Studies have indicated that humans evolved on a diet of PUFA with an n-6:n-3 ratio of approximately 1:1, while current estimates of the typical North American diet are 15:1 (reviewed in Simopoulos(Reference Simopoulos1)). The change in ratios is considered to be attributed to an increased intake of foods containing n-6 fatty acids such as vegetable oils, namely maize and soyabean, with a reduced intake of long-chain n-3 PUFA from foods such as fresh oily fish, namely salmon and mackerel (reviewed in Black & Sharpe(Reference Black and Sharpe2)). This high n-6:n-3 ratio in the diet has been suggested to play a role in the pathogenesis of a number of chronic inflammatory diseases including CVD, diabetes, Crohn's disease as well as allergy(Reference Kompauer, Demmelmair and Koletzko3–Reference Zamaria10). Indeed, an association has been observed between the level of n-6 PUFA in plasma phospholipids and hay fever and IgE levels(Reference Kompauer, Demmelmair and Koletzko3, Reference Yu and Bjorksten8). Furthermore, a high ratio of n-6:n-3 PUFA intake has been reported to be associated with significantly increased risk estimates for current asthma(Reference Oddy, de Klerk and Kendall6). Also, one study has shown an association between higher n-6 fatty acids in maternal serum and the appearance of allergic disease in their children during the first 6 years of life(Reference Yu and Bjorksten9).
These associations may be due, at least partially, to the fact that n-6 fatty acids give rise to PGD2, a major mediator of allergic inflammation produced by mast cells following activation(Reference Moon, Murakami and Ashraf11, Reference Tanaka, Hirai and Takano12). Both PGD2 receptors, D prostanoid receptor 1 (DP1) and DP2, influence inflammatory cell chemotaxis (T helper type 2 (Th2) cells, eosinophils and basophils), with DP1 inhibiting and DP2 mediating this effect(Reference Pettipher13–Reference Monneret, Gravel and Diamond16). DP2 is also called CRTh2 (chemoattractant receptor-homologous molecule expressed on Th2 cells) and is considered a marker of Th2 cells in humans(Reference Cosmi, Annunziato and Galli17). PGD2–CRTh2 signalling mediates the expression of the Th2 cytokines IL-4, IL-5 and IL-13(Reference Tanaka, Hirai and Takano12, Reference Xue, Gyles and Wettey14), and therefore this pathway may influence allergic sensitisation, since both IL-4 and IL-13 instruct B cells to isotype switch to IgE(Reference Del Prete, Maggi and Parronchi18, Reference Punnonen, Aversa and Cocks19).
There are a number of studies investigating whether re-balancing the n-6:n-3 ratio would prove beneficial and/or protective to the development of allergy. In mouse models of dietary supplementation with α-linoleic acid, significantly lower IgE antibody response against ovalbumin (ova), mortality from anaphylactic shock(Reference Watanabe, Sakai and Yasui20), reduced proliferation of CD4+ splenic T cells and increased expression of the inhibitor molecule cytotoxic T-lymphocyte antigen 4 (CTLA-4)(Reference Ly, Smith and Switzer21) have been observed. In one study, it has been shown that administration of aerosolised DHA reduced airway hyper-responsiveness in a mouse model of asthma(Reference Yokoyama, Hamazaki and Ohshita22).
Evidence for the potential efficacy of n-3 fatty acid administration in human subjects also exists. A case–control study of Japanese children showed that 10 months of EPA/DHA supplementation significantly improved asthma symptoms(Reference Nagakura, Matsuda and Shichijyo23), while a large cross-sectional study found an inverse association between fish intake and the prevalence of asthma and allergic rhinitis(Reference Miyake, Sasaki and Tanaka24, Reference Miyamoto, Miyake and Sasaki25). Recently, n-3 administration has been shown to reduce inflammation in response to low-dose allergen challenge in human subjects(Reference Schubert, Kitz and Beermann26). There are a number of studies investigating the effects of in utero exposure to maternal fish oil supplementation during pregnancy. This approach has been shown to result in inverse correlations between the level of DHA and both IL-4 and IL-13(Reference Dunstan, Mori and Barden27, Reference Krauss-Etschmann, Hartl and Rzehak28) as well as fewer children who developed allergic sensitisation(Reference Schnappinger, Sausenthaler and Linseisen29, Reference Furuhjelm, Warstedt and Larsson30). However, the mechanism(s) for these effects have not yet been described.
Here we report that isolated splenocytes from ova-sensitised mice receiving an n-3 fatty acid-enriched diet during early life exhibit lower IL-4 and IL-13 expression. DHA also attenuated IL-13 expression from human Th2 cells, through the inhibition of promoter activation and transcription factor binding. These data indicate the potential of n-3 fatty acids to attenuate IL-13 expression, which suggests that they may subsequently reduce allergic sensitisation and the development of allergic disease.
Materials and methods
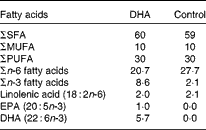
Dietary exposure of mice to a DHA-enriched diet
Experiments were performed according to the protocols approved by the Animal Ethics Board of the University of Alberta. Pregnant BALB/c mice timed to give birth on the same day were obtained from the Health Sciences Laboratory Animal Services breeding facility at the University of Alberta on day 14 of gestation. They were fed nutritionally adequate semi-purified high-fat (15 %, w/w of diet) diets being a DHA-enriched (1 %, w/w of diet, using Marinol®; Lipid Nutrition) or control diet (0 % DHA) starting at the time of delivery (Table 1). The macronutrient (including fat content) and micronutrient content of the diet is as reported previously(Reference Robinson, Clandinin and Field31), except for the fat composition provided in Table 1. Both diets provided similar polyunsaturated:saturated fat ratios (0·5) but differed in the ratio of n-6:n-3, due to the replacement of the n-6 fatty acid linoleic acid by DHA. The control diet had an n-6:n-3 fatty acid ratio of 13·2, while the n-6:n-3 fatty acid ratio for the DHA-enriched diet was 2·4. The complete fatty acid composition of the two diets, analysed by GLC, is provided in Table 1. Pups were then weaned to the maternal diet at 3 weeks, and this was continued until the end of the experiment. At 8 and 9 weeks of age, mice were sensitised with ova by an intraperitoneal injection of 0·9 % sterile saline (0·5 ml) containing 10 μg ova (Sigma) and 2 mg Al(OH)3 (MP Biomedicals), as we recently demonstrated that it is sufficient for allergic sensitisation(Reference Odemuyiwa, Ebeling and Duta32). After 14 d (week 10), single cell suspensions (1·25 × 106/ml) were prepared from the spleen as described previously(Reference Ruth, Taylor and Zahradka33). Cells were stimulated (48 h) with ova (100 μg/ml) or concavalin A (Con A, 2·5 μg/ml; MP Biomedicals). This concentration was based on pilot studies from our laboratory with rodent cells (from animals fed the high-fat control diet) stimulated for 48 h with a low (2·5 μg/ml, n 6) or high (5 μg/ml, n 8) concentration of Con A. We calculated a stimulation index (rate of [3H]thymidine uptake by stimulated cells/[3H]thymidine uptake by unstimulated cells) of 6·2 (se 2·0) v. 10·7 (se 4·0) and the amount of IL-2 in the media to be 900 (se 200) pg/ml v. 5000 (se 450) pg/ml for the low v. high concentration of Con A. The experiments were performed with 2·5 μg/ml, a concentration considered to represent an early response to stimulation. Levels of IL-13, IL-4, IL-10 and interferon-γ (IFN-γ) were detected by ELISA according to the manufacturer's instructions (eBioscience). The detectable range was 4–500 pg/ml and averages of duplicates were used for statistical analysis.
Table 1 Diet fatty acid composition (% total fatty acids)
Characterisation of mouse splenocytes
Flow cytometry was used to determine cell phenotypes in the isolated spleen cells as described previously(Reference Field, Thomson and Van Aerde34). All supplies were purchased from ThermoFisher Scientific with the exception of the antibodies. Monoclonal fluorescent antibodies against mouse CD3-PE, CD4-PE, CD8-PE.cy5, CD25-APC, CD19-FITC, MHCII-PE, CD86-PE.cy.5, CD11c-APC, CD11b-FITC and the corresponding isotype controls were purchased from eBioscience. Briefly, cells were washed twice with 4 % fetal calf serum (FCS) in PBS (4 g/l) and incubated (30 min) with the antibodies in combination. The cells were then washed again two times with buffer and fixed with a 1 % (w/v) paraformaldehyde PBS solution. The proportion of positive cells for each marker was determined by flow cytometry (FACSCalibur) and analysed using CellQuest software (Becton Dickinson). Unstained cells and isotype controls were used to adjust the gate on the splenocyte population and to determine background and colour compensation.
Primary human T helper type 2 cells
The present study was conducted according to the guidelines laid down in the Declaration of Helsinki and all procedures involving human subjects/patients were approved by the University of Alberta Human Ethics Board. Written informed consent was obtained from all subjects. Th2 cells were generated by isolating CD4+ T cells from peripheral blood mononuclear cells by negative selection using the CD4+ Isolation Kit II (Miltenyi Biotech). Purity was greater than 96 % as assessed by flow cytometry. Cytokines and antibodies were all from R&D Biosystems, Inc., except that anti-IL-12 was from eBiosciences, Inc. CD4+ T cells (2 × 106 cells/ml) were cultured in X-VIVO-15 medium (Lonza) supplemented with 10 % fetal bovine serum (Hyclone; Fisher Scientific) and 1 % penicillin, streptomycin and glutamine (Gibco; Life Technologies). Cells were primed for 3 d (2 × 106/ml) with antibodies against CD3 and CD28 (plate bound, 1 μg/ml) under Th2-polarising conditions (recombinant human (rh) IL-2, 5 ng/ml; rhIL-4, 10 ng/ml; neutralising antibodies against IFN-γ, 1 μg/ml; IL-12, 1 μg/ml; eBiosciences). Cells were rested for 4 d in rhIL-2 (5 ng/ml), IL-4 (10 ng/ml), anti-IFN-γ and anti-IL-12 (1 μg/ml). On day 7, CRTh2+ were isolated using the CRTh2 isolation kit and MACS columns (Miltenyi Biotech). CRTh2+ cells (2 × 106/ml) were similarly maintained on weekly cycles of 3 d priming (anti-CD3, anti-CD28 and rhIL-2) followed by a 4 d rest (rhIL-2, 5 ng/ml). The experiments were performed with five independently differentiated lines ranging from day 10 to day 66 of culture with Hyclone fetal bovine serum (10 %), which contained DHA (0·79 μg/ml), EPA (0·27 μg/ml) and arachidonic acid (AA, 1·62 μg/ml) as determined by GLC.
Chemoattractant receptor-homologous molecule expressed on T helper type 2 cells and T helper type 2 cytokine staining
Surface staining of CRTh2 was performed at 4 °C by first blocking (30 min) with normal rat IgG (Invitrogen; Life Technologies), then addition (30 min) of anti-CRTh2 primary antibody (CRTh2-biotin; Miltenyi Biotech) or isotype (rat IgG2a; ABD Serotech) followed by addition (30 min) of Streptavidin-APC (eBiosciences). To assess the influence of DHA on the Th2 cell phenotype, DHA (10 μm; Cayman Chemical) was added to the CRTh2+ cells during the resting phase, the time point of optimal CRTh2 expression (L Cameron, unpublished results).
For intracellular cytokine staining, Th2 cells were stimulated (1·3 × 106 cells/ml) for 4 h with phorbol myristate acetate (PMA) (20 ng/ml), ionomycin (1 μm) and brefeldin A (10 μg/ml) (Sigma Aldrich) in the presence or absence of DHA, AA or oleic acid (OA; 10 μm). Cells were fixed with paraformaldehyde (4 %; Sigma Aldrich) and permeabilised with saponin (0·4 %; Sigma Aldrich). IL-13-PE and IL-4-Alexa 488 antibodies (JES10-5A2 and 8D4-8, respectively; BD Pharmingen) and isotypes (rat IgG1k-PE and mouse IgG1k-Alexa 488) were incubated on ice (30 min), washed and fluorescence read immediately by flow cytometry (FACSCalibur; Becton Dickson).
Transient transfections
Jurkat T cells (clone E6-1; American Type Culture Collection) were transiently transfected (5 × 106) with a firefly luciferase reporter construct containing 369 bp of the IL-13 promoter located immediately upstream of exon 1 (IL-13-369pro/Luc; 10 μg) and a constitutive reporter containing Renilla luciferase (pRL-Tk; 10 ng; Promega) to control for transfection efficiency. Cells were electroporated with a square wave using one pulse of 50 ms (240 V, BTX, ECM 830; Harvard Apparatus, Inc.). These conditions resulted in transfection efficiency >30 %. In all experiments, cells were stimulated with PMA (20 ng/ml) and ionomycin (1 μm) with or without DHA, AA or OA (10 μm) immediately following transfection. Luciferase activity was assessed after 16–18 h incubation with the Dual Luciferase Assay (Promega) according to the manufacturer's instructions. Protein concentration was determined using the bicinchoninic acid protein acid kit (Pierce; Fisher Scientific). Firefly luciferase results were normalised for transfection efficiency (as determined by Renilla luciferase activity) and protein concentrations, and expressed as relative luciferase activity (luciferase counts/μg protein). All data are represented as fold induction by PMA/ionomycin (stimulated/unstimulated). For each experiment, the relative luciferase activity of a control sample without any fatty acid addition was set at 1 and the DHA, AA and OA samples were compared with this reference sample.
Nuclear extracts
Jurkat and Th2 cells (12 × 106) were stimulated with PMA (20 ng/ml) and ionomycin (1 μm) in the presence or absence of DHA (10 μm, 3 h). Cells were harvested, pelleted, washed in cold 1 × PBS and resuspended in ice-cold buffer A (10 mm-HEPES, 3·0 mm-MgCl2, 40 mm-KCl, 1·0 mm-dithiothreitol (DTT), 5 % glycerol, 0·20 % NP-40 (nonyl phenoxypolyethoxylethanol), 1 mm-phenylmethylsulfonyl fluoride (PMSF), 5 mm-β-glycerophosphate, 1 mm-benzamidine, 1 mm-orthosodium vanadate, 1 mm-sodium fluoride, 10 μg antipain/ml, 10 μg aprotinin/ml, 10 μg leupeptin/ml, 10 μg pepstatin/ml: 120 μl; Sigma Aldrich). PMSF and DTT were added to buffer A immediately before use. Cell lysis was monitored by trypan blue, and once swollen, cells were centrifuged at 13 200 rpm for 2 min at 4°C. Nuclear pellets were resuspended in an appropriate volume of ice-cold buffer C (420 mm-NaCl, 10 mm-HEPES, 25 % glycerol, 1·5 mm-MgCl2, 0·2 mm-EDTA, 1·0 mm-DTT, 1 mm-PMSF, 5 mm-β-glycerophosphate, 1 mm-benzamidine, 1 mm-orthosodium vanadate, 1 mm-sodium fluoride, 10 μg antipain/ml, 10 μg aprotinin/ml, 10 μg leupeptin/ml, 10 μg pepstatin/ml), incubated on ice (20 min) and centrifuged at 13 200 rpm for 20 min at 4 °C. PMSF and DTT were added to buffer C immediately before use. Supernatants containing nuclear proteins were recovered and aliquots frozen immediately in liquid N2 and stored at − 80°C.
Electromobility shift assay
Gel shift assays were performed using an oligonucleotide probe (PR) spanning -112/-87 (5′cggcattgatggaaattgatgatatttgaa) of the IL-13 5′-flanking region. Double-stranded oligonucleotides were 5′ end-labelled with [γ-32P]ATP. The binding reaction included nuclear extract (5 μg), NaCl (100 mm), glycerol (10 %), poly dIdC–dIdC (1 μg; Sigma Aldrich), bovine serum albumin (0·2 μg/μl; New England BioLabs), 1 × binding buffer (100 mm-HEPES, 0·5 mm-MgCl2, 1 mm-EDTA and 1 mm-DTT) and 1 μl of the probe (approximately 1·4 ng). This reaction was incubated with the probe (30 min, room temperature). Competitions and supershifts were performed by pre-incubating nuclear proteins with unlabelled oligonucleotides (100-fold molar excess) or transcription factor-specific antibodies (4 μg, 30 min on ice) before addition of the probe. Reactions were loaded onto 5 % non-denaturing polyacrylamide gels and run for 5–6 h at 18–19 mA with 0·5 × Tris borate EDTA (TBE) at 4°C. Antibodies for cyclic AMP response element binding (CREB, C-21), activating transcription factor 2 (ATF2, F2BR-1) and control antibody (IgG) were purchased from Santa Cruz.
Statistical analysis
Data are represented as means with their standard errors of the mean. Statistical analysis was performed using SigmaPlot software (version 11; Systat Software Inc.). Statistical differences in IL-13 and IL-4 expression from unstimulated compared with ova-stimulated splenocytes from animals fed the two diets were determined using the two-sample Student's t test; IL-10 and IFN-γ using the Mann–Whitney U test. Con A stimulations were examined for significant increases using the Mann–Whitney U test. Differences in the percentages of IL-13- and IL-4-expressing human Th2 cells and IL-13 promoter activity with or without DHA were determined using the paired Student's t test; experiments including lipid controls were determined with one-way ANOVA (repeated measures). P< 0·05 was considered as significant.
Results
Influence of a DHA-enriched diet on the relative abundance of spleen cell types
To characterise the effect of a DHA-enriched diet on the different cell populations within the spleen, flow cytometry was performed with antibodies for phenotypic markers. This analysis revealed no significant differences in the proportion of T cells, B cells, dendritic cells or macrophages, although there were trends for a reduction in the percentage of activated T cells and macrophages (Table 2).
Table 2 Profile of splenocytes (% total cells)‡ (Mean values with their standard errors)
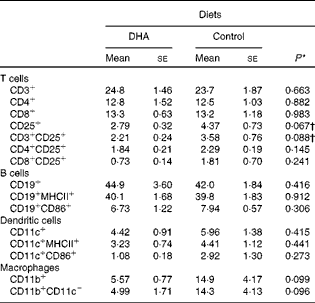
* P values determined by Student's t test
† P values determined by the Mann–Whitney U test.
‡ Fluorescence-activated cell sorter (FACS) analysis gated on all cells.
A DHA-enriched diet reduces antigen-specific production of T helper type 2 cytokines
Supplementation of pregnant dams with fish oil, a source of long-chain n-3 PUFA including EPA and DHA, has been associated with reduced levels of IL-13 in the cord blood(Reference Dunstan, Mori and Barden27). To follow up on these observations, we developed a mouse model to investigate whether dietary intake of DHA during early life could similarly modulate the immune system. To gain a broad understanding of the response, we examined Th2 and Th1 cytokine expression. Fig. 1(a) shows that there was an increase in IL-13 production from splenocytes from DHA-fed mice in response to ova stimulation (57·2 (se 21·7)) compared with unstimulated cells (12·1 (se 5·94), P< 0·05). An increase in IL-13 production was also observed from ova-stimulated cells from animals fed the control diet (161·5 (se 45·0)) compared with unstimulated cells (9·48 (se 2·5), P< 0·001). When the two diets were compared, significantly more IL-13 was produced by ova-stimulated cells from animals fed the control diet (161·5 (se 45·0)) than from animals fed the DHA diet (57·2 (se 21·7), P< 0·05). In the case of IL-4, unstimulated and ova-stimulated cells from mice on a DHA-enriched diet did not show a significant difference (4·95 (se 0·6) v. 7·33 (se 3·4), P>0·05), unlike cells from mice fed the control diet (9·74 (se 2·2) v. 33·2 (se 11·8), P< 0·05). Similar to IL-13, there was also significantly more IL-4 produced by ova-stimulated cells from animals fed the control diet (33·2 (se 11·8)) compared with the DHA diet (7·33 (se 3·4), P< 0·05). The production of IL-10 and IFN-γ in response to ova was not blunted in cells from animals on the DHA diet (Fig. 1(c) and (d)), although baseline IFN-γ levels from DHA-fed mice were significantly lower (Fig. 1(d)). The effect of the DHA diet was not observed after Con A stimulation (Table 3). These results indicate that a DHA-enriched diet coincides with lower allergen-induced production of Th2 cytokines, which may be due to the direct inhibition of cytokine expression or indirect by influencing the T-cell phenotype.

Fig. 1 DHA-enriched diet reduces splenocyte production of T helper type 2 cytokines. Splenocytes obtained from ovalbumin-sensitised mice fed either the DHA (1 %) or control diet were cultured for 48 h with (![]() ) or without (□, unstimulated) ovalbumin stimulation. Cells from mice fed the DHA diet showed lower levels of (a) IL-13 and (b) IL-4, but not (c) IL-10 or (d) interferon-γ (IFN-γ). Values are means (n 6–8 experiments), with standard errors represented by vertical bars. * Mean value was significantly different from that of control diet-fed mice (P< 0·05; Student's t test or Mann–Whitney U test).
) or without (□, unstimulated) ovalbumin stimulation. Cells from mice fed the DHA diet showed lower levels of (a) IL-13 and (b) IL-4, but not (c) IL-10 or (d) interferon-γ (IFN-γ). Values are means (n 6–8 experiments), with standard errors represented by vertical bars. * Mean value was significantly different from that of control diet-fed mice (P< 0·05; Student's t test or Mann–Whitney U test).
Table 3 Cytokine levels from splenocytes (Mean values with their standard errors)
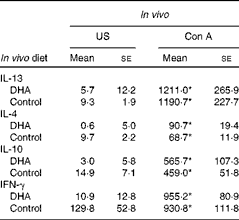
US, unstimulated; Con A, concavalin A; IFN-γ, interferon-γ.
* Mean value was significantly different from that of US splenocytes (P< 0·01; Mann–Whitney U test).
DHA does not influence the maintenance of T helper type 2 cells
To test the possibility of whether the effect of DHA on Th2 cytokine expression was through influencing the maintenance of Th2 cells, we assessed the expression of CRTh2, as a marker of human Th2 cells(Reference Cosmi, Annunziato and Galli17, Reference Messi, Giacchetto and Nagata35). Fig. 2(a) shows representative flow cytometry staining for CRTh2 on human Th2 cells after a 4 d rest with IL-2 (5 ng/ml), the time point of maximal CRTh2 expression, in the presence or absence of DHA (10 μm). This concentration is within or below the range used by numerous other in vitro studies investigating the influence of DHA on immune function(Reference Weise, Hilt and Milovanovic36–Reference Serini, Donato and Piccioni38). Fig. 2(b) shows that 50·1 (se 10·2) % of cells were expressing CRTh2 in resting conditions and 54·1 (se 8·6) % when DHA was added. These findings suggest that DHA does not affect the maintenance of the Th2 phenotype.

Fig. 2 DHA treatment does not inhibit CRTh2 (chemoattractant receptor-homologous molecule expressed on T helper type 2 cells) on human Th2 cells. Representative flow cytometry staining of CRTh2 on human Th2 cells cultured for 4 d in (a) IL-2 (□) or IL-2+DHA (![]() , 10 μm) and shows there was no reduction in (b) the percentage of CRTh2+ cells (n 4). Statistical significance was assessed by Student's t test. (A colour version of this figure can be found online at http://www.journals.cambridge.org/bjn)
, 10 μm) and shows there was no reduction in (b) the percentage of CRTh2+ cells (n 4). Statistical significance was assessed by Student's t test. (A colour version of this figure can be found online at http://www.journals.cambridge.org/bjn)
DHA inhibits the expression of IL-13 protein
Since there was no influence on the percentage of Th2 cells, we next assessed whether DHA could directly inhibit Th2 cytokine expression. The percentage of IL-13 expressing human Th2 cells stimulated (4 h) with PMA (20 ng/ml) and ionomycin (1 μm) in the presence or absence of DHA (10 μm) was assessed. Fig. 3(a) shows that this stimulation resulted in 41·4 (se 6·0) % of cells expressing IL-13, which was reduced in each experiment by DHA on average by 32 % (n 12, P< 0·01). Interestingly, the inhibitory effect was more potent (40 %) when the Th2 population was moderately IL-13 positive ( < 50 %) compared with highly IL-13 positive (>50 %, 9·5 %), although reductions were significant in both populations (P< 0·05). This response was DHA-specific since control experiments performed with diluent (ethanol), AA or OA (10 μm) showed no change (Fig. 3(b)). However, significant reductions in the percentage of cells expressing IL-4 when stimulated in the presence of DHA (n 9), AA or OA were not observed, although there was a trend (n 4, Fig. 3(c) and (d)). DHA treatment also did not affect total cell numbers (data not shown). These results show that DHA inhibits the expression of IL-13 in differentiated Th2 cells.

Fig. 3 DHA treatment inhibits the expression of IL-13 by human T helper type 2 (Th2) cells. Intracellular flow cytometry of phorbol myristate acetate (PMA) (P, 20 ng/ml)- and ionomycin (I, 1 μm)-stimulated Th2 cells in the presence or absence of DHA (10 μm). There was a significant inhibition of (a) IL-13 with DHA treatment (n 12), which was not observed with (b) other lipids (n 4). There was no difference in the percentage of IL-4-positive cells when stimulated in the presence of (c) DHA (n 9) or (d) other lipids (n 4). Statistical significance was assessed by (a, c) paired Student's t test or (b, d) one-way ANOVA. Values are means, with standard errors represented by vertical bars. ** Mean value was significantly different from that of the other treatments (P< 0·01). AA, arachidonic acid; OA, oleic acid.
DHA abrogates transcriptional activation of the human IL-13 promoter
Since we demonstrated that DHA could inhibit the protein expression of IL-13, we next asked whether this inhibition was due to direct interference with IL-13 promoter activation. We transiently transfected Jurkat T cells, a line commonly used for studying transcriptional regulation of T-cell genes(Reference Monticelli, Solymar and Rao39), with a luciferase reporter construct containing a fragment of the human IL-13 promoter (IL-13-369pro/Luc). Fig. 4(a) shows a 25-fold induction of IL-13 promoter activity in response to the stimulation of PMA (20 ng/ml) and ionomycin (1 μm); this induction was lower with cells stimulated in the presence of DHA (16-fold, P< 0·01). The effect of DHA was specific, since control lipids (AA and OA) did not reduce IL-13 promoter activity (10 μm; Fig. 4(b)). These data indicate that the n-3 fatty acid metabolite DHA can inhibit IL-13 promoter activation.

Fig. 4 DHA treatment and IL-13 promoter regulation. Jurkat T cells transiently transfected with an IL-13 promoter reporter construct (IL-13-369pro/Luc) showed a 25-fold increase in promoter activity in response to an overnight treatment with phorbol myristate acetate (PMA) (P, 20 ng/ml) and ionomycin (I, 1 μm), and this activity was inhibited (36 %) by (a) DHA (10 μM, n 7), but not by (b) other lipids (n 4). Statistical significance was assessed by (a) paired Student's t test or (b) one-way ANOVA. Values are means, with standard errors represented by vertical bars. Mean value was significantly different from that of the other treatments: * P< 0·05, ** P< 0·01. AA, arachidonic acid; OA, oleic acid.
DHA abrogates transcription factor binding to the human IL-13 promoter
Since DHA reduced IL-13 promoter activity, we hypothesised that it also affects transcription factor binding. Electromobility shift assays (EMSA) with nuclear extracts from PMA (20 ng/ml)- and ionomycin (1 μm)-stimulated Jurkat cells (Fig. 5(a)) and human Th2 cells (Fig. 5(b)) were performed. In silico analysis identified putative transcription factor sites and antibodies were used to confirm the binding. Fig. 5(a) and (b) shows a number of bands (lanes 2 and 8), compared with free probe (containing no extract; lanes 1 and 7). Specific binding was confirmed by competition with 100-fold molar excess of unlabelled oligonucleotide (lanes 3 and 9). A polyclonal anti-CREB antibody recognising a number of CREB family members revealed that three of the bands were CREB proteins, since they were supershifted (lanes 4 and 10). The fourth was the CREB family member ATF-2, since the band was lost with anti-ATF2 antibody (lanes 5 and 11). No alteration in these bands was observed with control antibody (IgG; lanes 6 and 12).

Fig. 5 DHA treatment inhibits transcription factor binding to the IL-13 promoter. Electromobility shift assay with nuclear extracts from phorbol myristate acetate (PMA) (20 ng/ml)- and ionomycin (1 μm)-stimulated (a) Jurkat and (b) CRTh2+Th2 cells show the binding of cyclic AMP response element binding (CREB) and activating transcription factor 2 (ATF2) to the IL-13 promoter. Nuclear extracts from CRTh2+Th2 cells stimulated in the presence of (c) DHA (10 μm) show that the binding of both CREB and ATF2 is reduced. Data are representative of four experiments. CRTh2, chemoattractant receptor-homologous molecule expressed on T helper type 2 cells; Ab, antibody.
Fig. 5(c) is an EMSA with nuclear extracts from Th2 cells stimulated with PMA/ionomycin in the presence or absence of DHA (10 μm). As shown in Fig. 5(b), ATF2 and CREB were identified by antibody supershift (lanes 3 and 4, respectively). The binding intensity of both these proteins was reduced with extracts from cells stimulated in the presence of DHA (lane 2) compared with stimulation alone (lane 1). The data are representative of four independent experiments using extracts generated from three different human Th2 lines. These findings indicate that the DHA effect on IL-13 transcription is mediated, at least in part, by the inhibition of CREB/ATF-2 binding to the IL-13 promoter both in Jurkat and Th2 cells.
Discussion
While there has been some controversy, various studies have indicated that n-3 fatty acid supplementation holds promise for treating and/or preventing allergic disease. An inverse association between fish intake and the prevalence of asthma has been reported(Reference Miyamoto, Miyake and Sasaki25), and n-3 fatty acid administration to children with asthma resulted in reduced symptoms(Reference Nagakura, Matsuda and Shichijyo23). More recently, investigators have focused on determining whether targeting sensitisation may be more effective than established disease. Indeed, n-3 supplementation during pregnancy was associated with lower levels of cord blood IL-13(Reference Dunstan, Mori and Barden27, Reference Krauss-Etschmann, Hartl and Rzehak28) and allergic symptoms(Reference Olsen, Osterdal and Salvig40) in children. Inverse correlations between fish intake and sensitisation have also been reported(Reference Schnappinger, Sausenthaler and Linseisen29, Reference Furuhjelm, Warstedt and Larsson30), and early introduction of fresh oily fish into children's diet was associated with protection from eczema at 2 years of age(Reference Oien, Storro and Johnsen41). The present results using a DHA-enriched diet are consistent with these findings (that used a mixture of EPA+DHA) and indicate that the effect may involve the inhibition of IL-13 and IL-4. Since these cytokines are essential for isotype switching to IgE(Reference Punnonen, Aversa and Cocks19, Reference Jabara, Fu and Geha42), the results suggest that DHA may reduce allergic sensitisation. However, further studies such as determining the presence of specific IgE, the effect on IgE-mediated mast cell activation and animal models of allergen challenge are needed to confirm whether the DHA-mediated reduction in Th2 cytokine production influences allergic sensitisation.
We selected a level of 1 % (w/w) DHA because our previous work using a rodent model demonstrated its influence on T-cell responses and altered the composition of DHA in the dam's milk(Reference Robinson and Field43). In the present study, the animals were exposed to elevated levels of DHA from birth until maturity (10 weeks) through the dam's milk and then due to being weaned onto the diet. Therefore, while this regimen is associated with lower IL-13 and IL-4 expression, we are not able to determine the relative importance of the early v. the chronic/prolonged feeding on allergic sensitisation. This is an interesting distinction that should be made in future studies, particularly in light of the data reported by Oien et al. (Reference Oien, Storro and Johnsen41) indicating that early introduction of fish to children's diet was highly protective against eczema.
We examined cytokine expression to determine the influence on Th2 immunity, and the present data clearly show that splenocytes from DHA-fed mice exhibited lower allergen-specific IL-13 and IL-4 expression, unlike activation with Con A. Although we used a time point and concentration of Con A that were previously determined to be optimal for splenocyte proliferation and IL-2 production, lower Th2 cytokine expression from cells of DHA-fed mice may have been observed with lower Con A concentration and/or time points. Alternatively, Con A and CD3 have recently been shown to use different pathways and kinetics for Ca influx and the recruitment of Ca-permeable non-selective cation channels(Reference Pang, Shin and Park44). Therefore, the complex regulation of Ca may play a role in the inability of DHA to inhibit Th2 cytokine expression in response to this concentration of Con A. There were no significant effects of the diet on the relative percentage or concentration of splenic T cells (CD4+ or CD8+), B cells or dendritic cells, or their expression of CD25, major histocompatibility complex II (MHC II) or CD86.
In vitro experiments with human Th2 cells treated with DHA demonstrated an inhibitory effect on the percentage of IL-13-expresssing but not IL-4-expressing cells. IL-4 is the crucial cytokine for Th2 differentiation, while IL-13 carries out Th2 effector functions. As such, the present data suggest that the in vivo effect was primarily on Th2 differentiation, while the in vitro effect on differentiated human Th2 cells was through the direct inhibition of IL-13 expression. We observed the DHA-mediated inhibition of CREB binding to the IL-13 promoter; however, CREB − / − mice showed no major alteration in IL-4 production(Reference Baumann, Kyewski and Bleckmann45). Therefore, the difference in IL-4 and IL-13 response to DHA may be attributed to their relative dependence on CREB; however, further experiments are needed to determine the underlying mechanism(s). Although Th2 cells stimulated in the presence of DHA exhibited a reduction in the percentage of IL-13-expressing cells in each experiment, there was an inverse relationship between the inhibitory effect of DHA and the percentage of IL-13-positive cells, indicating that higher concentrations of DHA may have resulted in stronger inhibition in these cells.
Our in vitro data with human Th2 cells suggest that the mechanism for the inhibitory effect of DHA on IL-13 involves interference with CREB/ATF2 binding, subsequently reducing transcriptional activation. Our previous studies showed that DHA can be incorporated into lipid rafts within the plasma membrane(Reference Schley, Brindley and Field46, Reference Ruth, Proctor and Field47) and others have demonstrated its effects on signalling events regulating transcription factor activation/phosphorylation. For instance, DHA interferes with CD40-mediated NFkBp50 nuclear translocation and IL-4R-mediated signal transduction activator of transcription 6 (STAT6) phosphorylation, resulting in a reduction of class switching to IgE(Reference Weise, Hilt and Milovanovic36). DHA treatment has also been shown to inhibit CREB binding to the IL-6 promoter(Reference Jia, Zhou and Shi48, Reference Shi and Pestka49), through reduced phosphorylation of CREB and its kinase Akt(Reference Jia, Zhou and Shi48). Akt is an important kinase in mediating T-cell activation through co-stimulatory signals such as CD28(Reference Kane and Weiss50). This is likely to interrupt fundamental regulatory components of the IL-13 core promoter, since T-cell activation and the subsequent phosphorylation of CREB have been reported to mediate the binding of basic transcriptional machinery such as CBP and p300(Reference Kwok, Lundblad and Chrivia51, Reference Lundblad, Kwok and Laurance52), which recruit RNA polymerase II(Reference Vo and Goodman53). Another possibility is that CREB family members have been shown to interact with nuclear factor of activated T-cells (NFAT) when regulating genes such as cyclo-oxygenase 2 and TNF(Reference Sharma-Walia, George Paul and Patel54, Reference Falvo, Lin and Tsytsykova55). NFAT is another factor that regulates IL-13 expression(Reference Monticelli, Solymar and Rao39, Reference Dolganov, Bort and Lovett56), and therefore the DHA effect on CREB phosphorylation and subsequent binding may also influence NFAT-mediated promoter activity. Our EMSA analysis investigated only one region of the IL-13 promoter and showed reduced binding of CREB/ATF2. However, it is also possible that some degree of the inhibitory effect of DHA on IL-13 expression resulted from influencing other regulatory elements. For instance, DHA inhibits the expression of NFkbp65, a transcription factor that induces IL-13 expression(Reference Draper, Reynolds and Canavan57, Reference Silbermann, Schneider and Grassmann58).
In addition to DHA acting as a direct inhibitor of IL-13 transcription, as we have shown, in vivo n-3 fatty acid supplementation may work through a number of mechanisms. For instance, the production of n-3 fatty acid metabolites such as Resolvin E1 has been shown to dampen allergic inflammation in a mouse model of asthma(Reference Aoki, Hisada and Ishizuka59). Another mechanism may be through counterbalancing the level of n-6 fatty acid metabolites such as pro-inflammatory PGD2 and cysteinyl leukotrienes (LTC4)(Reference Funk60). For instance, a recent report has shown that human leucocytes obtained following 12 weeks of n-3 supplementation produced less cysteinyl leukotrienes(Reference Schubert, Kitz and Beermann26).
There are also human studies which do not support a protective effect of n-3 fatty acids on the development of allergic disease(Reference Marks, Mihrshahi and Kemp61–Reference Albers, Bol and Bleumink64), some of which have been discussed in a recent meta-analysis(Reference Anandan, Nurmatov and Sheikh65). However, studies showing positive and negative effects vary greatly in study design, n-3 formulation, dose, time and length of administration, outcome measure and heterogeneity of the population. Therefore, the effectiveness of n-3 supplementation may well depend on one or more of these factors(Reference Yin, Liu and Goleniewska63, Reference Albers, Bol and Bleumink64). Careful reading of these studies indicates that conflicting results may be attributed to higher doses of DHA (2–4 %), compared with the present study (1 %). Furthermore, it is possible that an effect of n-3 fatty acid supplementation may not be observed in animal models if the sensitisation and/or challenge are too robust, overpowering the n-3 effect. In reality, sensitisation and the subsequent development of allergic disease are subtle events resulting from a tip in the balance between tolerance and sensitisation, and so experiments using animal models with mild sensitisation and challenge protocols may be needed to confirm the abrogation of sensitisation.
In conclusion, the present data suggest that dietary supplementation with n-3 fatty acids may help skew the balance away from allergic sensitisation by interfering with IL-13 promoter activation and IL-13 expression. These data are consistent with observational studies showing an association between n-3 fatty acid intake and reduced levels of cord blood IL-13 and the incidence of atopy and eczema(Reference Dunstan, Mori and Barden27–Reference Furuhjelm, Warstedt and Larsson30, Reference Olsen, Osterdal and Salvig40, Reference Oien, Storro and Johnsen41), though further experiments are needed to determine whether n-3 fatty acids reduce allergic sensitisation and the development of allergic disease.
Acknowledgements
This study was supported by grants to: L. C. from the SickKids Foundation/Canadian Institutes of Health Research, the Alberta Heritage Foundation for Medical Research and the American Academy of Allergy, Asthma and Immunology; C. F. from the National Science and Engineering Council of Canada; H. V. from the Canadian Institutes of Health Research. The authors also thank Susan Goruk, Chris Tse, Melanie Abel, Courtney Davidson and Jessica Storie for their technical assistance, as well as Donata Vercelli and Michael Kabesch for providing the IL-13-369pro/Luc reporter construct. E. M. performed the cell culture and EMSA analysis; N. M. performed the IL-13 reporter assays; H. V.'s laboratory performed the allergen sensitisation study; C. F.'s laboratory designed and generated the DHA and control diets, performed the splenocyte assays and the IL-13 ELISA; C. F. and H. V. also contributed to the manuscript writing; L. C. designed, coordinated and supervised the study and wrote the final manuscript. There are no conflicts of interest to declare.












