Gyms have become increasingly attractive, offering different types of planned and structured exercises to regulars, which has motivated both men and women to seek exercises that change their body composition(Reference Ruano and Teixeira1). This behaviour makes Brazil the second country in the world in terms of number of gyms, totaling more than nine million users(2).
The increasing popularity of gyms has generated enormous market potential for the dietary supplement segment(Reference Druker and Gesser-Edelsburg3). Several types of food supplements with different purposes can be found in markets, and those aiming to increase muscle mass, reducing body fat, prolonging physical resistance and improving muscle recovery stand out(Reference Hutson4). Thus, the use of dietary supplements has increased dramatically among gym users(Reference Ruano and Teixeira1,Reference Attlee, Haider and Hassan5) , often easily acquired without a prescription and correct guidance on their consumption(Reference Goston and Correia6,Reference Menon and Santos7) .
Despite the effectiveness of supplements in increasing muscle strength and lean mass(Reference Morton, Murphy and McKellar8,Reference Kreider, Kalman and Antonio9) , and the performance of high-intensity exercises(Reference Van De Walle and Vukovich10), evidence suggests that the gyms promote the consumption of dietary supplements, which culminates in the unnecessary consumption of these substances(Reference Attlee, Haider and Hassan5,Reference El Khoury and Antoine-Jonville11) . The lack of guidance and monitoring for the dietary supplements use can result in an abusive consumption of these products(Reference Druker and Gesser-Edelsburg3), raising concerns about safety, especially regarding kidney and liver function(12,13) .
Although several studies have shown the safety of supplements regarding both general heath and kidney and liver function(Reference Kim, Kim and Carpentier14–Reference Souza E Silva, Pertille and Barbosa16), some show that the abuse of some supplements can overwhelm liver and kidney functions by different mechanisms(Reference Gabardi, Munz and Ulbricht17–Reference Vasconcelos, Bachur and Aragão20). Studies have reported that the consumption of supplements such as creatine, protein, vitamins and sport drinks may lead to an acute kidney injury(Reference Gawad and Kalawy21). The studies analysed associated creatine supplementation with renal function impairment(Reference Thorsteinsdottir, Grande and Garovic22,Reference Taner, Aysim and Abdulkadir23) ; however, the conclusions regarded only one patient, requiring, therefore, a cautious interpretation. Another study reported that liver injury cases (modest increases in alanine aminotransferase (ALT) and aspartate aminotransferase (AST) values) could be attributed to dietary and medical supplements including vitamins, minerals and botanical extracts(Reference Navarro, Barnhart and Bonkovsky24). Thus, the safety of supplementation among gym users must be further investigated. This study aimed to examine the association between dietary supplements intake with alterations in the liver and kidney function markers among gym users.
Materials and methods
Participants
The participants of this cross-sectional study were gym users of thirty-one gyms registered in Regional Council of Physical Education of the 2nd Region – CREF2/RS, from the municipality of Santa Cruz do Sul, state of Rio Grande do Sul, Brazil. The gym users were invited to answer an online questionnaire about dietary supplementation and to perform biochemical tests by peers and friends at gyms, via emails and social media (including Instagram™ and Facebook™). Gym users had to be 18 years of age or older to be included in the study. Exclusion criteria were individuals with incomplete data, pregnant women, individuals with pre-existing liver and kidney diseases, individuals who used drugs that could cause changes in biochemical tests of liver and kidney function or anyone who used anabolic steroids. Of the total number of gym practitioners (n 594, 266 male and 328 female), a subset (n 242, 114 male and 128 female) gave blood samples, as described in Fig. 1. Supplementary tables were created to characterising the subset sample (online Supplementary Table S1) and comparing the subjects who performed and those who did not perform biochemical analysis (online Supplementary Table S2). Consent was obtained from all participants. The Research Ethics Committee of the University of Santa Cruz do Sul, Brazil (number 20·20·170) approved the study.

Fig. 1. Flow chart of gym users, inclusion process for the biochemical exams of renal and liver function.
Online questionnaire
An online questionnaire, available on the Google forms platform, was answered, containing questions adapted from previous articles about supplements use(Reference Attlee, Haider and Hassan5,Reference El Khoury and Antoine-Jonville11,Reference Welter, Neves and Saavedra25) . The questionnaire contained closed and open questions divided into three sections: (1) socio-demographic characteristics: age (in years), sex (female or men) and educational level (middle school, high school or higher education); (2) training habits: time of exercise (< 1, 1–3 or ≥ 4 years), physical exercise intensity (moderate or intense), type of physical exercise (aerobic, anaerobic or combined exercises), extra activities performed (none, walking/running, team sports, others); and (3) supplement use: supplement usage (yes or no), time of supplement intake (< 1, 1–3 or ≥ 4 years), number of dietary supplements used (no use, 1–4 types or ≥ 5 types), who provided supplement prescription (nutritionist, physician, coaches, self-prescribed or other providers), type of dietary supplements used (open answer), time of supplement use, reasons attributed to the use of dietary supplements (muscle mass increase, muscle mass recovery, health and performance).
Blood collection and anthropometry
For the second stage of the study, of all subjects (n 594), a subset (n 242, 114 male and 128 female) volunteered to performed a blood analysis to assess the biochemical markers of liver and kidney function. The same subset (n 242) also performed anthropometry that was used to characterise the sample. Blood collection and anthropometry were performed in the Laboratory of Biochemical of Exercise located at University of Santa Cruz do Sul, Brazil. Blood samples were drawn by a specialised professional, in the morning and after a 10–12 h fasting period.
Anthropometric evaluation
BMI and fat percentage were also assessed using an Omron® bioimpedance scale (model HBF-514C), with the respondent wearing no shoes and light clothing. To assess body height, a compact tape-tape stadiometer (MD®HT-01) was used with the individual in an upright position. BMI and fat percentage were classified according to the reference values indicated on the Omron® bioimpedance scale model HBF-514C(Reference da Silva, Molz and da Silva Schlickmann26).
Biochemical analysis
During blood collection, samples of 10 ml were collected per individual and immediately the blood was centrifuged in lab to obtain 3 ml of serum and perform biochemical analysis. The hepatic markers analysed were the enzymes ALT, AST, alkaline phosphatase and γ-glutamyltransferase. The renal markers analysed were creatinine and urea. Serum samples were evaluated in the automated equipment Miura One (ISE), using DiaSys® commercial kits (DiaSys Diagnostic Systems). For internal quality control, normal (Topkon N, Kovalent) and altered (Topkon P Kovalent) control was used. The analytes were dosed twice, respecting the CV (%) for AST (2·4 %), ALT (2·4 %), AKT (0·7 %), γ-glutamyltransferase (1·2 %), creatinine (0·83 %) and urea (2·2 %). For data analysis, the reference normal values for hepatic and renal markers according to manufacturer’s instructions were < 30 U/l to ALT, < 37 U/l to AST, 35–104 U/l (women) and 40–129 U/l (men) to alkaline phosphatase, < 32 U/l (women) and <49 U/l to γ-glutamyltransferase , 0·5–0·9 mg/dl (women) and 0·7–1·2 mg/dl (men) to creatinine and 17–43 mg/dl to urea(Reference Thomas27,Reference Newman, Price, Brutis and Ashwood28) . No subject presented values below references values to alkaline phosphatase and creatinine. Values obtained were categorised as normal, slight altered (1–2 times the reference value), altered (3–5 times the reference value) and strongly altered (more than 6 times the reference value). No subject presented classification altered or strongly altered to hepatic and renal markers. Therefore, in the data analysis, only the classification normal and slight altered for hepatic and renal markers were included.
Statistical analysis
The data were analysed using the Statistical Package for the Social Sciences version 23.0 (IBM). Categorical variables were expressed in prevalence and compared using the χ 2 test. The dependent variables ‘ALT’, ‘AST’, ‘FA’, ‘GGT’, ‘creatinine’ and ‘urea’ were used as categorical variables, classified as normal and slight altered. The independent variable ‘supplement intake’ was also categorical, classified as no-supplement use and supplement use. Binary logistic regression was used to test the association of the dependent variables with the independent variable, and values were expressed in terms of OR and 95 % CI. Crude analysis is shown in model 1, model 2 was adjusted for sex and age and model 3 was adjusted for sex, age and education. Significant values were considered for P < 0·05.
Results
In total, 594 participants answered the online questionnaire and performed the anthropometry evaluation. Gym users in this study had an age average of 37 (sd 14) years and were mostly female (55·2 %).
Table 1 shows the dietary supplement intake, number of dietary supplements used, supplements prescription, type of dietary supplement intake and reasons for using dietary supplements among both women and men. Dietary supplements were consumed by 36·0 % of gym users. Men presented the highest dietary supplement usage (42·5 %; P = 0·02) and 8·3 % reported consuming more (up to five types) dietary supplements. The dietary supplement prescription and the reasons for using dietary supplements were found to be associated with the sex of participants (P < 0·01).
Table 1. Dietary supplement intake, number of dietary supplements used, supplement prescription, type of dietary supplement intake and reasons to the use of dietary supplements among women and man (n 594) (Numbers and percentages; mean values and standard deviations)
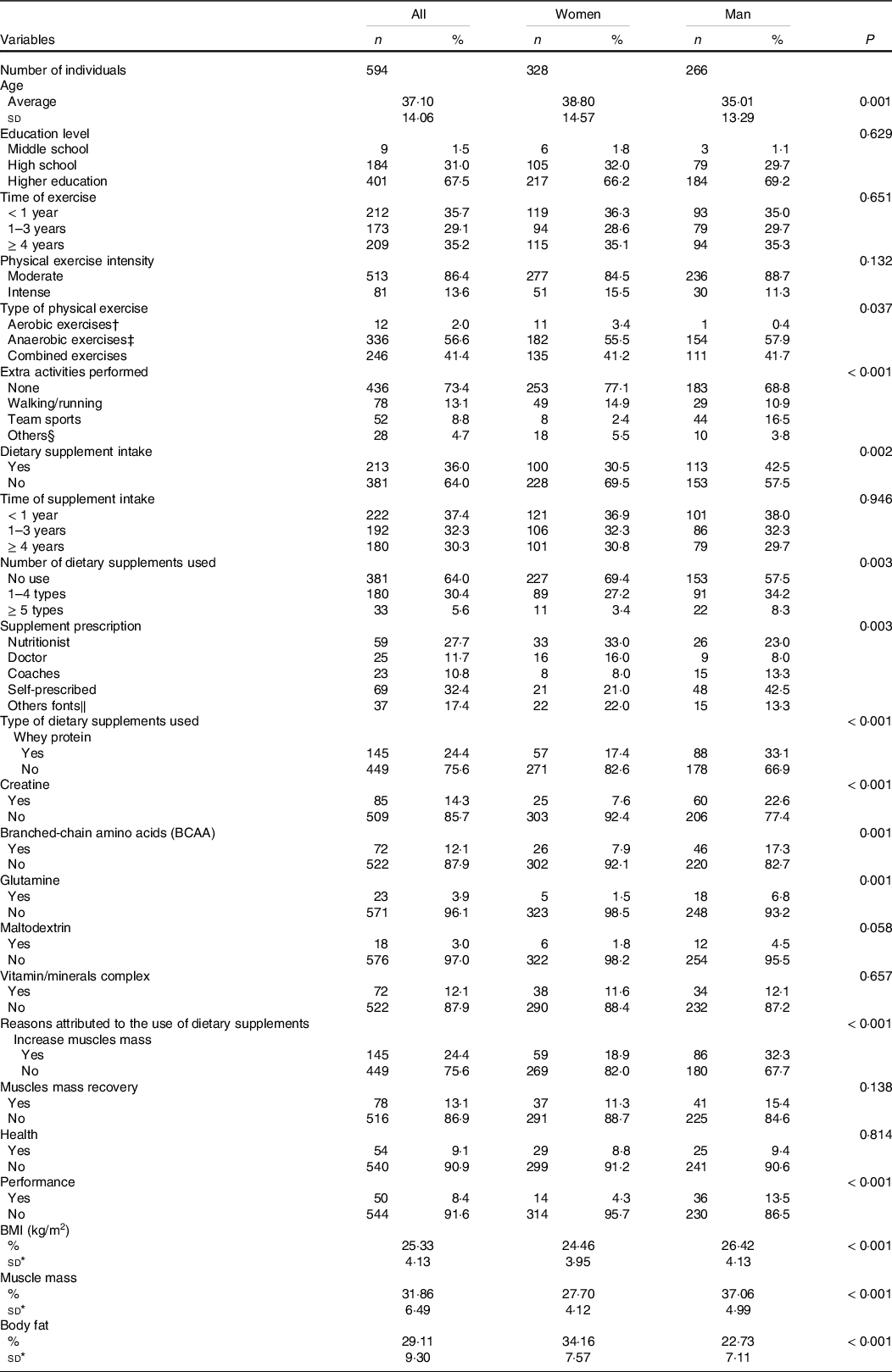
* Subset sample of 242 subject.
† Aerobics: treadmill; functional training; bicycle, dance; jump; step and localised gymnastics.
‡ Anaerobic: strength training; pilates; swimming; fight and yoga.
§ Others fonts: bicycle, dance, fight, yoga and skating.
‖ Others fonts: Internet, friends, pharmacist, or salesman of supplement store.
Significant at P < 0·05, according χ 2 test or Student’s t-test.
Table 2 shows the prevalence of biochemical markers of renal and liver function between supplement use and no-supplement use. The data show a higher prevalence of slight altered AST levels in the supplement use group (P = 0·012), whereas the other variables showed no difference between groups. We included the analysis of prevalence of renal and liver function markers between no-supplement use and supplement use among women and men as a complementary material. Online Supplementary Table S3 showed a higher prevalence of slight altered AST and ALT levels for both men and women, and urea in the supplement use group only among men.
Table 2. Prevalence of renal and liver function markers between no-supplement use and supplement use (Numbers and percentages, n 242)
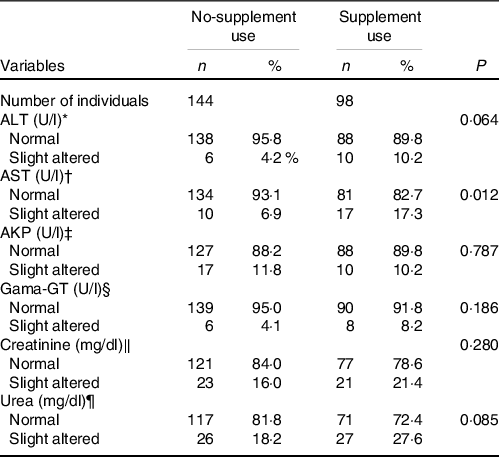
* Cut-off value to slight altered ALT > 30 U/l.
† Cut-off value to slight altered AST > 37 U/l.
‡ Cut-off value to slight altered AKP > 104 U/l (women) and >129 U/l (men).
§ Cut-off value to slight altered Gama-GT > 32 U/l (women) and > 49 U/l.
‖ Cut-off value to slight altered creatinine > 0 9 mg/dl (women) and > 1 2 mg/dl (men).
¶ Cut-off value to slight altered urea > 43 mg/dl.
Table 3 shows the associations between liver and renal function markers and dietary supplement intake. Individuals who intake dietary supplements show a higher prevalence of slight alterations in the AST enzyme after adjustments for sex and age (OR = 2·57; 95 % CI 1·09, 6·07). Similarly, gym users who consumed dietary supplements showed a higher chance for slight alterations in the urea in all models. Other hepatic function markers and the creatinine marker were not associated with dietary supplement intake.
Table 3. Association between dietary supplement intake, biochemical marker of liver and renal function (Odds ratios and 95 % confidence intervals, n 242)
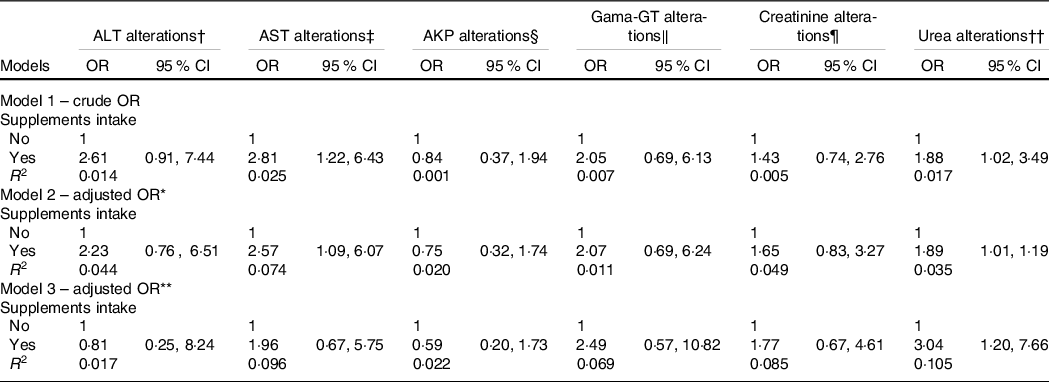
ALT, alanine aminotransferase; AST, aspartate aminotransferase; AKP, alkaline phosphatase; Gama-GT, γ-glutamyltransferase.
* Adjusted for sex and age.
† Cut-off value to slight altered ALT > 30 U/l.
‡ Cut-off value to slight altered AST > 37 U/l.
§ Cut-off value to slight altered AKP > 104 U/l (women) and > 129 U/l (men).
‖ Cut-off value to slight altered Gama-GT > 32 U/l (women) and > 49 U/l.
¶ Cut-off value to slight altered creatinine > 0 9 mg/dl (women) and > 1 2 mg/dl (men).
** Adjusted for sex, age and education.
†† Cut-off value to slight altered urea > 43 mg/dl.
Binary logistic regression analysis.
Discussion
The main findings of this study indicate that gym users who intake dietary supplements had a higher chance to present slight alterations in both liver (AST enzyme) and kidney (urea) function. However, these results should be interpreted with caution, since we cannot affirm that supplementation was associated with renal function impairment. Besides, these findings may only be interpreted as physiological adaptations, without any clinical relevance. The lack of knowledge about the safety of consumption of health supplements, combined with the lack of regulation on these products, must also be considered, which reflects the need for research in this area.
The prevalence of dietary supplement intake, in this study, was 36·0 % among gym users. Some studies conducted in Brazil and other countries have shown similar rates(Reference Goston and Correia6,Reference Welter, Neves and Saavedra25,Reference Abo Ali and Elgamal29,Reference Jawadi, Addar and Alazzam30) , whereas others indicate a higher rate of dietary supplement among gym users from the municipality of São Luís, state of Maranhão, Brazil (64·7 %(Reference Lacerda, Carvalho and Hortegal31)), and countries such as Portugal (43·8 %(Reference Ruano and Teixeira1)) and the USA (45·2 %(Reference Sassone, Muster and Barrack32)). According to Attlee et al. (Reference Attlee, Haider and Hassan5), these discrepant results could be explained by varied characteristics of participants that many times are influenced by physical appearance, combination of social and psychological factors, knowledge and financial conditions, and media influence(Reference Druker and Gesser-Edelsburg3,Reference Attlee, Haider and Hassan5) .
The analysis of liver and kidney markers of healthy gym users showed that the individuals who consumed dietary supplements presented slight alterations in the AST enzyme. Galati et al. (Reference Galati, Carreira and Galvão33) also evaluated the markers of liver function of gym users who used dietary supplements, regardless of sexes, and found that 18·5 and 26 % of individuals presented ALT and AST values, respectively, below of reference values according to the kit used. The authors also related that all gym users with ALT and AST values above of reference values presented excessive protein intake according to Dietary Reference Intakes. Navarro et al. (Reference Navarro, Barnhart and Bonkovsky24) showed that bodybuilding products are the most common cause for liver injury among those using dietary and herbal supplements including vitamins, minerals and botanical extracts, and observed a modest increase in the values of ALT and AST, showing changes especially among young men.
We have also observed a slight alteration in the renal function marker, urea, which is essential for the removal of residual nitrogen, caused by protein and amino acid metabolism(Reference Häberle, Boddaert and Burlina34). Abnormal urea levels have also been an indicative of high protein diets(Reference Liu, Mo and Wei35). At the same time, changes in renal markers have also been observed in individuals with high protein intake and may lead to adverse effects on long-term renal function(Reference Kirsztajn, Salgado Filho and Draibe36). Moreover, a review study found seventeen types of dietary supplements associated with kidney dysfunction and injury(Reference Gabardi, Munz and Ulbricht17). In this study, 84·5 % of gym users reported the usage of one to four supplements, mostly protein supplements. However, we could not find an association between the number of supplement use and the liver and renal function marker (data not shown). A study evaluating the high protein diet obtained from whole food or protein powder, combined with a heavy resistance training for the 8-week treatment period, has also not observed changes in any renal marker evaluated (including urea and creatinine)(Reference Antonio, Ellerbroek and Silver15). Following the same line, a review study concluded that creatine supplementation (an ergogenic supplement) does not induce renal damage in the studied amounts and durations from 1997 to 2013(Reference Souza E Silva, Pertille and Barbosa16).
This study has some limitations that must be considered. Firstly, we did not evaluate diet intake, specific supplements, dose supplemented and the use of other anabolic-androgenic steroids by biochemical exams. Besides, although the slight alteration in renal and hepatic function seems to be related to supplement intakes, the data reported in this study are observational and we cannot exclude the possibility that other factors may have contributed to this. Secondly, the sample size may also be a limitation to this study, once there were sample losses due to the low adherence of participants performing biochemical tests (loss of 40 %). In addition, the occurrence of changes in liver and kidney markers is low in individuals who practice physical exercise and uses supplements(Reference Antonio, Ellerbroek and Silver15, Reference Souza E Silva, Pertille and Barbosa16, Reference Navarro, Khan and Björnsson19, Reference Brown37), requiring a larger sample size to significantly detect changes in these markers. In this sense, due to the limited sample size of the present study, wide CI was found, demonstrating a great variability of the sample. Indeed, data must be interpreted with caution, and future studies should consider larger sample size. Thirdly, this study is composed of individuals who were undergoing moderate and intense resistance training. Therefore, these findings cannot be generalised to people with renal or hepatic disease, non-exercising/sedentary subjects, individuals in aerobic exercise training, older adults and other populations not examined in this study. Finally, this study did not aim to explore mechanisms. Therefore, studies should further explain the mechanisms related among dietary supplement intake and renal/hepatic alteration function. However, we emphasise strong points of this study, such as the different type of supplement assessment, since there are several types of supplements to bodybuilders and many gym users ingest more than one supplements at once.
In conclusion, our results showed that gym users who intake dietary supplements showed a higher chance to present slight alterations in the AST enzyme, and in urea. These data may contribute to the analysis of future studies, and be an alert for health professionals to outline goals and strategies that will contribute to the disease prevention and health promotion in this population. Although our findings have shown slight alterations in renal and hepatic markers, these results are not indicative of renal and hepatic damage; however, they indicate the need for biochemical monitoring of these markers to avoid possible alterations that could compromise the health of these individuals.
Acknowledgements
The authors gratefully acknowledge all the gym’s partners who participated in this study and all the team of the Laboratory of Experimental Nutrition.
This study was supported by grants from the Coordination for the Improvement of Higher Education Personnel Brazil (CAPES), National Council for Scientific and Technological Development (CNPq), Research Support Foundation of the State of Rio Grande do Sul (FAPERGS) and University of Santa Cruz do Sul (UNISC).
D. S. S. contributed to conception and design, data acquisition, analysis and interpretation, and drafting the article. P. M. contributed to conception and design, interpretation and drafting the article. C. B. contributed to analysis and interpretation and drafting the article. C. S. contributed to data acquisition and critical review of the article. T. G. S. contributed to data acquisition and critical review of the article. A. R. contributed to analysis and interpretation and critical review of the article. P. J. B. contributed to interpretation and critical review of the article. C. P. R. contributed to analysis and interpretation and critical review of the article. J. D. P. R. contributed to data acquisition, interpretation and critical review of the article. S. I. R. F. contributed to conception and design, interpretation and critical review of the article.
None of the authors has any potential conflict of interest.
Supplementary material
For supplementary materials referred to in this article, please visit https://doi.org/10.1017/S0007114521003652









