Fe deficiency is the most widespread micronutrient deficiency that affects both developing and developed countries( Reference Lopez, Cacoub and Macdougall 1 ). The aetiology of Fe deficiency is complex. However, in low and middle income countries, suboptimal intake of bioavailable forms of Fe is often assumed to be the cause of Fe deficiency( Reference Camaschella 2 ). This assumption is based on the observation that a significant proportion of population in developing countries rely on a predominantly plant-based diet. Besides, the bioavailability of Fe taken from such plant-based foods is compromised by the high content of absorption inhibitors like phytate and polyphenols( Reference Baye, Guyot and Icard-Vernière 3 , Reference Hurrell and Egli 4 ). Consequently, interventions that provide Fe through fortification or supplementation have been promoted( Reference Schauer and Zlotkin 5 , 6 ).
Surprisingly, Fe intake in Ethiopia is consistently found high, even among young children whose physiological needs are high( Reference Baye, Guyot and Icard-Verniere 7 , Reference Abebe, Haki and Baye 8 ). This higher intake occurs despite the reliance on a predominantly plant-based diet. One study has hypothesised that this could probably result from excessive contamination of grains with soil Fe( Reference Abebe, Bogale and Hambidge 9 ). As grains are not washed before processing, additional soil Fe is consumed by humans. Indeed, studies that have carefully washed cereal grains consumed in Ethiopia are highly contaminated with soil( Reference Baye, Mouquet‐Rivier and Icard‐Vernière 10 ). A particular process that is associated with high levels of soil contamination is the threshing of cereals, which in Ethiopia is traditionally done with oxen walking on the grains. This practice favours contamination and particularly leads to extreme contamination of tiny cereals like teff. Smaller grains have larger surface area of contact with soil and thus, are more susceptible to contamination( Reference Abebe, Bogale and Hambidge 9 , Reference Baye, Mouquet‐Rivier and Icard‐Vernière 10 ). Consequently, Fe values as high as >150 mg/100 g were reported for teff( Reference Abebe, Bogale and Hambidge 9 ). Subsequent contamination with soil during open drying of grains or with metallic Fe screw-wares during milling have also been reported( Reference Icard-Vernière, Hama and Guyot 11 , Reference Greffeuille, Kayodé and Icard-Vernière 12 ).
Earlier studies have illustrated that Fe from cooking pots (i.e. metallic) could contribute to improving Fe status( Reference Adish, Esrey and Gyorkos 13 , Reference Geerligs, Brabin and Omari 14 ). However, little is known of the biological utility of the additional Fe coming from soil contamination. This, unfortunately, is complicating the design and implementation of fortification programmes in Ethiopia and other African countries where contamination with soil is prevalent. For example, before implementing a fortification programme, a careful determination of the Fe intake of the population is required to set the dosage that will avoid both inadequate and excessive intakes( Reference Gibbs, Carriquiry and Capanzana 15 , Reference Allen, De Benoist and Dary 16 ). Such activities in Ethiopia have shown that excessive Fe intake is common, and fortification scenarios with Fe can substantially increase the proportion of the population at risk of excessive Fe intakes and potential toxicity( Reference Abebe, Haki and Baye 8 ). This practice highlights the need to have a better understanding of the bioavailability of this contaminant soil Fe to inform the planned fortification programme.
Commonly used bioavailability prediction equations and molar ratio are not very helpful as intrinsic and not extrinsic soil Fe was used when developing and validating them( Reference Baye, Mouquet‐Rivier and Icard‐Vernière 10 ). The rather poor exchangeability of soil Fe is also likely to limit the use of the gold-standard stable isotope method. However, sequential extraction, a common in vitro method used in Soil Sciences( Reference Tessier, Campbell and Bisson 17 ), can have the potential to indicate the mobility; and hence, the exchangeability of both intrinsic and extrinsic (soil) forms of Fe. This could further be complemented with a comparison of the bioavailability of contaminated and uncontaminated grains using the standard rat Hb depletion–repletion assay( Reference Forbes, Arnaud and Chichester 18 ), which is an in vivo method recommended for predicting Fe bioavailability in humans.
Therefore, the present study aimed to investigate the bioavailability of laboratory-threshed (clean) and field-threshed (soil contaminated) teff (Eragrostisis tef (Zucc) Trotter) to investigate the relative bioavailability of intrinsic and extrinsic (soil) Fe in teff.
Methods
Teff samples preparation
Red teff (E. tef (Zucc) Trotter) samples were collected from Adulala, Zekuwala Abo district, an area known for its teff production. The harvested teff was divided into two. The first was threshed manually in the laboratory (laboratory threshed), taking precautionary measures to prevent any adventitious contamination. The second was threshed in the field following the traditional practice of oxen walking on the teff. The grains were milled using stainless steel standard laboratory miller (FW100; Ohaus).
Iron content of the teff samples
Total Fe content in laboratory- and field-threshed teff samples was determined using flame atomic absorption spectrometry after dry ashing (FAAS – Perkin Elmer AAnalyst 800; Bodenseewerk PerkinElmer)( Reference Jorhem 19 ). Fe speciation into different fractions was done following the Tessier et al.( Reference Tessier, Campbell and Bisson 17 ) sequential extraction method as described in Simpson et al.( Reference Simpson, Sidhar and Peters 20 ). In brief, 1 g of teff flour was subject to a five-step solvent extraction. At each extraction step, the fraction was the supernatant obtained after 30 min centrifugation combined with a wash with deionized water (8 ml). The residue was then subjected to the next solvent extraction. The five fractions, in their order of application, were as follows.
Exchangeable
Soluble fraction in 8 ml of 1 m-magnesium chloride after shaking for 1 h at room temperature
Carbonate-bound (acid soluble)
Soluble fraction in 8 ml of 1 m-sodium acetate (pH 5·0) after shaking for 4 h at room temperature
Oxide-bound (reducible iron)
Soluble fraction in 20 ml of 0·04 m-hydroxylamine hydrogen chloride in 1 m-acetic acid; obtained after shaking for 24 h at room temperature
Organic bound (oxidisable iron)
Soluble fraction obtained after treating with: First, 3 ml of 0·02 m-nitric acid and 5 ml of 30 % H2O2 (pH 2·0 adjusted with nitric acid) with 3 h of incubation at 85°C with shaking in water bath; the mixture is then cooled, 5 ml of 3·2 m-ammonium acetate (in 20 % nitric acid) solution is added, followed by a dilution with 20 ml of deionised water and incubation at room temperature (with shaking) for 30 min.
Residual
The difference between the total concentration and the sum of the preceding fractions
Hb depletion–repletion rat assay
In vivo bioavailability of the laboratory- and field-threshed (soil contaminated) teff was investigated using the standard rat Hb depletion–repletion assay referred to as Hb regeneration efficiency (HRE) assay( Reference Forbes, Arnaud and Chichester 18 ). In brief, after 3 d of acclimatisation to individual cages and the diets, weanling rats were fed low Fe AIN-93G diets for 21 d to induce Fe-deficiency anaemia (Hb<60 g/l; depletion phase). This phase followed by a 14-d repletion phase, in which rats were randomly assigned (n 8/group) to one of three diets (treatments/control): laboratory-threshed teff, field-threshed teff and FeSO4 (control), all formulated to provide 35 mg Fe/kg based on the intrinsic Fe content of the teff. However, the field-threshed teff sample provided an additional 120 mg/kg diet of extrinsic (soil) Fe. Throughout the study, rats were provided with deionised water, ad libitum. Fig. 1 illustrates the experimental design followed.
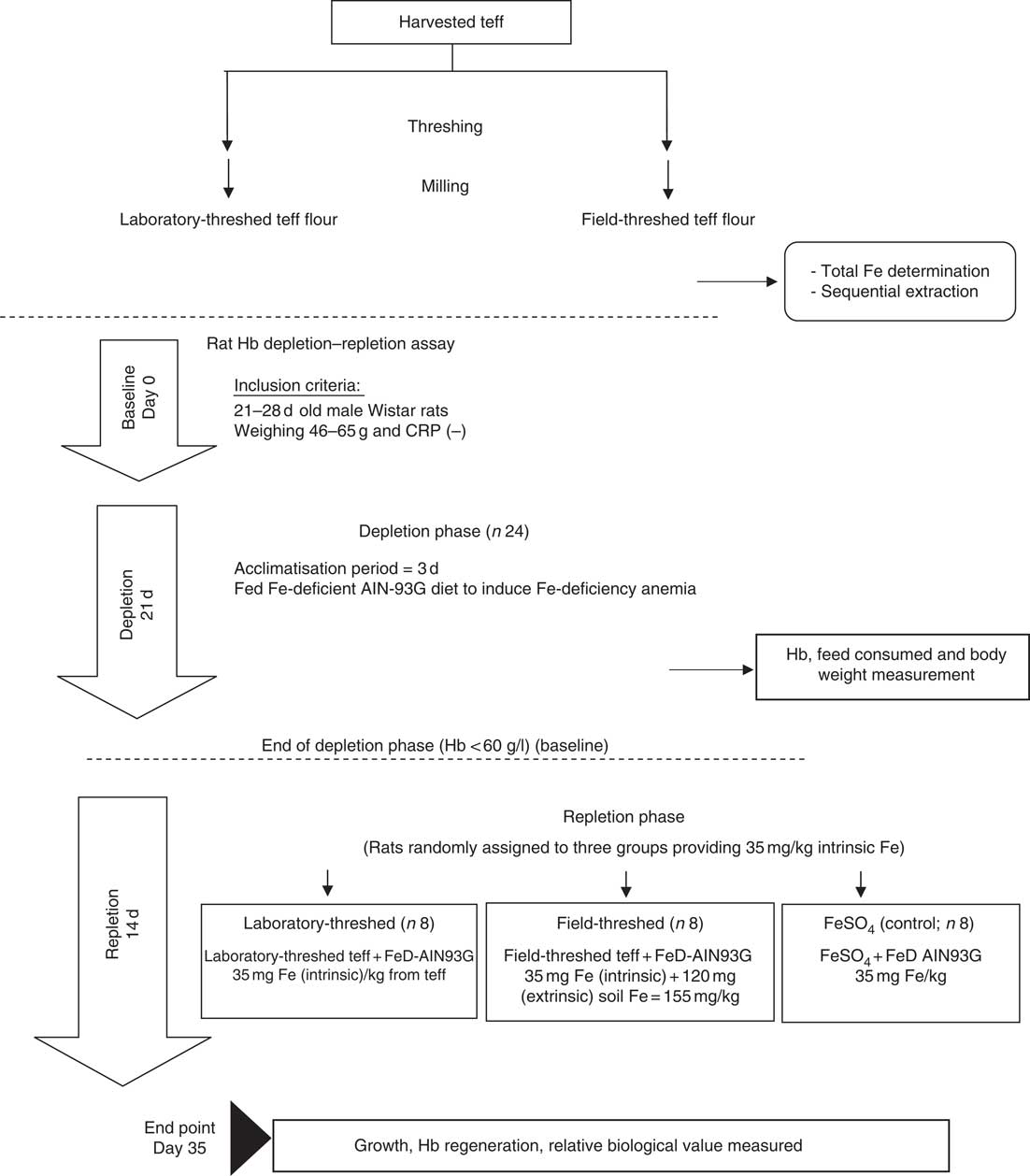
Fig. 1 Experimental design of the study. CRP, C-reactive protein; AIN-93G, American Institute of Nutrition formula for growing rats; FeD, iron-deficient diet.
Animals and diets
Weanling (21–28 d) twenty-four male Wistar rats (Rattus norvegicus, albinus variety, Rodent class), weighing 45–65 g, were housed individually in plastic cages at room temperature (22±2°C) with 12 h light–12 h dark cycles. For inclusion at baseline, rats were screened for markers of inflammation using a qualitative C-reactive protein (CRP) test (lot: 24108; Linear Chemicals SL). Rats with positive CRP results indicating the presence of inflammation/infection were excluded.
Diets conforming to AIN-93G purified diets( Reference Reeves, Nielsen and Fahey 21 ) without Fe, but with all other minerals, soyabean oil, and vitamins were obtained from Dyets Inc. For the repletion phase, teff flours were mixed, in our laboratory, with the Fe-free AIN-93 G diets to provide 35 mg Fe/kg diet (Table 1). This was achieved by mixing 526·3 g (laboratory/field-threshed) teff with 473·7 Fe-free AIN-93G diet. Three samples were randomly picked from the prepared batch and Fe was analysed to check homogeneity of mixing. The CV was <5%. Vitamin and mineral contents of the mixes (groups) were made similar to the FeSO4 diet by adding vitamin (AIN-93VX; Harlan Laboratories) and mineral mixes (LLC; catalogue no. 960400, lot no. 9634; MP Biomedicals). Total energy of the diets were also adjusted to make the diets isoenergetic by adding additional amount of soyabean oil. The diets were uniformly pelletised into a cylindrical shape (diameter 9 mm; length 6 cm), were dried in an oven at 50°C and stored in the refrigerator (4°C) until feeding( Reference Reeves, Nielsen and Fahey 21 ).
Table 1 Composition of experimental diets (/kg)
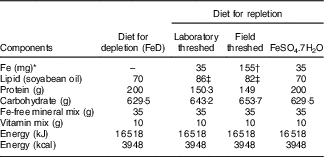
FeD, Fe-deficient; laboratory- and field-threshed teff were mixed with AIN-93G (American Institute of Nutrition formula for growing rats) in the formulations.
* Fe formulations are based on analysed values.
† The field-threshed teff provided 155 mg Fe/kg=35 mg intrinsic+120 mg extrinsic (soil) Fe/kg of diet.
‡ Soyabean oil was added to keep formulations isoenergetic.
Rat weight gain, iron intake and Hb measurements
Body weight was measured weekly, whereas feed intake was monitored daily during the repletion phase. Feed intake was then converted to Fe intake by using the Fe composition of the diets obtained from our analyses. Blood samples were taken at baseline, after depletion and repletion periods by tail vein incision( Reference Fluttert, Dalm and Oitzl 22 ). Hb concentrations were immediately measured in duplicate using Hemocue Hb 301 (HemoCue). The HemoCue machine was calibrated against a standard HMX Hematology analyzer (Beckman Coulter) using known standards of Hb (Coulter 5C cell control-7547117) before use.
Hb regeneration efficiency and relative biological value
Based on the measured Fe (intrinsic) intake and Hb concentrations, the HRE was calculated as follows by assuming that 6·7 % of rat’s body weight is blood and that Hb contains 3·35 mg of Fe/g of Hb( Reference Whittaker, Mahoney and Hendricks 23 ):
where:
 $$\eqalignno{&{\rm Hb}\,{\rm Fe}\left( {{\rm Initial}} \right)\,{\equals} \cr \quad {{\left( {{\rm initial}\,{\rm body}\,{\rm weight}\left( {\rm g} \right){\times}{\rm initial}\,{\rm Hb}\left( {{\rm g}/{\rm l}} \right){\times}6 \! \cdot \! 7{\rm }{\times}{\rm }0 \! \cdot \! 335} \right)} \over {1000}}.$$
$$\eqalignno{&{\rm Hb}\,{\rm Fe}\left( {{\rm Initial}} \right)\,{\equals} \cr \quad {{\left( {{\rm initial}\,{\rm body}\,{\rm weight}\left( {\rm g} \right){\times}{\rm initial}\,{\rm Hb}\left( {{\rm g}/{\rm l}} \right){\times}6 \! \cdot \! 7{\rm }{\times}{\rm }0 \! \cdot \! 335} \right)} \over {1000}}.$$
 $$\eqalignno{\quad{\rm Hb}\,{\rm Fe}\left( {{\rm Final}} \right){\equals} \qquad \qquad \qquad \qquad \qquad \qquad \qquad \qquad \qquad \cr \! {{ \left( {{\rm final}\,{\rm body}\,{\rm weight}\left( {\rm g} \right){\times}{\rm final}\,{\rm Hb}\left( {{\rm g}/{\rm l}} \right){\times}6 \! \cdot \! 7{\rm }{\times}{\rm }0 \! \cdot \! 335} \right)} \over {1000}}.$$
$$\eqalignno{\quad{\rm Hb}\,{\rm Fe}\left( {{\rm Final}} \right){\equals} \qquad \qquad \qquad \qquad \qquad \qquad \qquad \qquad \qquad \cr \! {{ \left( {{\rm final}\,{\rm body}\,{\rm weight}\left( {\rm g} \right){\times}{\rm final}\,{\rm Hb}\left( {{\rm g}/{\rm l}} \right){\times}6 \! \cdot \! 7{\rm }{\times}{\rm }0 \! \cdot \! 335} \right)} \over {1000}}.$$
The relative bioavailability of Fe from laboratory- and field-threshed teff was calculated as the rate of Hb repletion relative to repletion rate of FeSO4:
The HRE assay requires inclusion of test and control Fe sources at levels that provide similar amount of Fe. Thus, HRE and relative biological values (RBV) are calculated based on the intrinsic Fe content of laboratory- and field-threshed teff (35 mg/kg diet). Nevertheless, it should be noted that the field-threshed teff provided additional Fe from extrinsic (soil) sources; hence, HRE (%) values could differ if total Fe (intrinsic+extrinsic) was used in the calculations. For ease of interpretation, calculations were made based on intrinsic Fe values, and differences in HRE and RBVs between laboratory- and field-threshed reflected the changes in Hb regeneration due to the consumption of additional extrinsic Fe (soil).
Ethical considerations
This study was approved by the Ethical Committee of the College of Natural and Computational Sciences of Addis Ababa University (CNSDO/678/06/14) and the animals were maintained in accordance with the National Research Council of National Academies Guide for the care and use of laboratory animals( 24 ).
Statistical analyses
Descriptive statistics were used and results are presented as mean and standard deviation. Independent-samples t test was used to compare the total Fe contents of laboratory- and field-threshed teff. One-way ANOVA, Duncan’s multiple comparison test was used to compare means of the three experimental groups. Differences in means were considered statistically significant for P values<0·05. Data were analysed using the software Statistical Package for the Social Sciences (SPSS), version 20.
Results
Total and fractional iron concentrations in teff flours
The total Fe content (/100 g) of the field-threshed teff flour (29·4 mg) was >4× than that of the laboratory-threshed flour (6·7 mg; Fig. 2). The laboratory- and field-threshed teff also exhibited differences in their fractional Fe profile (Fig. 3). Relative to the laboratory-threshed, the field-threshed teff flour had higher Fe concentrations in all the five fractions obtained from the sequential extraction. The first three fractions considered to be potentially bioavailable were 4–5× higher in the field than in the laboratory-threshed flour (P<0·05).
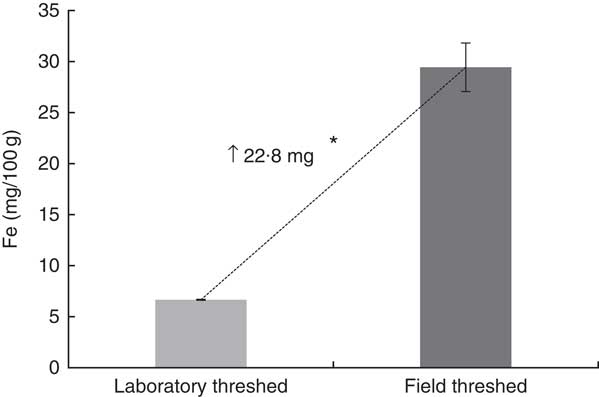
Fig. 2 Total iron of laboratory- and field-threshed teff. Iron was analysed after dry ashing using Atomic Absorption Spectrometry. Values are means of triplicate analyses of a single batch of laboratory- and field-threshed teff. The same teff sample, but threshed either in the laboratory or in the field was used. * Mean differences were statistically significant using independent (two-tailed) t test (P<0·05).
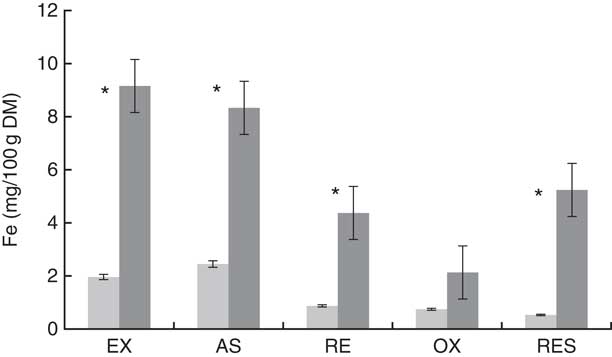
Fig 3 Iron fraction of laboratory-threshed (![]() ) and field-threshed teff (
) and field-threshed teff (![]() ) using sequential extraction. Values are means and standard deviation of triplicate values. Sequential extraction was performed following the modified Tessier et al.(
Reference Tessier, Campbell and Bisson
17
) method as described in Simpson et al.(
Reference Simpson, Sidhar and Peters
20
). EX, exchangeable; AS, acid soluble; RE, reducible; OX, oxidisable; RES, residual. * Differences between laboratory- and field-threshed teff are statistically significant (P<0·05), independent t test (two-way).
) using sequential extraction. Values are means and standard deviation of triplicate values. Sequential extraction was performed following the modified Tessier et al.(
Reference Tessier, Campbell and Bisson
17
) method as described in Simpson et al.(
Reference Simpson, Sidhar and Peters
20
). EX, exchangeable; AS, acid soluble; RE, reducible; OX, oxidisable; RES, residual. * Differences between laboratory- and field-threshed teff are statistically significant (P<0·05), independent t test (two-way).
Characteristics of the experimental rats after Hb depletion (baseline)
At the end of the depletion phase (21–28 d) all rats had a Hb concentration of≤60 g/l, and weighed 45–65 g. The mean Hb values and body weight were comparable across the three experimental groups (P>0·05), indicating effective randomisation (Table 2).
Table 2 Rats characteristics at baseline (end of depletion phase) (Mean values and standard deviations)

CRP, C-reactive protein.
a Mean values within a row with unlike superscript letters were significantly different (P<0·05).
Characteristics of the experimental rats after Hb repletion (endline)
There were no statistically significant differences in weight gain and feed efficiency ratio across the treatment groups (Table 3). The total feed and Fe intakes in the ferrous sulfate group was comparable with that of the field-threshed (intrinsic Fe), but was significantly higher than the laboratory-threshed group (P<0·05). Rats in the field-threshed teff group consumed additional 29 mg Fe from extrinsic sources.
Table 3 Feed intake, body weight and iron intake at the end of repletion (Mean values and standard deviations)
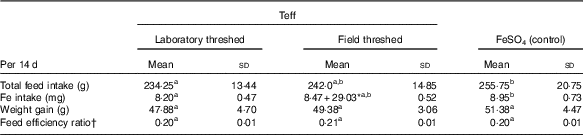
a,b Mean values within a row with unlike superscript letters were significantly different (P<0·05).
* The total Fe intake from field threshed was 37·47 mg, 8·47 from intrinsic and 29·03 from extrinsic (soil) sources.
† Feed efficiency ratio is calculated as weight gain divided by total feed intake.
Although baseline Hb concentrations were similar across the experimental groups, the ferrous sulfate group had the highest Hb gain (134 g/l), followed by the field-threshed (116 g/l) and laboratory-threshed teff (101 g/l) group. The HRE of the ferrous sulfate (control) and the field-threshed teff group were comparable and were significantly higher than the laboratory-threshed flour (Table 4). The RBV for the field-threshed teff (88 %) was significantly higher than that of the laboratory-threshed ones (68 %), suggesting that the 22·8 mg additional Fe coming from soil (extrinsic) contributed to a 21 % increase in RBV (Fig. 4).
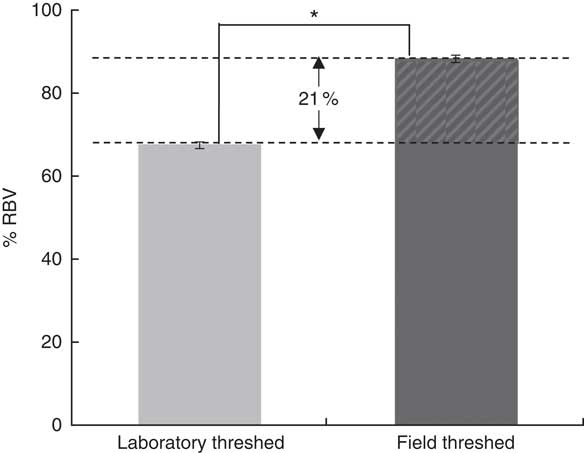
Fig. 4 Relative biological values (RBV) of laboratory- and field-threshed teff. * Statistically significant difference between means. The RBV are calculated based on Hb regeneration efficiency values accounting only for intrinsic iron concentrations; thus, the 20·8 % difference in RBV should be interpreted as the contribution of the 29 mg extrinsic iron.
Table 4 Hb-repletion of rats receiving 35 mg (/kg diet) intrinsic iron (laboratory threshed) and additional 120 mg/kg extrinsic iron (field threshed) (Mean values and standard deviations)
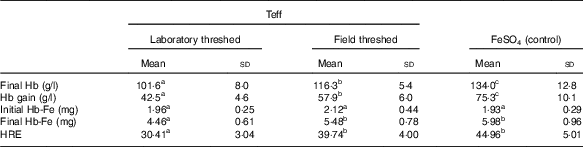
HRE, Hb regeneration efficiency, calculated using only intrinsic Fe intake from feed.
a,b Mean values within a row with unlike superscript letters were significantly different (P<0·05).
Discussion
Teff was a relatively good source of Fe, but a significant proportion of the Fe came from extrinsic contamination (soil Fe) that occurred mainly during the traditional threshing of the grain. This additional Fe coming from soil is shown to be mobile and potentially bioaccessible as reflected by the higher exchangeable, acid-soluble and reducible Fe fractions in the field- than in the laboratory-threshed teff. This was further confirmed by the rat Hb depletion–repletion assay that has shown higher HRE and RBV in the field- than in the laboratory-threshed teff flour.
Fe contamination is quite common in foods consumed in developing countries( Reference Abebe, Bogale and Hambidge 9 , Reference Harvey, Dexter and Darnton-Hill 25 ). However, the source and the bioavailability of this contaminant Fe is rarely investigated. For instance, Fe from screw-ware during milling (metallic), ground water (soluble) and soil Fe are not expected to be of similar bioavailability( Reference Icard-Vernière, Hama and Guyot 11 , Reference Greffeuille, Kayodé and Icard-Vernière 12 , Reference Karakochuk, Murphy and Whitfield 26 ). Studies have found high Fe contents in cereal grains from Africa that are partly attributable to contamination with soil Fe( Reference Abebe, Bogale and Hambidge 9 , Reference Gibson, Wawer and Fairweather-Tait 27 ). Indeed, the present study illustrates the extent to which traditional processing (i.e. threshing) can contribute to contamination of cereals like teff.
Because soil Fe is often found in the oxide form, it is often considered to be of low bioavailability( Reference Hooda, Henry and Seyoum 28 , Reference Lindsay and Schwab 29 ). However, in cases of high levels of contamination as is often the case with smaller cereals like teff, even if a small proportion of this extrinsic Fe is found bioavailable, it may have a non-negligible contribution to Fe status. Indeed, the Fe fractionation revealed significantly higher concentrations of exchangeable, acid-soluble (carbonate-bound) and reducible (oxide-bound) fractions in the field than in the laboratory-threshed teff sample. These fractions have been previously reported to predict the mobility, and hence the bioavailability of Fe in a rat model( Reference Simpson, Sidhar and Peters 20 ).
In the present study, the field-threshed teff flour did not only have a higher exchangeable fraction, but was also found to have a significantly higher HRE and RBV than the laboratory-threshed teff (rat assay). The additional 29 mg of extrinsic Fe intake from the field-threshed teff led to an increase of 21 % in RBV, suggesting that approximately 6 mg of this extrinsic Fe was bioavailable. Considering that adult humans require 1–2 mg of Fe/d to replenish their loss( Reference Camaschella 2 , Reference Abbaspour, Hurrell and Kelishadi 30 ), the seemingly low proportion of bioavailable Fe from soil cannot be neglected, especially in cases of high levels of contamination as seen in teff. This finding implies that more care is needed when using or generating food composition tables( Reference Gibson, Wawer and Fairweather-Tait 27 ). Food composition tables may not capture the variable Fe content that foods can have due to inadvertent soil contamination.
Recent studies have shown that other cereals like sorghum, barley, and wheat are also contaminated with soil to a varying extent( Reference Baye, Mouquet‐Rivier and Icard‐Vernière 10 ). As a result Fe intake estimates in Ethiopia have consistently shown very high Fe intakes, which seems to be confirmed by the relatively low Fe deficiency despite high-reliance on plant-based foods( Reference Abebe, Haki and Baye 8 , Reference Gashu, Stoecker and Adish 31 , Reference Abebe, Bogale and Hambidge 32 ). Indeed, a recent observation in Malawi suggested that depending on the soil type, soil may contribute to Fe status( Reference Gibson, Wawer and Fairweather-Tait 27 ). Altogether, these findings suggest that Fe contamination may contribute to Fe status. However, the present rat study cannot be directly extrapolated to humans, because of inherent differences in the digestive system of humans and rats. Clearly, human studies are needed to confirm the present findings. Although the rat Hb depletion–repletion assays have limitations in evaluating the effect of dietary factors promoting or inhibiting absorption, they have been successfully used to estimate the bioavailability of fortificants( Reference Zimmermann and Hilty 33 , Reference Hilty, Arnold and Hilbe 34 ), and thus relevant for the objective of the present study.
To our knowledge, this is the first study evaluating the HRE of contaminant soil Fe. The present findings clearly illustrate that extrinsic Fe from soil contamination can contribute to the pool of bioavailable Fe. This information can have implications to fortification or supplementation programmes in settings like Ethiopia, where soil contamination of foods is common. The contribution of soil to human Fe status needs to be investigated.
Acknowledgements
The authors acknowledge the support from Dyets Inc. for donating the Fe-free AIN-93G diet. Staffs of the Ethiopian Public Health Institute are also acknowledged for facilitating the access to their laboratory facilities.
This work would have not been possible without the support of the graduate programme of Addis Ababa University.
H. G. and K. B. designed the study and analysed and interpreted the data; H. G. performed that laboratory work; H. G. wrote the first draft, whereas K. B. critically reviewed the manuscript. Both authors have read and approved the final version of the manuscript.
The authors declare that there are no conflicts of interest.












