Antibody-mediated encephalitis (AME) is an inflammatory brain condition caused by antibodies directed against neuronal cell surface receptors. It can present sub-acutely with psychosis, seizures, memory and cognitive deficits, behavioral changes, and a decreased level of consciousness.Reference Dalmau and Graus1 This family of encephalitides encompasses a number of diagnostic subcategories, including limbic encephalitis (LE) associated with autoantibodies directed against neuronal cell surface antigens (e.g., alpha-amino-3-hydroxy-5-methyl-4-isoxazoepropionic acid receptor (AMPA-R)), inter-neuronal (cytoplasmic or nuclear), or neuronal antigens (e.g., Hu, Ri, Yo, Ma1/2). While the inflammatory changes of AME were traditionally thought to arise in response to a neoplasm, no neoplasm is found in substantial proportions of patients with AME.Reference Dalmau and Graus1,Reference Vincent, Buckley and Schott2 Rather, AME has been increasingly associated with triggers such as exposure to viral infections or individual-specific predisposition. Our case is an illustration of this latter category.
It is only in the last decade that antibodies against the GluA1 or GluA2 subunits of the AMPA-R have been associated with encephalitis.Reference Lai, Hughes and Peng3 This glutamate receptor is thought to be involved in learning and memory and also involved in seizure generation. Interestingly, a case outlining the association of anti-N-methyl-D-aspartate receptor (anti-NMDAR) encephalitis with systemic lupus erythematous (SLE) has recently been described.Reference Wu, Feng, Huang and Zhang4 In this case report, we discuss a case of seronegative SLE associated with AMPA-R antibody positive LE.
A 39-year-old female with a known history of seronegative rheumatoid arthritis and SLE was previously functioning independently at home. Her medications at the time included prednisone and plaquenil. She presented to the emergency department following a generalized tonic–clonic seizure with altered level of consciousness. Three months prior to her presentation, she had experienced worsening arthritis (the proximal interphalangeal joints and metacarpophalangeal joints), a maculopapular rash, palpitations, myalgias, and tremors. She had also noted weight loss, anorexia, and fever. Her tremors, palpitations, proximal weakness, and weight loss were thought to be due to her recent diagnosis of Graves’ disease (found to have elevated thyroid stimulating hormone receptor antibodies). Prior to her seizures, there was no history of neurological conditions or psychiatric abnormalities.
On admission, she appeared agitated and confused with a Glasgow coma score of 9/15 (E2V3M4). She had a temperature of 40°C and inspection yielded a diffuse maculopapular rash on the face and groin. She presented with opsoclonus, and proximal weakness in the upper and lower extremities. There was also an observed high-frequency irregular tremor. The remainder of her neurological exam including her cranial nerves, sensory exam, and cerebellar exam was unremarkable.
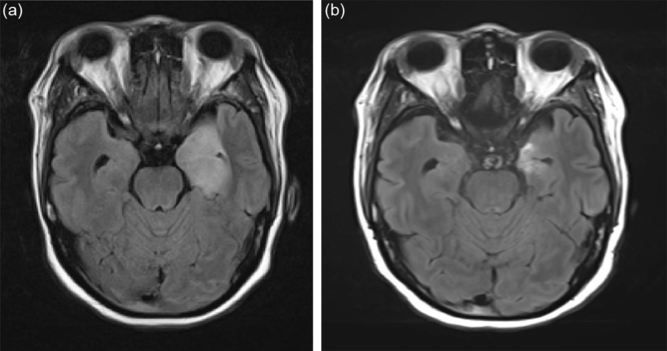
In the emergency department, the patient received ceftriaxone, vancomycin, ampicillin, and acyclovir as empiric treatment for infectious encephalitis. A magnetic resonance imaging (MRI) of the brain demonstrated abnormal hyperintense T2 signal of the medial left temporal lobe with involvement of the left hippocampus, fornix, and thalamus (Figure 1a and b). Lumbar puncture demonstrated a pleocytosis of 1687 × 106 cells/L, decreased glucose of 0.9 mmol/L, elevated protein of 2.55 g/L, and an elevated IgG index of 0.94 but with no oligoclonal banding. Cerebrospinal fluid gram stain, bacterial cultures, and viral panel were negative. In hospital, her thyroid scan demonstrated an enlarged thyroid gland in keeping with Grave’s disease and she was started on tapazole for a total of 3 days (discontinued due to neutropenia).

Figure 1: (a) Brain MRI (Fluid Attenuation Inversion Recovery Sequence) in a 39-year-old woman presenting with encephalopathy and fever. (b) Note the hyperintense signal involving the left mesial temporal lobe.
With antibiotic and antiviral treatment, the patient’s clinical status did not show any improvement. Antibiotics were discontinued and an electroencephalogram was performed, which demonstrated a background of moderate amplitude beta activity with polymorphic slow waves in the delta and theta frequency in the temporal regions of the brain bilaterally, with a predominance in the left temporal region.
A repeat lumbar puncture was completed and fluid was sent to the Mitogen laboratory for an investigation for antibodies, including NMDAR, AMPA-R, and antibodies associated with the voltage-gated potassium complex. Given the high clinical suspicion of autoimmune encephalitis, she was treated with a 5-day course of methylprednisolone 1 g and had five cycles of plasma exchange therapy (PLEX). She did show marked improvement in her clinical status following this treatment. Her antibodies eventually returned 3 weeks later and showed negative for NMDAR and VGKC antibodies, but showed a high positive for AMPA-R antibodies. She was eventually discharged with a Montreal Cognitive Assessment of 25/30 (points lost on visuospatial/executive, language, and delayed recall) and partial resolution of her proximal weakness. Repeat full-body computed tomography (CT) scans and positron emission tomography/CT scans have not demonstrated any malignancy. A transvaginal ultrasound did not demonstrate any pelvic masses. The patient was discharged 1 week after the completion of the PLEX treatment. She was eventually started on Rituximab (570 mg/m2 IV over two doses) which she received 7 days apart, and after 6 months, she received 645 mg/m2 IV divided over two doses. At last assessment 18 months following discharge, she is currently stable and has not suffered a relapse.
According to the 2016 consensus criteria, this patient met the diagnosis of definite AME.Reference Graus, Titulaer and Balu5 Presence of AMPA-R antibodies in patients with autoimmune encephalitis (AE) is specific for AMPA-R encephalitis as healthy individuals show low rates of seropositivity (<0.1%).Reference Dahm, Ott and Steiner6 The importance of early diagnosis cannot be understated. Overall, autoimmune AMPA-R encephalitis is responsive to treatment as compared to paraneoplastic anti-AMPA-R encephalitis associated with isolated AMPA-R antibodies. While frequent relapses are noted in this population, aggressive treatment regimens contribute to lower risk of relapses.Reference Hoftberger, van Sonderen and Leypoldt7
While our patient clearly had a predisposition to autoimmune disease (concurrent SLE and autoimmune thyroiditis), some researchers have hypothesized a genetic predisposition of AE. In particular, a link has been drawn between anti-leucine-rich glioma-inactivated 1 antibody encephalitis and human leukocyte antigen class II genes.Reference Dalmau and Graus1 However, the relationship between AME and SLE is still not clear and further research needs to be conducted to better understand the factors that promote formation of autoantibodies against neuronal cell surface antigens.
Disclosures
The authors have no conflicts of interest to declare.
Statement of Authorship
Dr. ZZ and Dr. CL drafted and revised manuscript. Dr. DA, Dr. GB, and Dr. ZAS provided clinical care for patient and revised manuscript