 90 min after TSST onset into ethylenediaminetetraacetic acid lavender-top tubes. Samples were placed on ice immediately after collection, after which they were centrifuged, aliquoted into plasma samples, and stored at –80°C. After data collection was completed, samples were assayed in duplicate for IL-6 using the Quantikine high sensitivity human IL-6 ELISA kits (R&D Systems, Inc., Minneapolis, MN) by the UCLA Cousins Center Inflammatory Biology Core. Intra- and interassay coefficients of variability were <7%. Outlier screening revealed a total of 11 samples across time points that had IL-6 values greater than 3 SDs from their respective means. These values were Winsorized and replaced with the value at 3 SDs. All values were natural log-transformed given the skewed distribution. As reported previously (Chiang et al., Reference Chiang, Bower, Irwin, Taylor and Fuligni2017), the TSST was effective in eliciting an inflammatory response, with participants exhibiting a 34% average increase in IL-6 from baseline to 90 min after TSST onset.
Psychological resources
Measures of psychological resources were completed during the laboratory visit and assessed perceived control, optimism, self-esteem, and positive reappraisal. Perceived control was assessed using the 7-item Pearlin Mastery Scale (Pearlin & Schooler, Reference Pearlin and Schooler1978). Participants indicated the extent to which they agreed with statements such as: “There is little I can do to change many of the important things in my life (reverse coded),” and “What happens to me in the future mostly depends on me,” using a 4-point scale (1 = strongly disagree, 4 = strongly agree). In the current study, internal consistency was adequate (α = .74).
Dispositional optimism was assessed using the 6-item Life Orientation Test (Scheier, Carver, & Bridges, Reference Scheier, Carver and Bridges1994). Using a 5-point scale (0 = strongly disagree, 4 = strongly agree), participants rated the degree to which they agreed with each item. Example items include “In uncertain times, I usually expect the best” and “Overall, I expect more good things to happen to me than bad.” This scale demonstrated good internal reliability in the present study (α = .83).
Self-esteem was assessed using the 10-item Rosenberg Self-Esteem Scale (Rosenberg, Reference Rosenberg1965). Participants rated items such as, “I feel that I have a number of good qualities” and “On the whole, I am satisfied with myself,” on a 4-point scale (0 = strongly agree, 3 = strongly disagree). Internal consistency of this scale was high (α = .93).
Positive reappraisal was assessed using the cognitive reappraisal subscale from the Emotion Regulation Questionnaire (Gross & John, Reference Gross and John2003). Using a 7-point scale (1 = not at all, 7 = a lot), participants indicated the extent to which they agreed with statements such as “To feel less negative emotion, I change what I think about” and “When I'm faced with a stressful situation, I make myself think about it in a way that helps me stay calm.” The Emotion Regulation Questionnaire has been well-validated and internal consistency in the present sample was high (α = .88).
Negatively worded items on each scale were reverse coded, such that higher scores indicated greater mastery, optimism, self-esteem, and positive reappraisal. Items were then summed for each scale. Principal-components analysis indicated that a single component explained 61.2% of the variance. Factor loadings were .83 for mastery, .83 for optimism, .73 for self-esteem, and .72 for reappraisal. Thus, we averaged standardized scores of the four scales to create a composite score indexing psychological resources.
Potential covariates
Sociodemographic characteristics (age, gender, ethnicity, socioeconomic status), health behaviors (exercise, caffeine consumption, cigarette smoking), adiposity (waist circumference), use of oral contraceptives, and time of day were considered as potential covariates given that these factors have been previously related to levels of cortisol and/or IL-6 (DeSantis et al., Reference DeSantis, Adam, Doane, Mineka, Zinbarg and Craske2007; Dowd, Simanek, & Aiello, Reference Dowd, Simanek and Aiello2009; Jacks, Sowash, Anning, Mcgloughlin, & Andres, Reference Jacks, Sowash, Anning, Mcgloughlin and Andres2002; Lovallo et al., Reference Lovallo, Whitsett, al'Absi, Sung, Vincent and Wilson2005; O'Connor et al., Reference O'Connor, Bower, Cho, Creswell, Dimitrov, Hamby and Sloan2009; Uhart, Chong, Oswald, Lin, & Wand, Reference Uhart, Chong, Oswald, Lin and Wand2006).
During the second wave visit, participants reported their own ethnicity and gender. Their parents reported participants’ date of birth, from which age was computed, and their own and spouse's educational attainment. Educational attainment was averaged across parents to index family socioeconomic status. Waist circumference was assessed twice at the midpoint between the iliac crest and lower rib and averaged. During the laboratory visit, study experimenters recorded participants’ time of arrival, and participants reported on the number of caffeinated beverages consumed that day, whether they smoked cigarettes during the past week (yes/no), and whether they currently use oral contraceptives (yes/no). Additionally, for three consecutive mornings before the laboratory visit, participants reported on whether they engaged in physical activity the previous day and the total number of days exercised was computed.
Analytic approach
Selection of covariates
Bivariate correlations among potential covariates and cortisol and IL-6 at each time point were examined to identify the panel of covariates to be included in primary analyses. Gender, parental education, time of day, exercise, smoking, and use of oral contraceptives were significantly or marginally associated with at least one of the cortisol measures across time points (all r = –.40 to .30, all ps < .06). Thus, these were included as covariates in all models with cortisol as the outcome. For IL-6, age, gender, parent education, ethnicity, and waist circumference showed marginal or significant correlations with at least one IL-6 measure across time points (all r = –.21 to .47, all ps < .06) and were therefore included as a covariate in models with IL-6 as the outcome. Models with and without covariates yielded similar results.
Primary analyses
Multilevel modeling was used to conduct analyses given repeated measurements within individuals. Level 1 modeled within-person changes in cortisol and IL-6 as a function of time, whereas Level 2 modeled between-person differences in cortisol and IL-6 responses based on experiences of stress (i.e., major life events, daily interpersonal stress, and early adversity), psychological resources, and their interactions. The intercepts in models represent baseline levels of cortisol and IL-6. Models with cortisol as the outcome included both a linear and a quadratic term for time given that sampling times captured cortisol reactivity as well as recovery (Chiang et al., Reference Chiang, Bower, Irwin, Taylor and Fuligni2017; Dickerson & Kemeny, Reference Dickerson and Kemeny2004). The linear term for time represents the rate of change in cortisol, whereas the quadratic term for time represents how the rate of change increases or decreases across time, or in other words, the curvature of cortisol responses. Thus, increased cortisol reactivity is reflected as a positive value for the linear term, and a slowing rate of cortisol increases (i.e., cortisol peaks then declines) is reflected as a negative value for the quadratic term. Models with IL-6 as the outcome only included a linear term for time given that sampling times captured only reactivity to the TSST, because IL-6 requires a longer period to return to baseline (Chiang et al., Reference Chiang, Bower, Irwin, Taylor and Fuligni2017; Marsland, Walsh, Lockwood, & John-Henderson, Reference Marsland, Walsh, Lockwood and John-Henderson2017).
We first tested the main effects of stress and psychological resources on cortisol and IL-6 response trajectories by examining the cross-level interactions between stress and time and between psychological resources and time. As reported in the following “Results” section, significant stress by time interactions emerged. These interactions were followed by tests of simple slopes in which the effect of time on responses to the TSST were examined among individuals above and below one-half of a standard deviation from the mean of major life events (low: n = 21; high: n = 21), daily interpersonal stress (low: n = 29; high: n =30), and early adversity (low: n = 32; high: n = 22).
We then tested whether psychological resources protected against the effects of stress by examining the three-way interactions among stress, psychological resources, and time. Significant interactions indicate that biological responses varied as a function of stress at varying levels of psychological resources. Observed interactions were followed by tests of simple interactions in which two-way stress by time interactions were examined separately in individuals above (n = 33) and below (n = 23) one-half of a standard deviation from the mean of psychological resources.
In all analyses, interaction effects were probed at one-half of a standard deviation above and below the mean of the moderator of interest because the number of individuals above and below one standard deviation were much fewer. All continuous predictor and covariate variables were grand mean-centered and separate models were tested for each measure of stress. Because major life events and daily interpersonal stress were assessed at varying times before the laboratory visit, we also assessed whether the duration between the second-wave visit of the parent study and the laboratory visit altered results. Results were unchanged when time between parent study and laboratory visit was added as a covariate. Therefore, results from models without this variable are reported.
Missing data
Rates of missing data were low for variables included in analyses. Variables that had missing data included IL-6 at all time points, parent education, and daily family stress. For IL-6, only 7 of the 364 (1.9%) samples had missing data. Only one (1.1%) participant had missing data on parent education, and only three (3.3%) participants had missing data on daily interpersonal stress. In all models, full information maximum likelihood estimation was used.
Results
Descriptive statistics are presented in Table 1 and bivariate correlations are displayed in Table 2. Overall, adolescents experienced a few major life events, had negative social interactions on 24% of days (i.e., 3.6 days), and experienced fairly low levels of early adversity. As expected, more major life events, daily interpersonal stress, and early adversity were positively correlated with one another and negatively correlated with psychological resources. Of the different stressors, only greater early adversity was associated with lower cortisol. Psychological resources were also not associated with cortisol. Neither stress nor psychological resources was correlated with IL-6.
Table 1. Sample characteristics and descriptive statistics of study variables.

Note: Cortisol and IL-6 reflect raw, un-Winsorized values.
Table 2. Bivariate correlations among primary study variables.
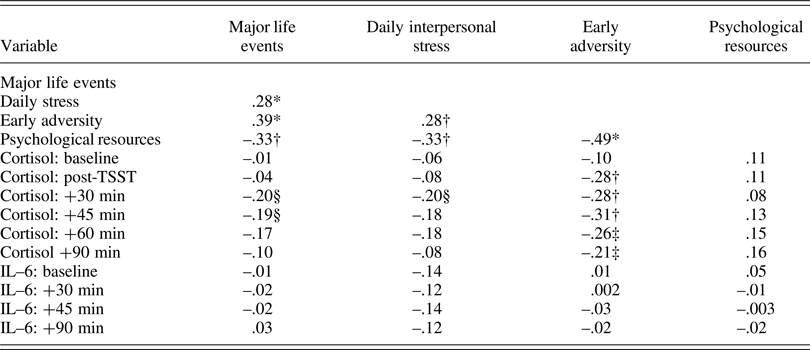
Note: *p < .001; †p < .01; ‡p = .05; §p < .063 – .072.
HPA reactivity
Major life events
As shown in Table 3 (column 1), neither major life events (p = .623) nor psychological resources (p = .870) were associated with baseline levels of cortisol. Cortisol reactivity to the TSST was also not modulated by psychological resources (linear: p = .297; quadratic: p = .201). However, there was a significant interaction between major life events and time (linear: p = .005; quadratic: p = .006). Follow-up tests revealed that adolescents who experienced more major life events exhibited smaller cortisol responses (linear: b(SE) = .07(.06), p = .195; quadratic: b(SE) = –.03(.01), p = .006) relative to those who experienced fewer major life events (linear: b(SE) = .21(.05), p < .001; quadratic: b(SE) = –.05 (.01), p < .001).
Table 3. Results of models predicting cortisol from stress, psychological resources, and their interactions.
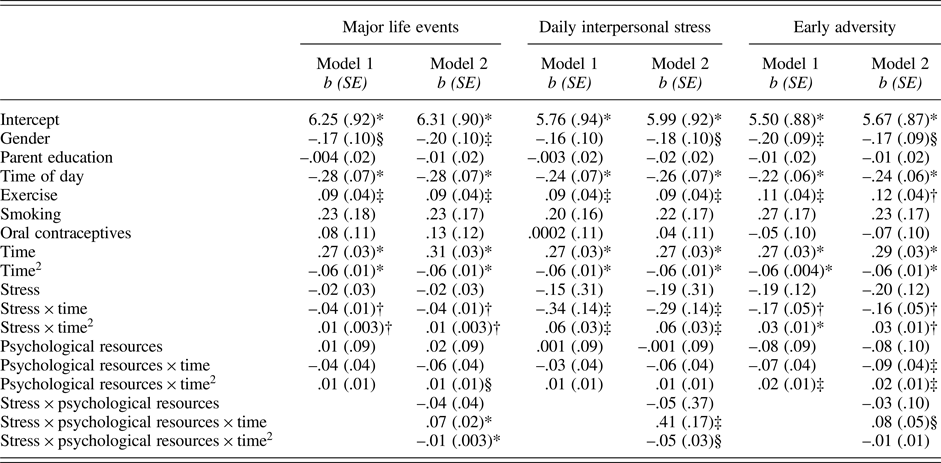
Note: *p ≤ .001; †p .01; ‡p ≤ .05; §p = .058 – .075.
Gender was coded as 0 = male and 1 = female; ethnicity was coded as 0 = European American and 1 = Latino.
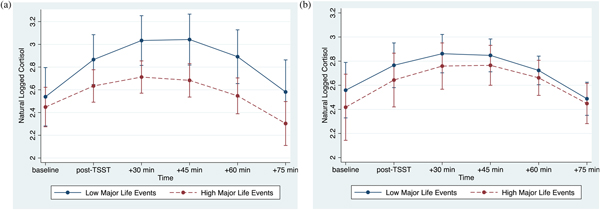
The association between major life events and cortisol responses were modulated by psychological resources, as evidenced by the significant interaction between major life events, psychological resources, and time (linear: p < .001; quadratic: p = .001; Table 3, column 2). At lower levels of psychological resources, cortisol responses varied as a function of major life (linear: b(SE) = –.07(.02), p = .002; quadratic: b(SE) = .01(.004), p = .008). By contrast, at higher levels of psychological resources, major life events were not associated with cortisol responses (linear: b(SE) = .01(.02), p = .715; quadratic: b(SE) = .0002(.004), p = .968). Visualization of these simple interaction effects shows that among individuals with low psychological resources (Figure 1a), those with more major life events exhibit smaller cortisol responses relative to their peers who report fewer major life events. However, among those with high psychological resources (Figure 1b), cortisol responses of those with more major life events more closely resemble those with fewer major life events.
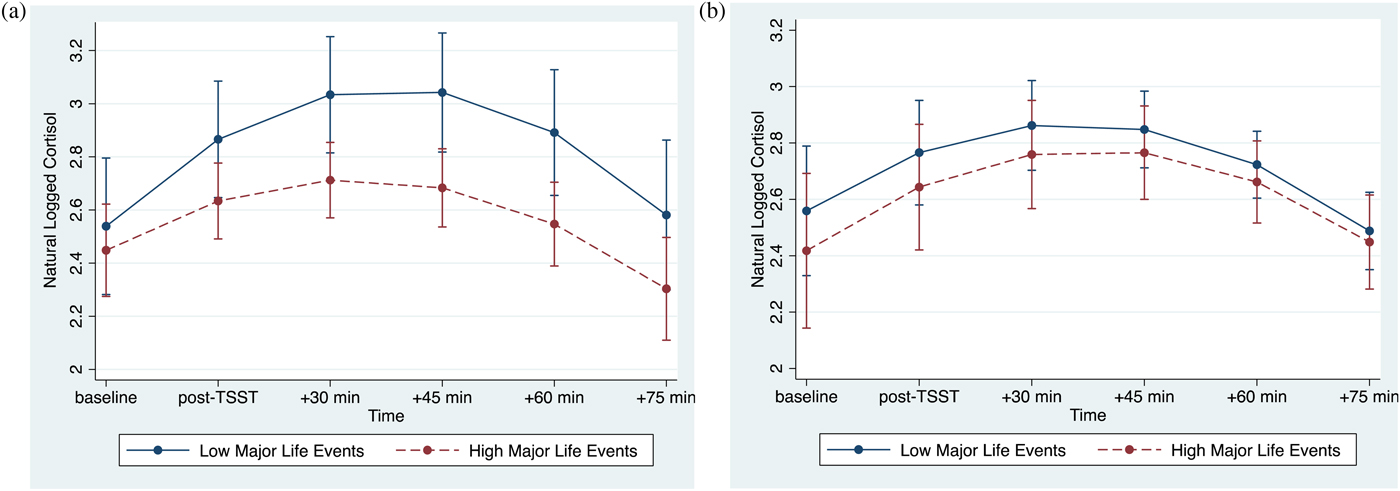
Figure 1. (Color online) (a) At lower levels of psychological resources, major life events are associated with decreased cortisol reactivity. (b) At higher levels of psychological resources, major life events are not associated with cortisol reactivity. Error bars reflect 95% confidence intervals.
Daily interpersonal stress
Paralleling findings for major life events, daily interpersonal stress operationalized as proportion of stressor days (p = .624) and psychological resources (p = .990) were not related to cortisol levels at baseline (Table 3, column 3). In addition, psychological resources were not associated with cortisol reactivity to the TSST (linear: p = .424; quadratic: p = .286). However, there was a significant interaction between daily interpersonal stress and time (linear: p = .017; quadratic: p = .011). Adolescents who experienced more interpersonal stress in their daily lives had smaller cortisol responses (linear: b(SE) = .19(.06), p = .001; quadratic: b(SE) = –.04(.01), p < .001) compared with their peers who experienced less interpersonal stress in their everyday lives (linear: b(SE) = .37(.04), p < .001; quadratic: b(SE) = –.07(.01), p < .001).
Variation in cortisol responses associated with daily interpersonal stress were further modulated by psychological resources, as reflected in the significant 3-way interaction among daily interpersonal stress, psychological resources, and time (linear: p < .015; quadratic: p = .070; Table 3, column 4). Tests of simple interactions indicated that daily interpersonal stress was associated with cortisol responses at lower levels of psychological resources (linear: b(SE) = –.60(.22), p = .006; quadratic: b(SE) = .09(.04), p = .020), but not at higher levels of psychological resources (linear: b(SE) = –.07(.25), p = .778; quadratic: b(SE) = .04(.04), p = .342). Visualization of the simple interaction effects revealed a similar pattern as that depicted in Figure 1.
We also examined whether summing the number of daily stressors across days yielded similar findings given that the cumulative exposure to stress across days might be distinct from the proportion of stressor days. These two measures of daily stress were highly correlated (r = .89, p < .001). Similar to proportion of stressor days, cumulative stress exposure modulated cortisol responses to the TSST (linear: b(SE) = –.01(.01), p = .028; quadratic: b(SE) = .002(.001), p = .027). Adolescents who reported a greater number of stressors across days had smaller cortisol responses (linear: b(SE) = .23(.07), p < .001; quadratic: b(SE) = –.04(.01), p < .001) compared with those who reported a smaller number of stressors across days (linear: b(SE) = .36(.04), p < .001; quadratic: b(SE) = –.07 (.01), p < .001). Contrasting findings for proportion of stressor days, however, there was no evidence that the variation in cortisol responses related to cumulative stress exposure across days was further modulated by psychological resources (linear: b(SE) = .01(.01), p = .119; quadratic: b(SE) = –.001(.001), p = .459).
Early adversity
Table 3 (column 5) shows that early adversity (p = .099) and psychological resources (p = .396) were not associated with baseline cortisol. Psychological resources were not associated with rate of change in cortisol (linear: p = .095), but was associated with quadratic change (p = .036). However, simple slope analyses revealed similar quadratic change at both high and low levels of psychological resources (quadratic: all bs(SE) = –.06(.01), all ps < .001).
Similar to major life events and daily interpersonal stress, early adversity also modulated cortisol responses to the TSST (linear: p = .002; quadratic: p < .001). Cortisol responses were smaller among those reporting higher levels of early adversity (linear: b(SE) = .20(.06), p = .001; quadratic: b(SE) = –.04(.01), p < .001) compared with cortisol responses among those reporting lower levels of early adversity (linear: b(SE) = .36(.04), p < .001; quadratic: b(SE) = –.07(.01), p < .001).
There was a trend toward a three-way interaction among early adversity, psychological resources, and time (linear: p = .073; quadratic: p = .310; Table 3, column 6). At low levels of psychological resources, early adversity was related to cortisol responses (linear: b(SE) = –.26(.08), p = .001; quadratic: b(SE) = .04(.01), p = .005). At high levels of psychological resources, early adversity was not related to cortisol responses (linear: b(SE) = –.07(.08), p = .406; quadratic: b(SE) = .03(.01), p = .072). Visualization of the simple interaction effects revealed a similar pattern as that depicted in Figure 1.
Inflammatory reactivity
Major life events
As shown in Table 4 (column 1), neither major life events nor psychological resources were associated with baseline levels of IL-6 (major life events: p = .812; psychological resources: p = .332) and IL-6 reactivity (major life events: p = .669; psychological resources: p = .609). There was also no major life events by psychological resources interaction effect on IL-6 reactivity (p = .098; Table 4, column 2).
Table 4. Results of models predicting IL-6 from stress, psychological resources, and their interactions
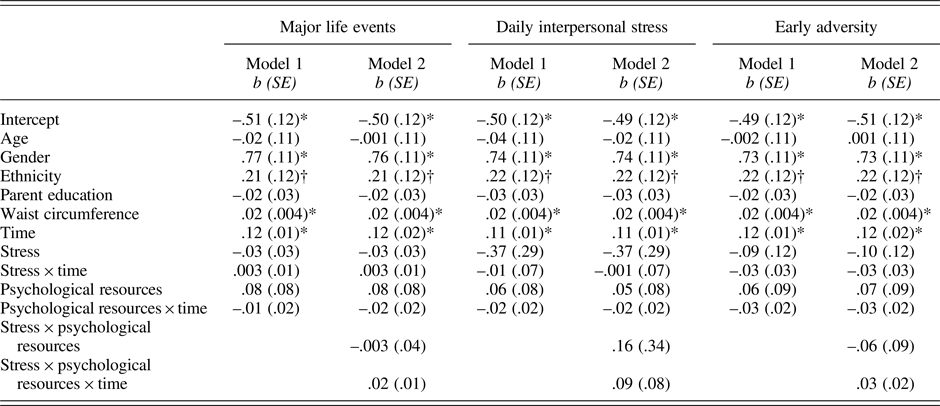
Note:*p .001; †p = .067 – .083. Gender was coded as 0 = male and 1 = female; ethnicity was coded as 0 = European American and 1 = Latino.
Daily interpersonal stress
Table 4 also shows that daily interpersonal stress operationalized as proportion of stressor days and psychological resources were not related to IL-6 at baseline (daily stress: p = .176; psychological resources: p = .452) and in response to the TSST (daily stress: p = .869; psychological resources: p = .408). Daily interpersonal stress and psychological resources also did not interact to influence IL-6 reactivity (p = .261). The cumulative number of stressors across the days similarly did not modulate IL-6 responses (b (SE) = −.001(.003), p = .619). This association was also not further modulated by psychological resources (b(SE) = .003(.003), p = .306).
Early adversity
Again as shown in Table 4, early adversity and psychological resources were not associated with baseline IL-6 (early adversity: p = .455; psychological resources: p = .494) and IL-6 reactivity (early adversity: p = .283; psychological resources: p = .248). There was also no interaction effect between early adversity and psychological resources on inflammatory reactivity (p = .10).
Sensitivity analyses
We tested whether higher psychological resources may be a proxy for better overall mental health by statistically adjusting for depressive symptoms and by testing whether depressive symptoms interacted with the various stressors to predict HPA reactivity. Results remained the same when including depressive symptoms as a covariate. There was also no evidence that daily interpersonal stress or early adversity interacted with depressive symptoms to influence cortisol responses (linear terms: all ps = .644–.796; quadratic terms: all ps = .564–.885). Depressive symptoms did, however, interact with major life events to predict cortisol responses (linear term: b(SE) = –.004(.001), p = .003; quadratic term: b(SE) = .001(.0003), p = .023). Major life events were related to cortisol reactivity only among those with higher depressive symptoms (n = 21; linear term: b(SE) = –.06(.03), p = .019; quadratic term: b(SE) = .01(.005), p = .074), but not among those with lower depressive symptoms (n = 33; linear term: b(SE) = .01(.03), p = .803; quadratic term: b(SE) = –.002(.005), p = .643).
Exploratory analyses
Compared with European-Americans, Latinos face higher rates of poverty and socioeconomic and racial discrimination and have different family values, which may lead to differential effects of stress and psychological resources on biological responsivity. Therefore, we examined whether ethnicity moderated the effects of stress and the effects of the stress by psychological resources interactions on cortisol and IL-6 responses. Overall there was no evidence that stress associations with cortisol (linear: all ps > .352; quadratic: all ps > .155) and IL-6 (all ps > .110) responses were different across ethnicity. The only exception to this pattern of findings was the association between early adversity and cortisol responses (linear term: b(SE) = –.31(.12), p = .009; quadratic term: b(SE) = .06(.02), p = .003). Early adversity was associated with decreased cortisol responses among Latinos (linear term: b(SE) = –.25(.07), p < .001; quadratic term: b(SE) = .05(.01), p < .001), but not among European-Americans (linear term: b(SE) = .01(.09), p = .947; quadratic term: b(SE) = –.01(.01), p = .699). There was no evidence of ethnic differences in stress associations with cortisol (linear: all ps > .590; quadratic: all ps > .255) and IL-6 (all ps > .065) responses moderated by psychological resources.
Discussion
The present study aimed to deepen our understanding of factors that may protect against the negative biological effects of psychosocial stress during adolescence, a developmental period important to the establishment of future adult health. Consistent with work on childhood and adolescent stress and HPA axis responses to subsequent acute stress (e.g., Cărnuţă et al., Reference Cărnuţă, Crişan, Vulturar, Opre and Miu2015; Carpenter et al., Reference Carpenter, Carvalho, Tyrka, Wier, Mello, Mello and Price2007; Elzinga et al., Reference Elzinga, Roelofs, Tollenaar, Bakvis, van Pelt and Spinhoven2008; Kraft & Luecken, Reference Kraft and Luecken2009), we found that experiences of various stressors, including major life events, daily interpersonal stress, and early adversity, were associated with smaller cortisol responses to an acute laboratory-based stressor. Individual psychological resources moderated the links from major life events and daily stress to cortisol reactivity, such that they were evident only among adolescents who reported lower levels of psychological resources. By comparison, these stress–cortisol reactivity associations were not apparent among those reporting higher levels of psychological resources. These findings were specific to the HPA axis and were not evident for inflammatory processes.
The present findings converge with work showing that among socioeconomically disadvantaged youth, positive reappraisal tendencies, and finding meaning in life were associated with reduced risk of poor health-related outcomes, such as obesity and cardiovascular risk (Chen et al., Reference Chen, Lee, Cavey and Ho2013, Reference Chen, McLean and Miller2015; Kallem et al., Reference Kallem, Carroll-Scott, Rosenthal, Chen, Peters, McCaslin and Ickovics2013). We extend this work by demonstrating that individual psychological resources may also protect young individuals from the effects of other stressors, including major life events, daily interpersonal stress, and to some extent early adversity, on reduced HPA responses to acute stress, which is thought to be one key mechanism linking stress to health and psychopathology (Ehrlich et al., Reference Ehrlich, Miller and Chen2016; Heim & Binder, Reference Heim and Binder2012).
The current findings raise the question of how psychological resources may counter the effects of chronic or repeated stress on HPA axis reactivity to subsequent acute stress. One explanation is that psychological resources foster effective coping strategies (Taylor & Broffman, Reference Taylor, Broffman, Olson and Zanna2011; Taylor & Stanton, Reference Taylor and Stanton2007). Having higher levels of optimism, self-esteem, self-mastery, and a tendency to positively reappraise negative or threatening situations likely biases one towards regulation of stress-evoked negative emotions, active problem-solving, and/or seeking support or other resources, over maladaptive ways of coping, such as rumination. In the face of major life events or repeated daily stress, these coping strategies may attenuate HPA reactivity and/or facilitate quicker recovery. To the extent that hypocortisolism results after extended periods of high levels of cortisol as a counterregulatory mechanism (Fries et al., Reference Fries, Hesse, Hellhammer and Hellhammer2005; Miller et al., Reference Miller, Chen and Zhou2007), attenuated reactivity to and/or quicker recovery from previous stress may reduce the amount of overall exposure to cortisol and prevent or delay hypocortisolism from emerging.
Psychological resources seemed to have stronger protective effects for major life events and daily stress relative to early adversity. Whereas significant interactions with psychological resources emerged for major life events and daily stress, there was only a marginally significant interaction between early adversity and psychological resources on linear cortisol changes, and there was no interaction for quadratic cortisol changes. Why we observed this pattern is not entirely clear, but may have to do with temporal distance between exposure to the different stressors and the assessment of cortisol responses. In the current study, major life events tapped into stressful events within the last year and daily stress assessed the everyday interpersonal in participants’ current lives. By contrast, early adversity assessed participants’ family climate from ages 5 to 15. Given the longer period between early adversity and assessment of HPA reactivity, the effects of early adversity may have had more sufficient time to “get under the skin” and solidify. As such, psychological resources may be particularly less effective for more temporally distal stressors relatively to more temporally proximal severe stressors. Of course, this remains speculative, and requires empirical examination.
One caveat to the observed pattern of findings is that there was some evidence that psychological resources per se did not buffer the effects of major life events on cortisol responses. Psychological resources and depressive symptoms were correlated fairly strongly (r ¼ −.60), and major life events interacted with depressive symptoms, such that major life events were related to cortisol responses only among those with higher depressive symptoms, but not among individuals with lower depressive symptoms. It may be that for the effects of major life events, psychological resources served as a proxy for overall adjustment, and lower mental health may be the important moderator that accentuates relations between major life events and HPA axis functioning. Psychological resources, on the other hand, may be effective in counteracting the adverse health-related effects not only of socioeconomic disadvantage, as shown in previous studies (Chen et al., Reference Chen, Lee, Cavey and Ho2013; Chen et al., Reference Chen, McLean and Miller2015; Kallem et al., Reference Kallem, Carroll-Scott, Rosenthal, Chen, Peters, McCaslin and Ickovics2013), but also of other chronic stressors such as more mundane daily stressors that may accumulate over time.
Interestingly, exploratory analyses revealed ethnic differences in the associations between early adversity and cortisol responses, but not in the buffering role of psychological resources. Specifically, the association between early adversity and cortisol reactivity was evident only in Latino adolescents, but not in their European-American counterparts. These findings may reflect ethnic differences in family values. Our measures of early adversity assessed overall family environment, and compared with European-American families, Latino families are characterized by higher levels of familism, or the view that the family constitutes the self and is the primary source of support; family duties, interconnectedness and unity among family members, and family needs rather than individual needs are also emphasized (Fuligni et al., Reference Fuligni, Tseng and Lam1999; Sabogal et al., Reference Sabogal, Marín, Otero-Sabogal, Marín and Perez-Stable1987). As such, early family-related stress may be more distressing for Latino adolescents. We found no ethnic differences in the protective role of psychological resources, which might suggest that psychological resources do not operate as a protective factor for Latino youth exposed to a harsh family climate. However, the current sample size may be a limiting factor. Future work should further probe psychological resources as a possible protective factor, particularly for Latino youth experiencing high levels of family-related stress.
Findings for stress and its interaction with psychological resources were specific to the HPA axis and not observed for inflammatory reactivity. At first, this may seem somewhat surprising given that the HPA axis regulates inflammatory activity (Irwin & Cole, Reference Irwin and Cole2011; Rohleder, Reference Rohleder2014). In particular, cortisol downregulates the production of cytokines when bound to its corresponding receptors in immune cells; as such, decreased cortisol reactivity might be expected to result in increases in circulating levels of IL-6 among those with higher levels of stress and lower levels of psychological resources. However, for cortisol to bind to its receptor in immune cells, cortisol must be unbound to carrier proteins. Yet, only a small fraction of cortisol (approximately 10%) remains unbound and biologically active (El-Farhan, Rees, & Evans, Reference El-Farhan, Rees and Evans2017). Furthermore, before modifying other biological systems, including the immune system, unbounded, free cortisol, must avoid being excreted and converted to its inactive form by counterregulatory mechanisms (Ehrlich et al., Reference Ehrlich, Miller and Chen2016; Miller et al., Reference Miller, Chen, Fok, Walker, Lim, Nicholls and Kobor2009). Once bound to its receptors, it must translocate to the nucleus of the cell, where it can then exert its effects. Measures of salivary cortisol, as used in the present study, do not offer any insight into these processes, and therefore the downstream effects of the smaller cortisol responses on inflammatory processes in adolescents with higher levels of stress and lower psychological resources cannot be determined in the present study.
That we observed no stress and IL-6 associations or moderation by psychological resources seems to diverge from prior studies showing significant interactions between socioeconomic status and psychological resources on inflammatory processes (Chen et al., Reference Chen, McLean and Miller2015; Elliot & Chapman, Reference Elliot and Chapman2016). However, these studies examined production of inflammatory cytokines in response to a bacterial stimulus in youth and circulating markers of systemic inflammation under tonic conditions in midlife adults. In the present study, we focused on circulating IL-6 in response to an acute psychosocial stressor in late adolescents. The lack of findings for IL-6 reactivity may have to do with the fact that systemic inflammation remains low in earlier life stages, whereas the HPA axis is known to be highly sensitive to the environment across the lifespan, even early in life when physical health is relatively intact (Gunnar & Quevedo, Reference Gunnar and Quevedo2007; Hostinar, Sullivan, & Gunnar, Reference Hostinar, Sullivan and Gunnar2014). As such, there may be delayed effects of stress on circulating markers of inflammation, and links may emerge in later in life. Consistent with this notion, associations between stress and circulating markers of inflammation are more consistently observed in adults compared with youth (Baumeister et al., Reference Baumeister, Akhtar, Ciufolini, Pariante and Mondelli2016; Coelho et al., Reference Coelho, Viola, Walss-Bass, Brietzke and Grassi-Oliveira2014; Slopen et al., Reference Slopen, Koenen and Kubzansky2012). That circulating inflammatory cytokines stem from both immune and nonimmune sources, such as adipocytes (Black, Reference Black2003), may also make it difficult to detect associations among stress, psychological resources, and inflammatory reactivity in young individuals. To the extent that stress biases immune cells specifically toward a pro-inflammatory phenotype (Miller et al., Reference Miller, Chen and Parker2011) and that psychological resources counteract the impact of stress (as described previously), effects in young persons may be better detected when stimulating immune cells, as in the study by Chen et al. (Reference Chen, McLean and Miller2015).
The current investigation was not without limitations. First, the observational nature of the study precludes any conclusion about causal relations and makes alternative explanations plausible. Although we adjusted for potential confounds and considered depressive symptoms as an alternative explanation, other unmeasured factors associated with stress and HPA axis functioning may have contributed to the observed findings. Second, given the one-time assessment of HPA axis reactivity to stress, we were unable to determine whether the effects of the various stressors on cortisol reactivity and the attenuation of these associations by psychological resources persist across time. As development continues, later experiences may alter the health trajectories that early experiences initially set in motion (Chiang, Chen, & Miller, Reference Chiang, Chen and Miller2018; Gouin, Caldwell, Woods, & Malarkey, Reference Gouin, Caldwell, Woods and Malarkey2017), making it important for future longitudinal studies to elucidate whether the current findings persist across time. Third, the clinical relevance of the study findings cannot be determined. We did not measure any physical health outcomes, and the clinical significance of the magnitude of cortisol decreases we observed remain unknown. Last, participants were European- and Latin-Americans from the Los Angeles area and of a narrow age range; thus, more work is needed to determine generalizability of our findings.
Despite these limitations, the current study contributes to previous literature. It builds on prior research on psychological resources by extending findings of the protective role of psychological resources to an adolescent sample. It extends work on adolescent stress biology by highlighting psychological resources as potential protective factors that may mitigate the association between stress and dampened cortisol reactivity. Results raise the possibility that intervention programs aimed at enhancing psychological resources among young individuals may ultimately help counteract the effects of certain stressors on HPA axis functioning, though the long-term health implications remain unclear.