Microvascular decompression surgery is a commonly performed operation for the treatment of trigeminal neuralgia in which the culprit vessel is mobilized away from the trigeminal nerve root and held in place by a Teflon implant. Although generally considered to be an inert substance, the Teflon implant used in microvascular decompression surgery has been implicated in rare instances of granuloma formation following implantation.Reference Chen, Lee, Lui, Yeh, Chen and Tzaan 1 - Reference Deep, Graffeo and Copeland 7
We report a case of a 77-year-old female with remote history of breast cancer and mastectomy who was referred to our center for evaluation and treatment of an unusual mass lesion located in the posterior fossa (Figure 1). She had an onset of trigeminal neuralgia beginning in 2000 for which she underwent a microvascular decompression in 2006; a shredded Teflon felt implant was placed between the culprit superior cerebellar artery and the underlying trigeminal nerve root. Of note, we occasionally use fibrin glue in conjunction with the shredded Teflon implant; however, this was not used in this case and not used routinely. She received limited pain relief and in 2007 underwent a Gamma Knife procedure in which an 80-Gy dose was prescribed to the proximal trigeminal nerve root. Neither computed tomography nor magnetic resonance imaging demonstrated any abnormality aside from the Teflon implant. She experienced minimal, delayed pain relief, and continued with medical management until undergoing radiofrequency rhizotomy in 2009, which provided minimal pain relief. This also resulted in corneal anesthesias and 80% preservation of sensation in V1 and V2 distributions and full preservation of V3. Over the ensuing years, she developed a progressive full sensory loss of all three trigeminal nerve distributions, temporalis and masseter muscle atrophy, accompanied by alleviation of her neuralgia. In 2015, she developed a progressive gait ataxia with magnetic resonance imaging revealing a lobulated, enhancing 2.2×1.8×1.6 cm mass in the region of the previously inserted Teflon implant along with significant edema involving adjacent brain.
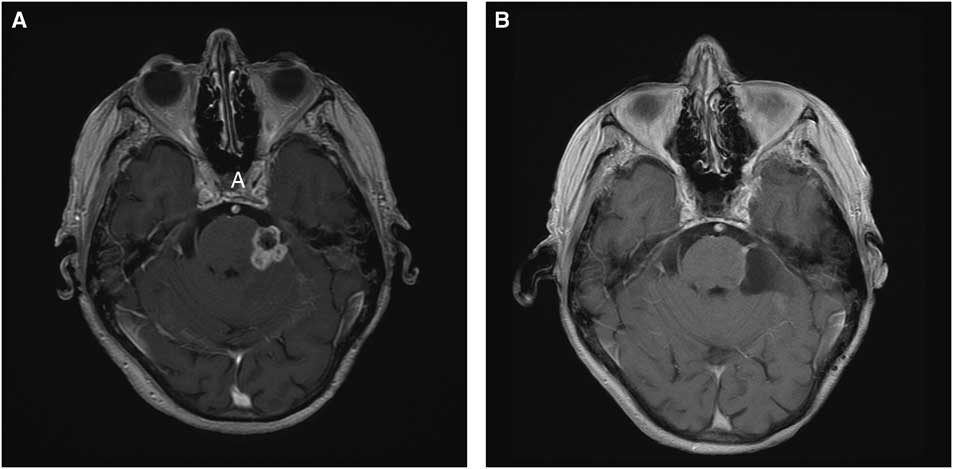
Figure 1 Panel A: Pre-operative Axial MRI with Gadolinium shows a 2.2×1.8×1.6 cm enhancing legion at the site of the trigeminal root entry zone. Panel B: Post-operative Gadolinium enhanced axial sequence showing minimal residual enhancing lesion.
Based on the clinical presentation and imaging findings, the differential diagnosis included metastasis, meningioma, chronic infectious process involving Teflon implant, inflammatory or granulomatous lesion, and radiation-induced pathology. Given her progressive gait ataxia along with unknown diagnosis, we elected to proceed with posterior fossa exploration through a retrosigmoid craniectomy with the goal of alleviation of mass effect and tissue sampling for diagnosis. The lesion was noted to have a firm capsule surrounding the inner contents of the tumor, consisting of soft white material intermixed with the Teflon implants. The lesion was adherent to the cerebellum, pons, trigeminal root entry zone, and superior cerebellar artery. After internal debulking and meticulous dissection of the tumor from the neurovascular structures, a thin rim of residual tumor was left adherent to the nerve and superior cerebellar artery.
The patient tolerated the procedure well and left hospital after several days with no new deficits. At 6-month follow-up, she reported a mild improvement of her gait instability however still required the use of a four-wheel walker for mobility.
Neuropathology investigation revealed mixed inflammatory infiltrates in a dense collagenous tissue (Figure 2). The infiltrate was composed of lymphocytes, histiocytes, neutrophils, and plasma cells. Multiple necrotic foci were noted, which were likely associated with previous Gamma Knife treatment. No microorganisms were demonstrated on both hematoxylin and eosin stain, as well as special stains for bacterial (Gram stain), fungal (Grocott’s methenamine silver stain), or mycobacterial (Ziehl-Neelsen stain). Based on the absence of microorganisms, it is reasonable to conclude that granulomatous inflammation was associated with the Teflon implant.
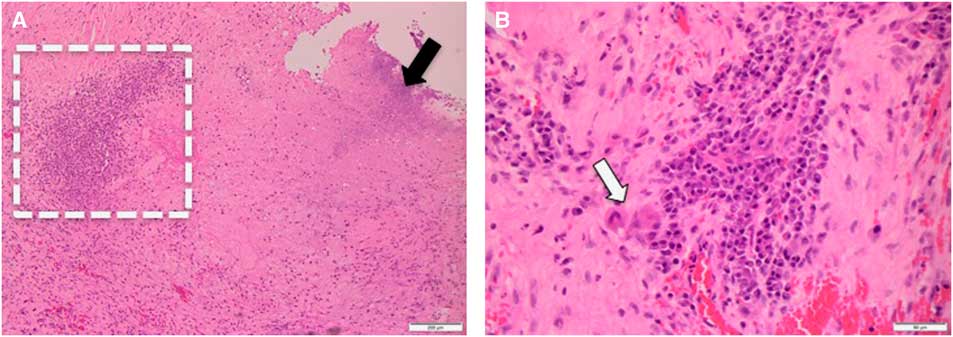
Figure 2 Panel A: Medium power view (at 100×) shows necrosis (black arrow) and inflammatory infiltrate (dashed rectangle). Panel B: High power view (at 400×) of the inflammatory infiltrate. Note presence of multinucleated giant cells (white arrow).
This case represents an interesting presentation, management, and histopathology of a rarely reported lesion associated with a Teflon implant several years after microvascular decompression and Gamma Knife radiosurgery for the treatment of trigeminal neuralgia. After more than 1000 microvascular decompression cases by the senior author, this is the first case we have observed of granuloma despite use of Teflon in all of our cases. We theorize that radiosurgery, in conjunction with presence of a foreign body, may have contributed to a heightened inflammatory response and resultant granuloma formation in this patient. We have, however, treated patients who had previously received Gamma Knife to the trigeminal nerve, with microvascular decompression without resultant granuloma formation. Furthermore, cases of Teflon-associated granuloma have occurred in the absence of radiosurgery treatment; we can therefore only speculate and not make any concrete conclusions with regard to radiosurgery as a contributing factor. Other rare cases of Teflon-associated granuloma have been reported in the literature, the majority of which are in reference to small granulomatous lesions discovered during posterior fossa exploration following recurrence of trigeminal neuralgia symptoms, as opposed to the larger granulomatous lesion seen on imaging in our case.Reference Chen, Lee, Lui, Yeh, Chen and Tzaan 1 - Reference Smucker, Bonnin and Pritz 5 , Reference Capelle, Brandis, Tschan and Krauss 8
We report a case of a large solid lesion encapsulating the Teflon implant and trigeminal nerve, resulting in mass effect on the brainstem and cerebellar peduncle with associated progressive ataxia and complete sensory loss in the trigeminal nerve distribution. We identified only one such similar case in our review of the literature presenting with progressive gait unsteadiness secondary to Teflon-associated granuloma.Reference Deep, Graffeo and Copeland 7 , Reference Capelle, Brandis, Tschan and Krauss 8 Furthermore, to our knowledge, this is the first case of a patient presenting with Teflon-associated granuloma in the context of previous Gamma Knife radiosurgery.
Although rarely reported, Teflon granuloma is a potential complication of microvascular decompression surgery. It should be suspected in the differential diagnosis of a patient presenting with recurrent trigeminal neuralgia or progressive neurological deterioration in the context of a history of previous microvascular decompression. Teflon granuloma can be amenable to safe surgical resection with associated clinical improvement.
Disclosures
The authors do not have anything to disclose.






