Mitochondrial encephalomyopathy, lactic acidosis and stroke-like episodes (MELAS) is a rare progressive maternally inherited mitochondrial disease that clinically harbours various neurological and systemic manifestations.Reference Testai and Gorelick1
We report the case of a 32-year-old female with a previously established diagnosis MELAS (confirmed A-to-G tRNA Leucine (UUR) point mutation at np-3243) who was admitted to the neurology ward in the context of new-onset global aphasia. Cerebral imaging revealed an acute right temporal stroke-like lesion that did not correspond to a specific vascular territory and that was confined to the cortex (Figure 1). Susceptibility-weighted imaging was normal. Head and neck vascular imaging did not reveal any abnormalities. The patient’s medications included l-arginine, Co-enzyme Q10, a thromboprophylaxis agent and no antithrombotic medication.
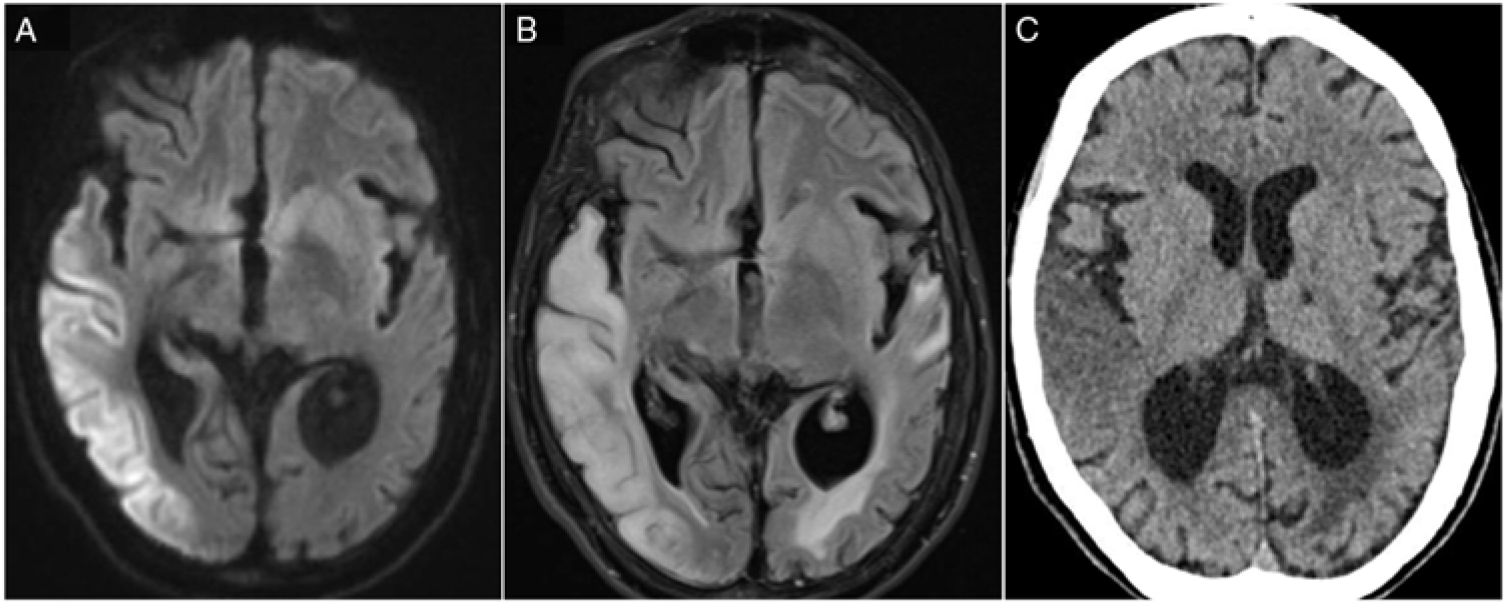
Figure 1: (A) DWI hyperintensity affecting right temporal, parietal and occipital cortices. (B) Corresponding T2-FLAIR hyperintense lesions with additional vasogenic oedema. (C) The same lesion on a CT scan.
Twelve days after her admission, her neurological condition deteriorated through marked confusion and gait problems. Brain CT revealed a new intraparenchymal haemorrhage (IPH) that occurred within the infarcted zone, suggesting haemorrhagic transformation (Figure 2). The patient died a few days after, being surrounded by family and with appropriate palliative care.
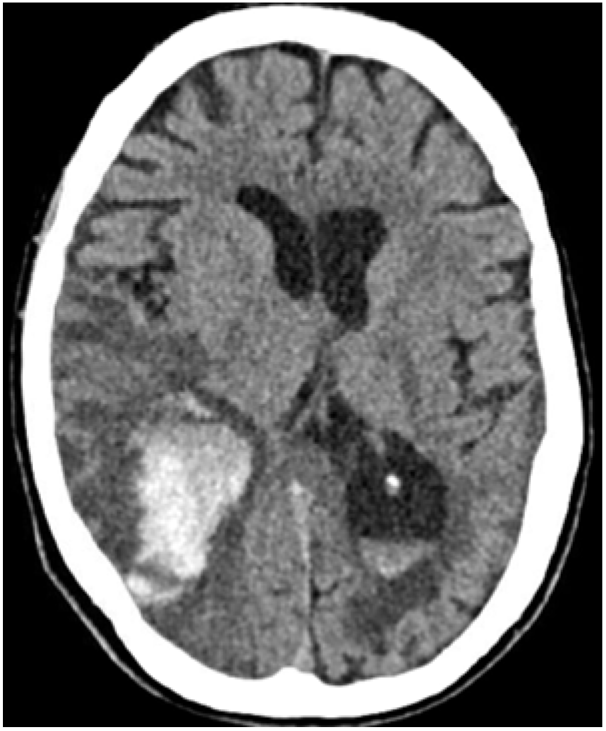
Figure 2: Haemorrhagic transformation within the stroke-like lesion with effacement of the ipsilateral occipital horn of lateral ventricle and intraventricular haemorrhage.
MELAS stroke-like events are characterized by cortical hyperintense diffusion-weighted imaging (DWI) associated with vasogenic oedema. They do not follow a defined arterial territory distribution and have a posterior predilection.Reference Yoneda, Maeda, Kimura, Fujii, Katayama and Kuriyama2 Unlike ischaemic stroke processes, cerebral perfusion has been shown to be preserved in imaging series.Reference Ito, Mori and Harada3 The precise cascade through which cellular damage occurs in stroke-like events is not fully elucidated but appears to be multifactorial. Oxidative phosphorylation failure due to mutation-induced mitochondrial protein translational defect leads to decreased oxygenation of cerebral tissue and eventual neuronal death.Reference Sano, Ishii, Momose, Uchigata and Senda4 Moreover, neuronal hyperexcitability was observed in the context of a progressive DWI hyperintensity and has been suggested to contribute to increasing cellular energy demands.Reference Iizuka, Sakai, Kan and Suzuki5 Finally, mitochondrial angiopathy has been pathologically demonstrated in MELASReference Ohama, Ohara, Ikuta, Tanaka, Nishizawa and Miyatake6,Reference Takahashi, Shimada and Murakami7 and is thought to contribute to vessel hyperaemia and impaired perfusion in the microvasculature.Reference El-Hattab, Adesina, Jones and Scaglia8
Given this distinct pathophysiology, stroke-like events should not be expected to have the same biological behaviour as typical acute ischaemic strokes, where strong disruption of blood–brain barrier and reperfusion injury are the leading mechanisms of haemorrhagic transformation. Whereas gyral laminar necrosis and microhaemorrhagic lesions several months after a stroke-like episode have been previously reported, Reference Iizuka, Sakai, Kan and Suzuki5,Reference Apostolova, White, Moore and Davis9 the case reported herein is the first description of haemorrhagic transformation of an acute stroke-like lesion in a MELAS with a m.3243A>G mitochondrial DNA mutation. Furthermore, a case of MELAS with MT-ND5 mutation was reported to display recurrent IPHs, with the last one being fatal.Reference Pénisson-Besnier, Reynier and Asfar10 Altogether, these elements suggest that MELAS stroke-like episodes may have a propensity to cerebral haemorrhages and that haemorrhagic transformation of stroke-like episodes may be underreported.
Statement of Authorship
MRB has done the required academic literature review for the conception of the article and drafted the article. MCC and MS have done a critical revision of the article and performed the final approval of the version to be published.
Disclosures
The authors have no conflicts of interest of declare.






