Introduction
In traditional livestock farming systems, animals were driven across land on foot, receiving food and water en route, but nowadays they are nearly always transported by road or rail. This is usually from a farm to a saleyard or market, or abattoir, or from a farm specialised in breeding stock to one reserved for fattening. Such journeys can cover thousands of kmReference Self and Gay1–Reference Knowles, Warriss, Brown, Kestin, Edwards, Perry, Watkins and Phillips3 and take several days. Food and water provision is nearly always suspended during transportation and it is also common practice to deny sheep and cattle access to feed and water for several hours before transport. The practice of feed and water deprivation (FWD) before transport was first called a ‘curfew’ by WythesReference Wythes4. This is distinct from undernutrition (‘a prolonged inadequate supply of nutrients to sustain good health and, in the case of immature or underweight animals, growth potential’)Reference Agenas, Heath, Nixon, Wilkinson and Phillips5 and malnutrition (‘a deficit, imbalance or excess of nutrients with consequential adverse effects on health and growth potential’)Reference Agenas, Heath, Nixon, Wilkinson and Phillips5.
The imposition of a period of FWD before transport has two main aims. The first is to reduce digesta load in the gastrointestinal tract in an attempt to reduce fouling of other animals, the trucks and roads over which they pass, and carcass contamination. The second, in situations where animals are sold by weight, is to permit a more accurate prediction of carcass weight.
The short-term interruption to nutrient supply associated with FWD will in particular affect functioning of the rumen and the rest of the digestive tract, tissue homeostasis and control of enteropathogenic bacteria by rumen microbes. Effects on metabolism in animal muscle will also influence meat quality. The process of gathering animals on a farm, holding them in yards, often with unfamiliar companions, loading them aboard unfamiliar vehicles and then transporting them, subjects the animals to multiple stressors. These are manifested by substantial increases in the circulating levels of corticosteroids, notably cortisol, and the release of catecholamines such as adrenalineReference Ferguson, Bruce, Thompson, Egan, Perry and Shorthose6. Hence, any effects of FWD may be influenced by the additional stressors associated with the transportation process. Animals need to recover quickly from the effects of FWD in order to maintain efficiency of production and to ensure meat quality and meat safety.
The present review will consider the animal's systems that may be affected by FWD, the mechanisms that the animal initiates to minimise those effects, and the rate of recovery when feed and water become available.
Effects on live weight
The most obvious effect of FWD is a loss in live weight, especially during the first 12 hReference Wythes4. A loss in sheep of 5·5 kg over 12 h compared with control animals was recorded by Cockram et al. Reference Cockram, Kent, Waran, McGilp, Jackson, Amory, Southall, O'Riordan, McConnell and Wilkins7, and losses of this magnitude occur whether or not transportation is involvedReference Knowles, Brown, Warriss, Phillips, Dolan, Hunt, Ford, Edwards and Watkins8. After the rapid initial loss, live weight continues to decrease steadily and relatively constantly for the next 36 h but declines more slowly after thatReference Wythes4. For instance, steers weighing 396 kg and fasted (with water available) for 12, 24, 36 and 48 h lost 23, 30, 46 and 57 kg, respectivelyReference Wythes, McLennan and Toleman9. In cattle transported for 31 h, including a 1 h rest period with water and feed available after 14 h, Knowles et al. Reference Knowles, Warriss, Brown and Edwards10 observed an 8 % loss in live weight. Some 70 % of this loss occurred in the first 14 h, 89 % in the first 21 h and 95 % by the 26th hour. These weight changes are greater than observed following major changes in the type of feed provided to cattle; for example, Balch & LineReference Balch and Line11 recorded a 30 kg live-weight loss over 3 d when cows were changed from a conserved forage and concentrate diet to grazing.
The live weight of herbivores is dynamic. They daily consume a weight of feed comprising approximately 4 % of body weight, plus, for lactating animals at least, an even greater weight of water. However, they are likely to show no net change in body weight over 24 h. This is because the feed is fermented, metabolised and excreted in urine, faecesReference Phillips12, bodily secretions and expired air. Furthermore, the distribution of weight in the animal varies during the day as digesta that accumulate in the rumen are gradually moved to more distal sections of the tract. Wether sheep grazing lush pastures may excrete 3–6 litres/dReference Beeston and Hogan13, may urinate twenty to forty times daily, with an average volume of approximately 150 mlReference Sears14. Female animals appear to urinate less frequently than castrate males but excrete a greater volume per urination (JP Hogan, unpublished results). Carbon losses from sheep through respiration and other mechanisms have been estimated by GrahamReference Graham15. Typically, sheep receiving energy at approximately the maintenance level had the following carbon exchange (g/d): intake, 248; faeces, 63; urine, 12; methane, 11; carbon dioxide, 160. In this example, the weight loss per d as respiration gases would comprise approximately 18 g as methane and 587 g as carbon dioxide, representing a significant part of the daily weight variation. However, as Blaxter & GrahamReference Blaxter and Graham16 indicated (Table 1), food deprivation for 2–4 d would reduce the output of carbon dioxide and methane, respectively, from (g/d) 709 and 18 to 267 and 1.
Table 1 Changes in output of respired gases in sheep before, during and after a 96 h fast (calculated from Blaxter & GrahamReference Blaxter and Graham16)
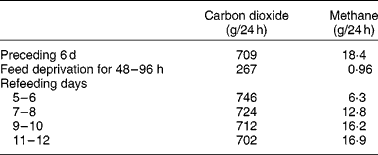
During FWD some live-weight loss is expected due to cessation of feed and water intake. However, major live-weight loss is due to catabolic processes, which occur at a declining rate as the supply of metabolites for respiration is reducedReference Blaxter and Graham16. Defaecation also gradually declines. In cattle after 48 h FWD, 70 % of faecal output occurred in the first 24 hReference Cole, Phillips and Hutcheson17. Water deprivation for 4 d resulted in a partial voluntary feed intake restrictionReference Weeth, Sawhney and Lesperance18, presumably because the cattle attempted to maintain rumen osmolality. As the daily intake of hay fell from 6·6 to 0·5 kg, there was a decline in output of urine from 7 to 2 litres/d and faeces from 16 to 2 kg/d. The water content of faeces fell from 85 to 72 %, reflecting water reabsorption during a longer retention time of digesta in the large intestine, so that the output of water in faeces declined from 14 to 1 litre/d.
Rumen content and function
The extent of change in rumen function during FWD depends on the amount and composition of digesta initially present. In ewes fed hay and concentratesReference Boyne, Campbell, Davidson and Cuthbertson19 and in cows fed hayReference Makela20 (Table 2), the weight of digesta in the whole tract was equivalent to approximately 19 % body weight. The digesta in the rumen formed about 70 % of the total. Changes in the weight of rumen contents reflect both the daily intake of feed and the rate at which residues are removed from the rumen, which in turn are dependent on the nature of the diet and on the physiological state of the animal. WestonReference Weston21, in reviewing factors regulating feed intake in animals, expressed the weight of rumen digesta (RD) in relation to the live weight of the animal free of rumen digesta (RFW), that is, the RD:RFW ratio. Data from eighteen studies with sheep and fourteen with cattle fed ad libitum showed that the RD:RFW (g/kg) varied from 100 with feeds of high digestibility to 300 with very mature forages. It is clear that the weight of digesta in the rumen at the start of FWD can be affected by the age and physiological state of the animal and by plant factors such as anatomy, stage of maturity and nutrient adequacy for animals. With lambs fed chopped straw or lucerne hay, Weston et al. Reference Weston, Lindsay, Peter and Buscall22 observed that, although the intake of organic matter was only about half that of adult sheep, RD:RFW was higher. However, when data were expressed relative to metabolic body size, i.e. live weight to the power of 0·75, the values for sheep and lambs became almost identical. Feed intake and digesta load are greater in lactating than non-lactating ewesReference Weston23. Sheep and cattle ate significantly more leaf than stem from tropical grassesReference Poppi, Minson and Ternouth24 but had similar amounts of digesta in the rumen. Research with a tropical legumeReference Hendricksen, Poppi and Minson25 has shown that, although the intake of leaf by sheep is much greater than that of stem, the RD:RFW ratio was similar. For cattle it was less for leaf than for stem. The provision of an N supplement to cattle fed a mature tropical grass increased both feed intake and RD:RFWReference Kennedy, Boniface, Liang, Muller and Murray26.
Table 2 Weights of digesta in the different sections of the gastrointestinal tract of sheepReference Boyne, Campbell, Davidson and Cuthbertson19 and cattleReference Makela20*
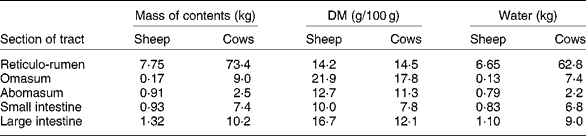
* Ewes of 58 kg ate 400 g hay and 1150 g concentrates daily. Dairy cows (mean weight 554 kg) ate 10 kg DM as hay daily.
The RD:RFW ratio also varies with time of day in animals grazing or fed forages ad libitum. For instance, cattle with 65 kg digesta in the rumen at 09.30 hours had increased the load by 21 % by 15.30 hoursReference Weston, Ellis and Cantle27. Similarly, a digesta load of 2·9 kg in the rumen of sheep sampled at daylight, before grazing had commenced, increased by 70 % when morning grazing ended 4 h laterReference Hogan28. Therefore, there is likely to be less excreta voided, and hence less vehicle and hide contamination, when livestock are transported early in the morning than later in the day.
If feed intake is known, the DM in the rumen: DM intake ratio provides an indication of the average time spent by feed residues in the rumenReference Thornton and Minson29. In these studies, the ratio of DM or organic matter in the rumen to the corresponding intake ranged from 0·5 with immature forage oats to 1·4 with straw. Calculations from the data in Table 2 suggest that the sheep of Boyne et al. Reference Boyne, Campbell, Davidson and Cuthbertson19, fed hay plus concentrates, had in their rumen a DM equivalent to approximately 0·8 × daily intake. Similarly the hay-fed cattle of MakelaReference Makela20 had approximately 1·06 × intake in their rumen. With cattle fed tropical grasses or legumesReference Poppi, Minson and Ternouth24, Reference Hendricksen, Poppi and Minson25, the DM in the rumen of animals fed the leaf fraction was equivalent to 1·1 to 1·5 × intake, whereas with the stem fraction, the corresponding values were 1·8 to 2·1. Sheep were apparently able to move feed more readily through the rumen as the digesta load for the leaf fraction was 0·5–1·1 × intake and the stem fraction 0·8–1·43. Presumably feed retained in the rumen for an extended time in the fed animal is discharged relatively slowly during FWD.
Jersey–Friesian steers removed from pasture for varying times before slaughter experienced a 27 % reduction in the weight of reticulo-rumen contents (initially 55 kg), over 17 h of FWD, and the DM content was reduced by 40 % (initially 8 kg)Reference Bass and Duganzich30. There was little change in the weight of digesta with 31 h of further FWD, but DM mass declined to 3 kg. However, the full extent of DM loss from the rumen may be partly masked by inflow of saliva, as confirmed by Weston et al. Reference Weston, Ellis and Cantle27 in studies of pregnant and control cows placed under FWD after being on pasture. Losses in mass of rumen contents during 16–17 h of FWD were 31 to 40 %, but the corresponding losses of DM were 49 to 60 %. In a study with 350 kg heifers, Janloo et al. 31 indicated that 36 h after withdrawing a diet containing 84 % rolled maize, the net loss in weight of 19 % from the rumen comprised 2·8 kg water, but 3·8 kg DM. It is of interest in the context of continuing microbial fermentation in the rumen during FWD that, although only 2·5 kg acid-detergent fibre was normally maintained in the rumen, 1·1 kg of that fraction of feed was still present in the rumen after 36 h of FWD.
Few studies have separately involved FWD, but water intake will be reduced anyway if feed intake is restrictedReference Hecker, Budtz-Olsen and Ostwald32, and conversely the withdrawal of water depresses feed intakeReference Weeth, Sawhney and Lesperance18. However, Warner & StacyReference Warner and Stacy33 had the opportunity to compare deprivation of feed plus water with feed alone. In a study using two sheep, one did not drink, whereas the other drank three times during feed deprivation. No appreciable differences were seen between the sheep in plasma osmotic pressure (OP) (mean 295–300 mOsm/kg) or rumen pH, which gradually rose from about 6·6 to 7·1. However, the two sheep differed physiologically. In the sheep that refused to drink, rumen OP rose to 280 mOsm/kg during the first 30 h but subsided to 245 mOsm/kg subsequently. This contrasted with a rise to 300 mOsm/kg in the second sheep before a drink of water lowered it to 240 mOsm/kg. The OP then rose but was lowered each time by water consumption. The increased rumen OP derived mainly from saliva which, though hypotonic to plasma, was hypertonic to rumen contentsReference Warner and Stacy34. Presumably the sheep that reduced rumen OP without drinking differed from the other in the composition and rate of secretion of saliva.
Homeostasis
FWD challenges the ability of the animal to regulate homeostasis. This is primarily concerned with the maintenance, within defined limits, of physiological features such as pH, osmotic pressure and acid–base balance. These are controlled by the movement of ions, notably Na, K, chloride and bicarbonate through intracellular fluids (ICF) and extracellular fluids (ECF). In single-stomached animals, ICF is defined as that part of the body water plus dissolved solutes within cell membranes. The ICF maintains homeostasis particularly through its high content of K. The ECF describes all body fluids outside the cells including plasma and interstitial fluid and can be regarded as an ultrafiltrate of blood plasma. Ruminants have a special consideration relating to whether the water in the digestive tract and especially in the rumen is considered part of ECF, which will be discussed later.
Homeostasis is challenged by loss of water from the body and hence during FWD, by dehydration leading to thirst. Thirst, defined as a longing or compelling desire to drink, is induced by both extracellular and cellular dehydrationReference Blair-West, Burns, Denton, McBurnie, Tarjan and Weisinger35. Denton et al. Reference Denton, Shade, Zamarippa, Egan, Blair-West, McKinley and Fox36 indicated that an increase of 1–2 % in plasma osmolality causes animals to drink. A reduction in the volume of ECF has a similar effect, but decreases of 10 % in blood volume or pressure are needed. Thirst is induced by signals emanating from the hypothalamus and associated brain structures and mediated by angiotensin 11. However, there is evidenceReference Coghlan, Blair-West and Denton37 that glucocorticoids such as cortisol inhibit the renin–angiotensin–aldosterone axis. Some corroborating evidence was provided by Parker et al. Reference Parker, Hamlin, Coleman and Fitzpatrick38. However, the concentrations of cortisol generated in response to the intravenous infusion of adrenocorticotropic hormone in their work were much higher than reported for sheep or cattle undergoing FWD plus transportation. Nevertheless, it seems possible that, depending on their relative levels of cortisol and angiotensin 11, animals under transport might show increased water loss through a cortisol-induced diuresisReference Parker, Hamlin, Coleman and Fitzpatrick39 without developing angiotensin-controlled sensations of thirst. Individually confined cattle, which are known to be stressed because of isolationReference Friend, Dellmeier and Gbur40–Reference Ladewig and Smidt42, have increased Na appetiteReference Phillips, Youssef, Chiy and Arney43 due to the natriorexigenic effect of heightened pituitary–adrenal secretion. During stress, Na concentration of saliva declines to restrict lossesReference Richter, Hinton and Reinhold44. Although the glucocorticoids do not themselves induce a Na hunger, they do so in conjunction with the mineralocorticoid aldosteroneReference Schulkin45, which is released in response to stress and is responsible for increasing Na appetite during Na depletionReference Michell, Phillips and Chiy46. The effect of stress on Na requirements is not unique to ruminants; the imposition of stress by movement restriction or injection of adrenocorticotropic hormone or corticotropin-releasing hormone also increases the Na appetite in rabbitsReference Tarjan and Denton47 and ratsReference Weisinger, Denton, McKinley and Nelson48.
Na in ECF must be regarded as the main regulator of homeostasisReference Michell, Phillips and Chiy46, and the investigation of possible threats to homeostasis exerted by FWD must involve a study of the distribution of Na in the ruminants' fluid pools. In ruminants the techniques used to measure ECF are complicated by the presence of a substantial pool of water in the rumen. It is currently unclear whether liquid in the digestive tract can be regarded as part of ECF.
The volume of water present in ICF is calculated as the difference between total body water and ECF, the estimation of each of which depends on measuring the dilution of reference substances or ‘markers’ introduced into the body. After injection intravenously, a marker is diluted exponentially. The concentration is measured in plasma collected at intervals, and extrapolation back to the time of injection indicates the concentration of marker that would have been achieved at zero time if mixing of the marker in that pool had been instantaneous. The amount of marker lost in urine during the study is estimated and the calculation made as follows:

It is accepted that 2H-labelled water and 3H-labelled water are suitable markers for the estimation of total body water because, within 6 h of intravenous injection, even in cattle, the concentration of those markers in plasma has equilibrated with that in other pools, including the rumenReference Panaretto and Reid49. However, there is no general agreement on markers to measure ECF. As Walser et al. Reference Walser, Seldin and Grollman50 indicated, a marker for ECF must penetrate the membrane of the capillary wall but not the cell membrane. Those authors quote reports that ionic markers such as bromide, thiosulfate or thiocyanate penetrate cell walls to some extent. Other larger molecules such as mannitol probably do not penetrate cells but may exert undesirable osmotic pressure on the cell. The use of thiocyanate in sheepReference Macfarlane, Morris, Howard, McDonald and Budtz-Olsen51 is further questioned by the observationReference Panaretto52 that some thiocyanate enters the rumen during the 4 h required for the measurement. By the time the concentration in the rumen reaches equilibrium with that in plasma, after 20 h or more, an unacceptably high proportion of the dose has been excreted in the urine. The use of 82Br injected as sodium bromideReference Coghlan, Fan, Scoggins and Shulkes53 may have overcome criticism of the use of bromide to measure ECF. Data from Coghlan et al. Reference Coghlan, Fan, Scoggins and Shulkes53 on Merino crossbred sheep indicated a mean ECF volume, excluding the rumen, of 245 ml/kg live weight. Applying that value to these data of the 58 kg sheep of Boyne et al. Reference Boyne, Campbell, Davidson and Cuthbertson19 (Table 2) which had 6·65 litres water in the rumen and assuming that the concentrations of Na in plasma and rumen fluid were 145 and 110 mEq/l respectivelyReference Rogers and Van't Klooster54, Reference Payne, Dew, Manston, Vagg and Phillipson55, the 14·2 litres ECF would contain 2060 mEq Na. This is approximately three times the 730 mEq in the rumen.
The significance of the removal of Na from plasma and hence from ECF has been considered by ScottReference Scott, McDonald and Warner56. The rate at which Na is extracted from ECF into saliva is affected by the animal's chewing activities. Saliva production at rest is appreciably lower than during eating, particularly forages, or ruminationReference Bailey57, and is affected by stress levelsReference Richter, Hinton and Reinhold44. Data from a 40 kg sheep with an oesophageal fistula, examined six times over 7 months, indicated rates of salivation that varied from approximately 7 ml/min at rest to 30 ml/min when vigorously eating chopped lucerne hay (JP Hogan, unpublished results). Hence that sheep was able to return approximately 2 litres saliva/h to the rumen. This volume would have been about one-fifth of the water calculated for ECF in the 58 kg sheep in Table 2. These calculations are consistent with the report of ScottReference Scott, McDonald and Warner56 that ECF and plasma volume can decrease by 10–20 % during a meal, a sufficient reduction to induce thirstReference Denton, Shade, Zamarippa, Egan, Blair-West, McKinley and Fox36.
Urine excretion can be observed through an exteriorised ureterReference Brook, Waites and Stacy58. Consumption of lucerne hay by sheep caused the almost complete cessation of urine formation and the conservation of Na in the kidneyReference Stacey and Brook59. The effect was associated with the release of arginine vasopressin. There was possible involvement also of the renin–angiotensin–aldosterone system with stimulation of the regions of the hypothalamus controlling thirstReference Blair-West, Burns, Denton, McBurnie, Tarjan and Weisinger35, Reference Fitzsimmons60. This may explain why sheep under a 24 h FWD did not drink until after they had eatenReference Knowles, Warriss, Brown and Edwards10, Reference Knowles61, Reference Knowles62.
Assuming that the Na content of mixed saliva in sheep is similar to that in cattle, 105 mEq/lReference Bailey and Balch63, saliva production at the rate of 2 litres/h during eating would extract in 1 h about one-tenth of the Na in ECF. However, it is not known whether in response to aldosteroneReference McSweeney and Cross64 the concentration of salivary Na might have been reducedReference Richter, Hinton and Reinhold44 and that of K increased. This would help to explain the decrease in Na levels in the rumen when sheep were re-fed after an 18 h fastReference Warner and Stacy33. The picture might be further complicated if elevated plasma cortisol levels depress aldosterone activity, as Parker et al. Reference Parker, Hamlin, Coleman and Fitzpatrick38 suggested. There is clearly a need for a comprehensive investigation of the hormonal control of thirst, kidney function and saliva secretion in FWD animals re-fed after transport.
Little is known about the movement of Na from the digestive tract to the plasma during FWD. In the fed sheep, Na is returned to the plasma only gradually. Transport of Na cations from rumen to blood occurs against not only both osmotic and concentration gradients but also a potential difference that is positive from blood to rumenReference Parthasarathy and Phillipson65. In sheep, Na transport may be as little as 14 mEq/h except for the period after feed intake, during which the osmotic pressure in the rumen exceeds that of plasmaReference Warner and Stacy33 and the K content is highReference Scott, McDonald and Warner56. However, neither hyperosmolarity nor high K content is likely to occur during FWD. Na transport may be relatively more rapid in cattle than sheep during the period of hyperosmolarityReference Dobson, Sellers and Shaw66, but the relative rates at which Na is returned from rumen to plasma in sheep and cattle during FWD is not known. Presumably during FWD, Na transactions in the rest of the digestive tract proceed in a manner similar to those in the fed animal but at a reduced rate as digesta flow is reduced. Na is transported across the wall of the omasumReference Engelhardt, Hauffe, McDonald and Warner67 and possibly the abomasum. Net losses of Na from the omasum plus abomasum were 20–25 % of the amounts in digesta leaving the rumen in sheep fed forages ad libitum (RH Weston and JP Hogan, unpublished results), but losses during FWD are not known. During FWD, Na presumably continues to cross the wall of the small intestine from blood to lumen in secretions and to be reabsorbedReference Kay, Pfeffer and Phillipson68. In the large intestine of the fed animal, the osmotic pressure reflects the concentration of osmotically active molecules such as Na and K, and is reduced as those ions are transported across the intestine wall. Presumably the same mechanism is maintained during FWD. If so, the intestinal contents relative to that of plasma ranges from isotonicity in the caecum and proximal colon to hypotonicity in the rest of the digestive tractReference Brouwer and Van Weerden69. It seems probable that Na transferred from ECF to the digestive tract is returned only gradually. This suggests that the digestive tract, although a major storage site for Na, is not a readily accessible extension of ECF. By contrast, Coghlan et al. Reference Coghlan, Fan, Scoggins and Shulkes53 reported that in sheep only about 15–20 % of 250–670 mEq Na removed via fistula of the parotid salivary duct was withdrawn from ECF. The availability of Na in the rumen during FWD may need to be reassessed. It may also explain the maintenance of acid–base balance observed by Parker et al. Reference Parker, Hamlin, Coleman and Fitzpatrick70 during 48 h FWD plus transport in steers.
When animals come under the restrictions of FWD, rumination is greatly reduced and practically ceases within 24 hReference Welch and Smith71. FWD thus removes not only the stimulus to drink induced by eating, but also that to salivate associated with eating and rumination. The passage of particulate material and water from the rumen continues and with reduced amounts of water entering the rumen, the weight of contents falls. However, after approximately 24 h the weight of rumen contents tends to stabiliseReference Bass and Duganzich30, 31, indicating that the rate of passage of digesta has become approximately equal to the inflow of saliva.
For a period, perhaps the first 24 h of fasting, Na continues to pass to the plasma and thence to ECF from the digestive tract. With no large-scale withdrawal induced by eating and ruminating, the loss of Na in faeces and urine presumably elicits no alarm signals to the kidneys from the areas of the hypothalamus sensitive to Na deficiency. Certainly in fasted yearling Hereford heifersReference Weeth and Lesperance72, plasma Na levels increased by 4 mEq/l in the first 48 h without change in the concentration of Na in urine, even though the rate of excretion of urine had declined appreciably and the osmotic pressure in urine had increased by 40 %. However, by day 4 of the fast, when plasma Na had risen by a further 8 mEq/l, the concentration of Na in urine increased five-fold. Despite this, the osmotic pressure in urine did not exceed 1289 mOsm/kg, which is appreciably lower than that observed with fasted sheepReference Macfarlane, Morris, Howard, McDonald and Budtz-Olsen51, and it seems probable that sheep can achieve far higher osmotic pressures in urine than cattle. At 48 h after water was provided in the fasted heifer study, water intake was above pre-fasting levels and the osmotic pressure in urine had returned to normal. However, the volume of urine was less than half and the Na content more than three times greater than before fasting began. In consequence, Na excretion was about 40 % above the pre-fasting rate.
There is some uncertainty as to the source of water loss during fasting. In depriving heifers of water for 4 d, Weeth et al. Reference Weeth, Sawhney and Lesperance18 reported a weight loss of 39 kg, including 18 kg or 47 % of the total from the thiocyanate space, which had been equivalent to about 34 % of body weight pre-fasting. Similarly, in 50 kg wether sheep, which had a mean thiocyanate space of 13·2 litres, equivalent to almost 26 % of body weight, loss of weight from this pool on fasting was 3·8 kg or 34 % of total weight lossReference Macfarlane, Morris, Howard, McDonald and Budtz-Olsen51. This would imply that ECF is a major source of water lost during fasting. By contrast, ColeReference Cole73, who fasted wethers for 3 d, observed that of 7·7 kg weight loss, 80 % or 6·2 kg was water, with 29 % derived from the digestive tract and 57 % from the ICF pool. This would imply that ECF contributed no more than 14 % to the total water loss. The extensive loss of ICF would be consistent with the observed excretion of K in urine, which was much greater than could be accounted for by losses from the digestive tract. The confusion may reflect the inadequacy of techniques to measure ECF. There would seem to be a need to resolve the question of whether ICF or ECF is the main source of water lost during fasting and, hence, what is the relative significance of Na or K in maintaining homeostasis.
Hunger
Ruminants consume feed in many meals per d. The total amount of feed eaten generally reflects the frequency and duration of periods of eating and, hence, depends on signals emanating from the lateral hypothalamus to start and stop eatingReference Baile and McLaughlin74. Such signals, as reviewed by MatteriReference Matteri75, are generated by appetite-stimulating and appetite-suppressing hormones and neurotransmitters produced in the central nervous system and periphery. As examples, leptin, originating in adipose tissue, inhibits the action of many neuropeptides that stimulate eatingReference Rhind, Archer and Adam76. By contrast, an appetite stimulant is ghrelin, a twenty-eight-amino acid peptide produced in the oxyntic cells of the stomach of ratsReference Miura, Tsuchiya, Sasaki, Kikuchi, Kojima, Kangawa, Hasegawa and Ohnami77 and presumably in corresponding cells in the ruminant abomasum78. This peptide in acylated form functions as an endogenous ligand for the growth hormone secretagogue receptorReference Wren, Small and Ward79. When injected into Holstein heifersReference ThidayMyint, Yoshida, Ito and Kuwayama80, ghrelin immediately stimulated a dose-dependent release of growth hormone.
In the context of the present review, it is of interest that leptin concentration in plasma falls during periods of feed restriction and fastingReference Rhind, Archer and Adam76, Reference Marie, Findlay, Thomas and Adam81, Reference Adam, Findlay, Young and Mercer82. Conversely, fasting in steers, even for 18 h, increased plasma levels of ghrelin and of NEFA78. BassettReference Bassett83 similarly observed elevated levels of plasma NEFA with fasted sheep, with associated marked rises in the concentration of growth hormone. The abomasum may thus be one source of hunger signals in the fasted ruminant. This could coincide with a reduction in the volume and acidity of gastric juiceReference Hill84, reflecting a reduced flow of volatile fatty acids (VFA) in digesta passing through the omasum from the rumenReference Margan85. If increased ghrelin secretion, with subsequent growth hormone release, stimulates sensations of hunger in fasted animals, the effect is probably transitory, as ghrelin levels in the plasma of Holstein heifers declined 49 to 60 min after feedingReference Miura, Tsuchiya, Sasaki, Kikuchi, Kojima, Kangawa, Hasegawa and Ohnami77. The subsequent regulation of feed intake then probably involves the interplay between energy transactions and rumen functionReference Weston86, Reference Forbes87. There appears to be no information on possible modifying effects of elevated levels of plasma cortisol on the activities of hormones such as leptin and ghrelin. However, any effects are probably slight, as sheep and cattle subjected to the stressors associated with transportation appear willing to commence feeding at the end of the journeyReference Knowles, Warriss, Brown and Edwards10, Reference Knowles61, Reference Knowles62.
Nitrogen metabolism
ColeReference Cole73 found that sheep fasted for 3 d had 57 % less N in the digestive tract than fed controls, with two-thirds of the deficit due to changes in the rumen contents. Some of the loss would be unfermented dietary N passed in feed particles through the omasum. Protein in microbial cells would have been removed by the same process. Part of the loss would be ammonia derived from the fermentation of feed protein, the ingestion of bacteria by protozoaReference Coleman, McDonald and Warner88 and the autolysis of bacteriaReference Nolan, McDonald and Warner89 due to lack of substrate. This ammonia would be transported through the rumen wall to contribute to the N pool. Little is known of the effects of a short-term fast on the removal of protein from the tissues, but it seems probable that some tissue catabolism occurs. The pattern of excretion of N was similar in faeces and urine, with about 32 % of the total in a 46 h fast excreted in the first 11 h and 62 % in the first 22 hReference Cole, Phillips and Hutcheson17. Those authors also showed that fasted steers excreted 30 % more faeces and 54 % more urine when transported for 46 h compared with non-transported controls. The output of N in faeces was 30 % higher and in urine 26 % higher in transported animals. The observation suggests that stress, evidenced in transported animals by elevated levels of cortisol, might cause not only a diuresis but also increased tissue catabolism. Such an increase would be consistent with observations of elevated levels of urea in plasmaReference Knowles, Brown, Warriss, Phillips, Dolan, Hunt, Ford, Edwards and Watkins8. In normally fed ruminants, a significant amount of urea circulating in plasma is recycled to the rumen where, after deamination, it can be re-incorporated into microbial protein. However, microbial protein synthesis requires a source of energy released as ATP during the fermentation processes that leads to the formation of VFAReference Bauchop and Elsden90, Reference Hungate91. Hence, in the fasted animal only a very limited incorporation of ammonia from recycled urea would occur. In that situation urea returned to the whole digestive tract would presumably be deaminated then returned to the liver as ammonia for re-incorporation into urea.
Undernutrition, with a consequent reduced supply of nutrients to the tissues, is associated with the loss of N from the liver indicated by a reduction in concentration of liver enzymes, particularly those associated with N metabolismReference Payne and Laws92. There seems to be a lack of information on the extent to which a similar reduction might occur during short-term fasting, but any need to restore the full complement of liver enzymes could contribute to the delay that animals experience when the fasting period ends before the return to full feeding.
Rumen microbial ecology
The sudden cessation of feed supply to the rumen affects the size and composition of the rumen bacterial populationReference Meiske, Salsbury, Hoefer and Luecke93. This will have its most immediate effect on those bacteria that derive nutrients from starch or low-molecular-weight carbohydrates, because those substrates are the most rapidly fermented. Their removal no doubt results in the death of many bacteria of this type, but some non-cellulolytic bacteria survive, deriving substrate from the cellulolytic activity of commensal bacteria. For instance, Selenomonas ruminantium cannot survive alone on a cellulose substrate, but can do so if Bacteroides (Fibrobacter) succinogenes is present to provide the initial release of cellobiose from celluloseReference Schelfinger and Wolin94, Reference Russell95. Cellulolytic bacteria probably remain in greater numbers in the rumen, adhering as they do to feed particlesReference Miron, Ben Ghadalia and Morrison96. Even though much of the particulate matter passes relatively quickly out of the rumen, some remains for extended periods. Bass & DuganzichReference Bass and Duganzich30 indicated that 2·7 kg DM compared with an original 7·6 kg remained in the stomach after a 48 h fast. Even allowing for digesta in the omasum and abomasum, and minerals plus microbial mass in the rumen, most of this would undoubtedly have been plant material in the rumen. After a 48 h fast, methane production is reduced to about 10 % of pre-fasting levels but does not completely ceaseReference Blaxter and Graham16, Reference Cole and Hutcheson97. Warner & StacyReference Warner and Stacy33 observed significant VFA presence (concentrations of 10–20 mm) in two sheep fasted for 68 h. Even though, under the slightly alkaline conditions pertaining in the rumen, the rate of absorption of VFA would be slower than under more acid conditionsReference Barcroft, McAnally and Phillipson98, the maintenance of even low levels of VFA in the rumen indicates continuing fermentation.
When feed is again offered after a fast, several days generally elapse before feed intake returns to pre-fast levels or, where a change of diet has occurred, reaches its maximum level. The extent to which rumen microbes are involved in low initial feed intakes is not clear. Meiske et al. Reference Meiske, Salsbury, Hoefer and Luecke93 observed a decline in fermentation rate in vitro to about one-third of pre-fast levels as a consequence of fasting. By contrast, Fluharty et al. Reference Fluharty, Loerch and Dehority99 showed that, although feed intake on arrival in a feedlot was only 61 % of that 7 d later, in situ disappearance of feed from fibre bags in the rumen was 59 % compared with 58 % pre-fasting. The discrepancy may perhaps be explained by the limitations of the two techniques in monitoring rumen microbial activity. The in vitro technique exposes a relatively large amount of feed to a relatively small amount of rumen liquor, whereas the in situ technique exposes a relatively small amount of feed to theoretically all the microbes in the rumen. It is possible that rumen microbial activity is not the first factor limiting feed intake because ColeReference Cole100 obtained no improvement in feed intake following replacement of rumen contents from fasted sheep with those from unfasted sheep.
Even though feed intake returns to pre-fasting levels, the composition of the microbial population may have undergone changes. Blaxter & GrahamReference Blaxter and Graham16 (Table 1) observed that, although carbon dioxide output returned to pre-fast levels within 9 d of re-feeding, methane production was resumed only slowly and, in fact, even after 12 d had reached only 92 % of pre-fast levels.
As will be discussed later, it is desirable for the animal transported to lairage before slaughter to resume feeding as an aid to controlling enteropathogenic bacteria in the digestive tract and to replenish the glycogen reserves in muscle. Research is needed to determine whether experience pre-FWD with the type of diet to be offered in lairage would improve the rate at which the transported animals achieve a desired level of feed intake. In sheep in particular, acclimatisation is preferably at the plant species level, since experience of a grass species accelerates the acclimatisation of ewes and their lambs to that speciesReference Phillips and Youssef101. There may be a need to specifically formulate lairage feeds to match those previously fed on the farms, or even to transport a small amount of feed with the stock.
Enteropathogenic bacteria
Ruminants are exposed to such pathogenic bacteria as Clostridium spp., Salmonella spp. and various strains of Escherichia coli. The normal population of rumen microbes seems to be able to exert some control over numbers of pathogenic bacteria that enter the rumen. Bullen et al. Reference Bullen, Scarisbrick and Maddock102 observed the more rapid disappearance of Clostridium welchii than of a marker designed to be approximately identical to C. welchii cells in size, specific gravity and electrical charge. Salmonella spp., when introduced into the rumen of cattle fed at above maintenance level on lucerne hay, were rapidly eliminated from the rumen and few viable cells appeared in the faeces. However, when feed supply was reduced or interrupted for 1 or more days, the capacity of the animal to control the pathogens was greatly reducedReference Brownlie and Grau103. Fasting permitted the establishment of Salmonella in the intestine.
In recent times, emphasis has shifted from Salmonella to E. coli and especially to its Shiga toxin-producing serotype O157:H7. As Brownlie & GrauReference Brownlie and Grau103 found with Salmonella, the withdrawal of feed for 6 to 48 h leads to an increase in E. coli numbersReference Callaway, Elder, Keen, Anderson and Nisbet104, Reference Vanselow, Krause and McSweeney105. The mechanisms by which normal rumen bacteria control pathogens may be related to toxicity of VFA, especially at acid pH levelsReference Wallace, Falconer and Bhargava106. Diet influences the numbers of E. coli in the digestive tract, with lower pathogen numbers in animals fed roughages than in those fed concentratesReference Diez-Gonzales, Callaway, Kizoulis and Russell107. The estimation of Shiga toxin-producing serotypes has been made possible by the identification of the Shiga toxin genes stx1 and stx2 and the accessory virulence factors eaeA and ehxA. Gilbert et al. Reference Gilbert, Tomkins, Padmanabha, Gough, Krause and McSweeney108 have shown clearly that diets containing large proportions of starch support greater populations of serotype O157:H7. Presumably the availability of starch provides some nutritional advantages to the pathogens. However, such diets would probably sustain higher levels of VFA or even lactic acid and bring about lower pH values than roughage-fed animalsReference Briggs, Hogan and Reid109, so enhanced E. coli numbers are a little surprising. A theory that does not seem to have been investigated involves competition for substrate between normal rumen bacteria and pathogenic bacteria. Concentrate diets containing starch or molasses would provide readily available energy sources for the pathogens, whereas with roughage diets when the small amount of readily fermentable carbohydrate was exhausted, the pathogens would have to compete with the non-cellulolytic rumen microbes for limited energy sources, such as cellobiose. Survival in that situation would depend on the relative abilities of pathogenic and non-pathogenic bacteria to acquire nutrients present in low concentration. The situation would be different with diets containing much higher levels of starch. Such substrates would support the generation of a large population of pathogenic bacteria while creating conditions of low pH and high acidity inimical to their survival. Even though mortality rates might be high, the numbers of pathogens surviving could represent a relatively fixed proportion of a bigger population.
In the work of Gregory et al. Reference Gregory, Jacobson, Nagle, Muirhead and Leroux110, the numbers of E. coli (log 10/g) in rectal contents of cattle moved from pasture were: 3·7 when kept for 48 h on hay; 5·0 when sampled directly from pasture; 6·6 when fasted for 24 h. Rumen pH was not greatly affected by treatment. The stocking density of cattle on pasture was very high, 40/ha, and it seems likely that hay feeding increased total DM intake, which probably increased the concentration of micro-organisms in the rumen. Although Gregory et al. Reference Gregory, Jacobson, Nagle, Muirhead and Leroux110 recommend that animals at pasture should be fed on hay for a few days before transportation to reduce E. coli numbers, it seems most likely that the effect was due to increased intake. Adequate feeding of digestible fibre pre-transportation is probably more important than feeding hay per se. Hay feeding was also recommended by Callaway et al. Reference Callaway, Elder, Keen, Anderson and Nisbet104 and Vanselow et al. Reference Vanselow, Krause and McSweeney105 regarding cattle held in feedlots, but Jacobson et al. Reference Jacobson, Nagle, Gregory, Bell, Le Roux and Haines111 acknowledge that, to be effective, the hay or silage offered had to be of high nutritional value. In a review of forage supplementation of grazing cattle, PhillipsReference Phillips112 concluded that if there is adequate high-quality pasture available, little or no hay or other conserved forage will be consumed. Also, if the cattle are unused to consuming hay, it may take as many as 3 d for pasture-fed cattle to commence eating itReference Chapple and Wodzicka-Tomaszewska113. Thus, there would seem to be a need to familiarise grazing animals to hays or silages some time before final removal from pasture. A nutrition-based health approach is attracting increasing attention following the widespread slaughter of animals that followed the outbreaks of BSE and foot and mouth disease in England. In particular, achieving a rapid digestion and absorption of nutrients is important, as this reduces the amount of substrate remaining in the gastrointestinal tract which could be used by pathogenic micro-organismsReference Adams114.
Meat quality
As HarperReference Harper115 indicated, meat quality is largely judged by the perception of tenderness developed by the consumer in the brief period that meat spends between the jaws. Tenderness is to some extent genetically controlled but can also be affected by age, sex, conditions of growth and in particular by pre- and post-slaughter treatment. Critical to the maintenance of tenderness of muscle is the attainment, post-mortem, of pH levels below 5·7 by the anaerobic conversion of glycogen to lactic acidReference Shorthose116. Any pre-slaughter treatment of animals that reduces muscle glycogen mitigates the attainment of a desired level of acidity in muscle post-mortem and hence threatens tenderness. The treatments that produce the stressors previously discussed include removal from familiar to novel surroundings, curfew, mixing with unfamiliar companions with associated agonistic behaviour, loading and unloading into and from vehicles and transportation. There is subsequently a repetition of many of these stressors associated with transfer to lairage. Of these, the stressors eliciting the greatest cortisol responses probably arise during handling and unloadingReference Eicher117. In reviewing responses to such stressors, Ferguson et al. Reference Ferguson, Bruce, Thompson, Egan, Perry and Shorthose6 have indicated the involvement of the two neuroendocrine systems, the sympatho–adrenalmedullary and the hypothalamic–pituitary–adrenal axes. The first of these operates in situations of acute stress such as in fighting and is marked by the release of catecholamines, such as adrenaline. The second, more involved with continuing stress, affects the release of glucocorticoids, especially cortisol from the adrenal cortex. Adrenaline increases the rate of breakdown of glycogen and also accelerates the rate of utilisation of glucose, thereby affecting the supply of precursor for the subsequent recovery of muscle glycogen. The effect of adrenaline release pre-slaughter with the conversion of glucose to lactic acid is to produce meat that is bright red in colour, of low pH and generally toughReference Grandin118. Cortisol increases glycogenolysis and induces protein catabolism to release amino acids, some of which serve as precursors for gluconeogenesis. Increased protein catabolism would be consistent with elevated levels of urea in plasmaReference Knowles, Brown, Warriss, Phillips, Dolan, Hunt, Ford, Edwards and Watkins8 and presumably with the enhanced excretion of N in urine and faeces in response to transport mentioned previouslyReference Cole, Phillips and Hutcheson17. As Ferguson et al. Reference Ferguson, Bruce, Thompson, Egan, Perry and Shorthose6 indicated, meat produced following glycogen depletion through elevated levels of cortisol tends to be dark red in colour, dry and undesirably tough, especially if showing an ultimate pH above 5·9. Such meat is referred to as dark, firm and dry.
Animals finish a period of transport and curfew showing the effects, not only of depleted glycogen reserves, but also of dehydration. Loss of 68 kg or 10 % of body weight in transported bullocks was reduced to 6 % of body weight following 3·5 h access to water and stabilised at 6·8–7·3 % with 28 and 32 h accessReference Wythes, McLennan and Toleman9. Rehydration increased the water content of muscle from 76 to 78 % and increased hot carcass weight from 369 to 383 kg. Recovery of depleted glycogen reserves may be a slower process. Data collated from various sources by Ferguson et al. Reference Ferguson, Bruce, Thompson, Egan, Perry and Shorthose6 suggest that dark, firm and dry meat is produced when muscle glycogen falls from a normal level of 60–120 μmol/g to less than 40–57 μmol/g. The repletion rate will probably range between 0·1 and 1·0 μmol/g per h, even with access to feed. Hence, depending on the extent of glycogen depletion, a substantial period of rest with access to water and feed of reasonable nutritional value may be needed before slaughter if dark, firm and dry meat is to be avoided. Cattle offered poor-quality hay with molasses poured over it showed little desire to eat and no lower ultimate muscle pH after 96 h rest than after 48 hReference Shorthose, Harris and Bouton119. Further information is needed on the genetic or nutritional factors controlling the levels of muscle glycogen pre-FWD, the rates of depletion of glycogen during FWD and transportation, and the repletion of glycogen during rest periods in lairage.
Recovery from feed and water deprivation
Recovery from FWD is often judged by the rate of return of live weight to pre-FWD levels. However, reliance on this criterion is complicated by the fact that the relocation of an animal from one environment to another, for example, from farm to feedlot, or from mature pasture to immature pasture may lead to substantial reductions in the weight of rumen contents. In such situations, live-weight change is of limited value as a predictor of recovery from FWD. A better measure of recovery is the comparison of daily feed intake from the start of re-feeding with that 7 d later, as adopted by Fluharty et al. Reference Fluharty, Loerch and Dehority99.
As indicated earlier in the present review, the animal needs to recover from the fatigue of transportation and to adjust to its new surroundings, companions, feed and, in some situations, drinking waterReference Petherick, Holroyd and Swain120. Internally it needs to rehydrate tissues, to rebuild its microbial populations in the rumen and large intestine, to restore any losses of electrolytes and of enzymes in the liver and muscle and to ensure the restoration of kidney function. Research into the possible role of electrolyte therapy in the process of recovery has been reviewed by Schaefer et al. Reference Schaefer, Jones and Stanley121, but they did not claim any practical application for the practice. The rate of recovery of the animal will largely depend on the quality and quantity of feed offered. It is a common observation that, when sheep or cattle are presented with a change of diet, there will be a delay of some days before adjustment to the new diet is complete. Information currently available is insufficient to establish the extent to which this delay will differ between animals changed directly to another diet compared with those changed after a fast or changed after undergoing fasting plus the stressors inherent in transportation. This information would provide a useful basis for developing better rations for sheep and cattle to overcome problems with enterotoxic bacteria, to improve meat quality by restoring muscle glycogen levels and to enhance the efficiency of conversion of feed into live-weight gain.
Conclusions and recommendations for future research
There is a lack of robust science relating to the impacts of FWD on different species and classes of livestock. However, it is clear that deprivation of food for about 1 d appears to provide an environment in the gastrointestinal tract that promotes the survival, and even enhances the proliferation, of pathogens such as Salmonella spp. and E. coli. A combination of these pathogens and other stress responses may negatively impact on immunocompetence, and trigger disease, such as shipping fever.
There is limited evidence that some individuals, stock species and classes will tolerate FWD better than others. In particular, it appears that sheep are more tolerant of water deprivation than are cattle. It may be possible to identify individuals that are better able to cope. Stock with low digesta loads are likely to be particularly susceptible to FWD. This includes young animals, deer in winter, stock that are first subjected to FWD early in the morning and undernourished stock, and possibly animals that have been selected for rapid growth rates and high feed efficiency. It is emphasised, however, that few comparative studies have been conducted.
From an economic perspective, FWD contributes to dehydration which results in lower carcass weights. FWD of more than 24 h also appears to severely negatively impact on the length of time that it takes cattle entering feedlots to regain live weight. The recommendation of any maximum curfew time will depend on the subsequent time off food and water during transport and subsequent lairage or yarding. Hence a maximum time off food and water is preferable to any specific recommendations on curfew time. Provision of food and water at points of unloading or at their ultimate destination will therefore affect curfew times. Based on the potential for enteropathogen growth and the potential for an increase in stress to the animal, it appears prudent to ensure that total time off food and/or water does not exceed 24 h.
There is a need to establish ways in which the timing of supply of water and feed can contribute to pathogen control. Feeding low-quality forage before transportation will increase digesta load and reduce enteropathogens, and is beneficial for the production of drier faeces, thereby reducing hide and fleece contamination, and nutrient supply to tissues, which may have implications for meat quality.
Given the limited scientific literature in relation to the impact of FWD and the potential for major human and animal health and welfare impacts, further research should be undertaken with a high priority. Research should be multidisciplinary to take into account the many interacting factors. In particular, quantification of the risk of carcass contamination with different levels of gastrointestinal contents should be a high priority, including an assessment of enteropathogen growth. It is particularly important to examine whether livestock with low-digestibility diets can tolerate FWD more than livestock with high-digestibility diets. It is also necessary to determine the effects of feed type before FWD on digesta load and release of nutrients into the rumen and caecum with different classes and species of stock. The impact on survival of pathogenic bacteria will be particularly important. Both adult and juvenile animals should be investigated, as the former may be more resistant to novel foods introduced shortly before curfews. Further research is required to determine how FWD affects normal rumen transfer of water and electrolytes, whether it is affected by drinking, whether changes in plasma osmotic pressure during FWD are physiologically significant and what can be done to inhibit or prevent losses in carcass weight through dehydration and respiration. Increased N excretion in faeces during curfews is of unknown origin. It could derive from microbial mortality or elimination of liver and muscle enzymes as substrate diminishes. Either could limit feed intake on resumption of access to normal food supplies, which is of economic significance. Finally, the possibility of developing a vaccine to the relevant E. coli and Salmonella strains could be explored, although the practical difficulties with application may outweigh the benefits.
Acknowledgements
The authors are grateful to Meat and Livestock, Australia, for commissioning the present review.






