Worldwide government initiatives are strongly advocating an increase in dementia diagnoses to be made at earlier stages of the condition.1, 2 The National Institute for Health and Care Excellence (NICE) states ‘People should be told their diagnosis as clearly and honestly as possible. Without this knowledge, people cannot begin to make sense of what is happening, nor can they plan effectively for their future’.3 The Memory Services National Accreditation Programme publish standards for memory clinics, but do not address the communication of the diagnosis other than ‘the outcome of the assessment is communicated to all relevant parties in a timely manner’.Reference Hodge, Hailey, Colwill, Walker and Orrell4
Previous studies have demonstrated that dementia presents a special set of considerations in breaking diagnostic news.Reference Karnieli-Miller, Werner, Aharon-Peretz and Eidelman5 Early symptoms are often noticed by family or friends who present to the doctor on the patient's behalf.Reference Perry-Young, Owen, Kelly and Owens6 People with dementia may not acknowledge the extent of their difficulties and resist going to the memory clinic.Reference Cahill, Gibb, Bruce, Headon and Drury7 Most will have impaired short-term memory, attention and language processing and production.Reference Karnieli-Miller, Werner, Aharon-Peretz, Sinoff and Eidelman8 Currently in the UK, the clinician communicating the diagnosis will often be meeting the patient for the first time at diagnostic feedback and will have no pre-existing relationship to guide the conversation.Reference Bailey, Dooley and McCabe9 In the light of these complexities, the aim of this study was to microanalyse video recordings of diagnostic feedback consultations in memory clinics to describe how a diagnosis of dementia is communicated.
Method
Data collection
Data were video-recorded diagnostic feedback meetings collected through the National Institute for Health Research funded Shared Decision Making in Mild to Moderate Dementia (ShareD) study (PB-PG-1111-26063). Data collection took place in nine UK-based secondary care memory clinics in Devon (site A – a semi-rural and rural setting) and London (site B – an urban setting) from 2014 to 2015. The memory clinics followed the NICE pathway for dementia diagnosis,3 with specialist services performing brain scans, cognitive testing and patient histories before meeting as a multidisciplinary team. Doctors fed back the diagnosis to the patient and management was discussed. In site A, tests and feedback took place on the same day in a ‘one-stop shop’ clinic. In site B, the patient attended separate clinic visits for testing and diagnosis feedback.
All clinicians who delivered diagnoses in the participating memory clinics were approached. Consecutive sampling was used for patients. All patients attending the memory clinic for diagnosis feedback were eligible, except for patients needing interpreters because of the added complexity of the communication. Information sheets were sent with patient appointment letters, and researchers approached patients and their companions to obtain informed consent. Diagnostic feedback meetings were video recorded using Go Pro cameras. Camden and Islington Research Ethics Committee approved the study (13/LO/1309).
Data analysis
Data were analysed using conversation analysis. Conversation analysis is a method of microanalysing verbal and non-verbal communication to provide insight into what people say and how they say it. A transcription company transcribed the consultations verbatim. Sections related to the diagnosis were transcribed in detail for conversation analysis by the first author (57%) and a conversation analysis transcription company (43%).Reference Jefferson and Lerner10 Visual features such as gaze and posture were also analysed. This enabled a description of the structure of the diagnosis feedback meeting, as well as a detailed description of the practices doctors use to deliver dementia diagnoses. Independent sample t-tests were used where relevant to identify whether the use of different communicative strategies was linked with patient cognitive test scores.
The inclusion of data from different doctors in a variety of clinics, as well as comparison with studies of diagnosis deliveries in other settings, enhanced reliability.Reference Peräkylä and Silverman11 Validity was addressed through repeated analysis within and beyond the research team.Reference Sidnell and Stivers12 Findings were discussed with participating doctors. This did not change the results but aided the analysis by contextualising the communication practices within service structures and cultures.Reference Pomerantz and Rintel13
The conversation analysis transcripts presented have been simplified. The markers for prosody, stress and speed have been removed, leaving the markers for the overlapping speech (represented by square brackets) and length of silences (represented in seconds in brackets, with full stops representing pauses under 0.2 s).
Results
Participant characteristics
The consent rate for clinicians participating in ShareD was 88%. This data-set included 9 doctors from site A and 11 from site B (Table 1). There was a mean of four patients per doctor, ranging from one to nine. There were three doctors where only one patient was recruited. Of 423 patients approached, 216 took part (51%). Of these, 101 patients were diagnosed with dementia, with the remaining patients being referred for further testing or receiving diagnoses of mild cognitive impairment, psychological conditions or not receiving a diagnosis. The first 81 consultations of dementia diagnosis feedback were analysed in this study as part of a PhD project. A total of 43 patients were from site A and 38 from site B. In 75% (n = 61) of meetings doctors were meeting patients for the first time. Participant information is displayed in Table 2.
Table 1 Doctor characteristics

Table 2 Patient and companion characteristics

Structure of the diagnostic feedback meeting
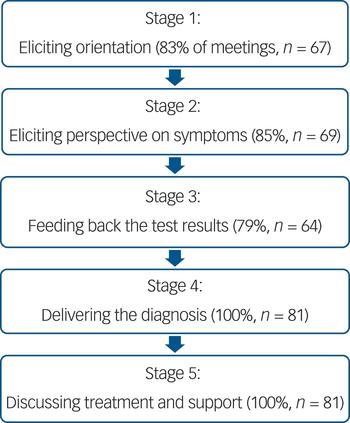
Figure 1 displays the five core stages in the diagnostic feedback meetings, with the corresponding frequencies. Across the two sites there was less than 5% difference between these frequencies. In stages 1 and 2 doctors elicited patient orientation to the meeting and their perspective on their symptoms. In stage 3 the test results were fed back. The diagnosis was delivered in stage 4. In stage 5 treatment and management were addressed.

Fig. 1 Stages of the diagnostic feedback meeting.
Communication of the dementia diagnosis
The communication of diagnostic information occurred in stages 1–4 with systematic practices occurring across the 81 meetings. There was no significant difference in how often these practices were used in site A and B. Additional examples of the practices described are available as supplementary material with the online publication of this article.
Stage 1: Eliciting orientation
In the majority of meetings doctors elicited the patient's orientation to the purpose of the meeting, often explicitly asking about the patient's expectations (extract 1, line 1). If the patient (PT) did not display orientation, the doctor (DR) provided this information before proceeding (extract 1, lines 4–10).
Extract 1
1 DR: do you remember what this is all about today
2 (0.9)
3 PT: er (0.6) no not really
4 DR: ah well I'll tell ya
5 (1.2)
6 DR: you came here
7 PT: mm [m]
8 DR: [a] while back
9 PT: that's right [yes]
10 DR: [ab]out your memory
As in Extract 1, 62% (n = 50) of patients demonstrated some uncertainty as to the purpose of the diagnosis feedback at this stage of the meeting. Although this could be attributed to short-term memory loss, in some cases it was evident other factors were involved. In extract 2, the patient does not respond after a significant pause (line 2) and her daughter (DAU) explains she had told the patient that the meeting was for the brain scan results. The patient had therefore not been informed of the possibility of a diagnosis (lines 3–4).
Extract 2
1 DR: are you clear about what it – what the appointment is
2 (0.7)
3 DAU: no I just said it was obviously the results of the er
4 (.) the brain scan
Stage 2: Eliciting perspective to forecast the diagnosis
Similar to other settings, doctors forecasted the diagnosis prior to delivery. Forecasting is a tool that allows recipients ‘to estimate and predict what the news will be’, and thus ‘ultimately facilitates realisation’.Reference Maynard14
Forecasting usually occurred in stage 2 of the meeting as doctors elicited the patient perspective on their symptoms, and then co-implicated their perspective in the diagnostic communication. In extract 3, the doctor asks if the patient agrees that her memory is not ‘as good as it used to be’ (lines 1–2). The patient shows some disagreement (lines 3–8). In these cases, doctors did additional work to demonstrate the problem: the doctor here presents test results that contrast with the patient's view (lines 9–12). Doctors have been found to present evidence in this way to manage potential resistance and prepare patients for diagnosis.Reference Maynard14, Reference Peräkylä15
Extract 3
1 DR: do you think that that's right (0.4) that the
2 memory is not as good as it used to be
3 (2.0)
4 PT: I don't think
5 (3.8)
6 PT: I don't think so but
7 DR: you don't think it's a problem
8 PT: it could be
9 DR: what I've heard is that (0.6) sometimes (.) you
10 know I did some tests with you before (.) some of
11 the things were a little bit difficult on the memory
12 tests
Conflict sometimes arose when doctors explored patient perspective while demonstrating prior knowledge of their situation. In extract 4, the doctor's perspective elicitation includes symptom descriptions reported by the patient's daughter (lines 1–2, clarified in line 9 ‘family mentioned that’). The patient disagrees, indirectly questioning where the doctor got his knowledge (‘I never said that’, lines 4–8). When the doctor changes to an open question (lines 9–10) and the patient reports having good memory (line 12), the doctor takes a different tack asking if the patient has ‘any problems’ (line 14). The patient then describes a single recent incident (line 16), which, while still in conflict with the doctor and daughter's time frame of 9 months, the doctor can still use to build up to the diagnosis.
Extract 4
1 DR: from what I understand your memory problems started
2 about nine months ago?
3 (2.8)
4 PT: no I've never said that - I've never said that
5 DR: no it's the yeah
6 (0.3)
7 PT: I've [never sa]id fo-
8 DR: [probably]
9 DR: family mentioned that but in your (0.5) observation
10 (.) how is your memory
11 (0.3)
12 PT: good!
13 (0.3)
14 DR: any problems?
15 (1)
16 PT: only just recently when I lost my (0.8) oh my wallet
Stage 3: Feeding back to the test results to forecast the diagnosis
Doctors also forecasted the diagnosis as part of stage 3 in feeding back the test results: explicitly stating the patient has significant memory problems (extract 5, lines 1–2, 4–5). This is an important part of the meeting as it may not be clear to patients which test provides the basis for the diagnosis.Reference Peräkylä16
Extract 5
1 DR: there were some significant problems in a couple of
2 areas
3 PT: mm
4 DR: specifically around memory you were performing
5 below where we would expect
Stage 4: Delivering the diagnosis
All doctors named the dementia diagnosis in stage 4 of the meeting. The clear majority oriented their gaze and posture towards the patient on delivery, thus delivering the diagnosis to the patient and not their companion.
In 25% (n = 20) of meetings doctors asked patients if they wanted to know the diagnosis immediately before naming the diagnosis as dementia (extract 6, line 1).
Extract 6
1 DR: do you want to know what we'd call that memory problem
2 PT: yeah
3 DR: yeah so we - we'd call it a vascular dementia
None of the patients explicitly stated they did not want to know their diagnosis, and thus in all these cases the diagnosis was named.
Two diagnosis delivery formats were identified in the analysis: indirect and direct. The indirect, more delicate, format was more common (59% of meetings, n = 48; extract 7, lines 1–3). It involves presenting the symptoms or test results and labelling them as ‘dementia’. This format requires some patient inference: they have these symptoms, and these symptoms are dementia, thus they have dementia. In other settings, it is a common way of delivering diagnoses in order to avoid strong emotional or resistant responses.Reference Monzoni, Reuber, Graf, Sator and Spranz-Fogasy17
Extract 7
1 DR: the most common cause for that kind of picture (0.4)
2 and this kind of (.) picture on the (.) on the memory
3 tests (.) is a problem called Alzheimer's disease
By contrast, the direct format (41%, n = 33) involved directly attributing the ‘dementia’ label to the patient, by using phrases such as ‘you have’ (extract 8, line 1). A direct format requires less patient inference to understand the diagnosis, but is interactionally more blunt and thus likely to increase emotional or resistant responses.Reference Maynard18
Extract 8
1 DR: we think that you have a dementia
Most doctors used different formats for different patients, with six doctors using the same format for all their patients (excluding the doctors where only one meeting was recorded, n = 3).
The relationship between the diagnosis format and patient scores on the Addenbrooke's Cognitive Examination (ACE)-IIIReference Mathuranath, Nestor, Berrios, Rakowicz and Hodges19 cognitive test was explored using an independent samples t-test. Too few patients were assessed on the Mini-Mental State ExaminationReference Folstein and Folstein20 to analyse these scores. ACE-III scores were lower among patients with whom doctors used a direct (mean score 64, s.d. = 13.32) v. an indirect format (mean score 71, s.d. = 13.31) (t(63) = 2.07, P = 0.042).
Although the evidence for a diagnosis had been presented prior to naming dementia, doctors often re-referred to the evidence in the diagnostic utterance (55%, n = 45; extract 9, lines 1). Explicating the evidence makes the doctor's reasoning more visible and tends to be used in the face of potential resistance.Reference Peräkylä15 This may also assist understanding among those with difficulty holding information in short-term memory.
Extract 9
1 DR: because of the changes we've seen in your scan (0.4)
2 I think the most (.) likely cause (0.6) is (0.4) er is
3 one of vascular dementia
The diagnosis was often characterised as uncertain (38%, n = 31) by doctors using phrases such as ‘the most likely’ or ‘this probably is’ (extract 10, line 1).
Extract 10
1 DR: the most likely diagnosis that we can come up with is a
2 mild Alzheimer's dementia
An emphasis on dementia as a ‘condition’ or ‘illness’ was also common (49%, n = 40; Extract 11, line 1). Emphasising that dementia has a medical cause delineates symptoms from ‘just old age’, which is commonly how people explain dementia symptoms.Reference Spanswick21
Extract 11
1 DR: what you've got is a condition called Alzheimer's
2 disease
Doctors were seen to reassure patients that they had ‘mild’ dementia, including when patients scored well below the cut-off point on cognitive tests (42%, n = 34; extract 12, line 1). This enabled doctors to frame the diagnosis positively, and differentiate the patient's situation from negative images of late-stage dementia.
Extract 12
1 DR: it's looking like an early form of a dementia
2 PT: yeah
Stage 5: Delivering the diagnosis using good news exits
Doctors used good news to exit the diagnosis discussion, emphasising the positive aspects of receiving treatment and support (53%, n = 43). This involved describing an ‘optimistic projection’ of the patient's future.Reference Jefferson22 In extract 13, the doctor delivers the diagnosis and pursues a response by providing more information (lines 1–5). The patient passes up two opportunities to speak (lines 2, 5) and the doctor progresses to assess the diagnosis as ‘good’ because the patient will be able to start medication (lines 6–14).
Extract 13
1 DR: you probably have early Alzheimer's disease
2 (0.6)
3 DR: which is a disease in the brain which affects
4 memory
5 (1)
6 DR: um (0.8) and (.) I think that's (.) it's good
7 to start thinking about that as a possibility
8 because there are some (.) things that we can try to
9 do
10 (0.3)
11 medications that we can try
12 (.)
13 which can help to (0.6) slow down the progression of
14 the memory problem
Stage 6: Delivering the diagnosis - discussing prognosis
Prognosis was explicitly discussed in 62% (n = 50) of meetings and was approached sensitively with qualifications. In extract 14, the doctor talks generically – ‘generally speaking’ (line 1) ‘for most people we expect it to get a little worse’ (lines 4–5) – rather than describing specifics. The deterioration is minimised, saying the dementia will get ‘a little worse’ (lines 4–5) over ‘many years normally’ (lines 7–8).
Extract 14
1 DR: generally speaking this is a condition that changes
2 over time
3 PT: mhm [mhm]
4 DR: [and] for most people we expect it to get a little
5 worse over time.
6 (0.5)
7 DR: but that means (.) over the space of many years
8 normally
Prognosis was not discussed in 14% (n = 11) of meetings. In 24% (n = 19) of meetings, prognosis was indirectly invoked when discussing the potential of medication to ‘slow the progression of this memory problem’ (extract 15, lines 3–4).
Extract 15
1 DR: now what I wanted to talk to you about (.) today (0.4)
2 among other things (0.3) was that we do have some
3 medication (0.4) that could slow (.) the progression
4 (.) of this memory problem
Medication was not offered to patients in 17% (n = 14) of the meetings, because of their diagnosis not being eligible for treatment using cholinesterase inhibitors. Prognosis was discussed explicitly in 71% of these meetings (n = 10/14), a higher proportion of those where medication was discussed (60%, n = 40/67).
Discussion
Main findings and comparison with previous studies
All doctors in the study clearly named dementia. Doctors deployed specific strategies to make the diagnosis clear to patients, but often downplayed or avoided prognosis. Doctors elicited patient orientation to the purpose of the meeting. This has not been described in work examining the structure of primary care consultations,Reference Robinson23 indicating that orientation is generally assumed in primary care but not in memory clinics. Over 60% of patients showed some uncertainty about the meeting purpose, which may be because patients have non-medical symptom explanations or companions are more proactive in seeking help.Reference Samsi, Abley, Campbell, Keady, Manthorpe and Robinson24 Additionally, as shown in extract 2, patients may not be informed as to the purpose of the diagnostic meeting. When patients do not expect a diagnosis, this can lead to more distressReference Robinson, Gemski, Abley, Bond, Keady and Campbell25 and difficulty accepting the diagnosis and its consequences.Reference Bunn, Sworn, Brayne, Iliffe, Robinson and Goodman26 Hence, eliciting orientation and forecasting the diagnosis prior to diagnosis delivery is important. However, guidelines advocate patient preferences for information should be ascertained prior to the diagnostic feedback meeting.Reference Guss27 Given that in 20% of meetings the doctors were asking if patients wanted to know the diagnosis immediately prior to delivery, this may not be happening in practice.
The common use of direct deliveries (‘you have dementia’) is different from cancer or HIV, where they are considered blunt and less sensitive.Reference Maynard18, Reference Gill and Maynard28 That direct deliveries occurred more often when patients had poorer cognitive functioning suggests doctors are overriding the normative, sensitive approach for a more blunt approach that may enhance understanding. Doctors also clarified the diagnosis by restating the evidence and differentiating the diagnosis from normal ageing. However, as the number of consultations is relatively small per doctor, it was not possible to analyse how doctors varied their approach with different patients. Additionally, previous work examining dementia diagnosis delivery has shown other aspects of communication, such as fractured sentences and hesitations, may negatively affect understanding,Reference Karnieli-Miller, Werner, Aharon-Peretz and Eidelman5 an aspect that was not explored in this study. Further work examining patient responses with a larger data-set, both before and after the consultation, would be necessary to draw conclusions on the effect these factors have on patient understanding and their emotional response.
That doctors are using strategies to enhance diagnostic understanding contrasts with previous research, which illustrates doctor avoidance of dementia diagnosis discussions.Reference Dooley, Bailey and McCabe29, Reference Peel30 Although this may be because of the presence of video cameras, a study using video recordings by Peel also illustrated systematic avoidance of the ‘dementia’ label in data collected in 2012.Reference Peel30 This may therefore reflect a cultural shift, potentially because campaigns such as the National Dementia Strategy have emphasised the importance of receiving a diagnosis so people can plan and access support. These campaigns are having an effect on the perception of dementia among both the public and clinicians,Reference Stites, Johnson, Harkins, Sankar, Xie and Karlawish31, Reference Cheston, Hancock and White32 which may be improving open diagnostic communication.
Indirect allusion to, avoidance of, and downplaying prognosis has been found previously in dementiaReference Karnieli-Miller, Werner, Aharon-Peretz and Eidelman5 and other settingsReference Leydon33 where doctors often follow diagnostic news with positive discussions of treatment.Reference Maynard and Maynard34, Reference Heritage, Freed and Ehrlich35 Whereas this could be compounded by the fact not all people with dementia are eligible for medication, explicit discussions of prognosis occurred in slightly more meetings where medication was not offered. This indicates that a lack of treatment may not be the only reason that prognosis is avoided. Although how much people want to know about prognosis will vary,Reference Bunn, Sworn, Brayne, Iliffe, Robinson and Goodman26 avoiding the subject means people may miss the chance to plan for their future.Reference Detering, Hancock, Reade and Silvester36 There have been initiatives to engage people in advance care planning at diagnosis, but doctors reflect that this is too early.Reference Brown37 However, given concerns that appropriate post-diagnostic support is not always available, if prognosis is not discussed at diagnosis people may have difficulty coping as the dementia progresses.Reference Stokes, Combes and Stokes38 More work is needed on how and when prognosis should be discussed.
Patients and companions will have a variety of explanations for dementia symptoms, from biological descriptions about brain changes, to social factors such as living alone, to psychological factors such as stress.Reference Cahill, Gibb, Bruce, Headon and Drury7, Reference Harman and Clare39 These may affect how doctors communicate and also how patients and companions respond and adjust to the diagnosis.Reference Harman and Clare39, Reference Sabat40 Although the diagnosis in this study was primarily delivered to patients (as judged by gaze on delivery), research has shown that companions become increasingly involved in treatment and support discussions.Reference Karnieli-Miller, Werner, Neufeld-Kroszynski and Eidelman41 This study did not analyse the role of the companion in detail. However, where patient and companion expectations differ, there is potential for more difficult communication, for example in extract 2 the daughter had withheld the purpose of the meeting from her mother, and in extract 3 the daughter had given the doctor information that the patient did not agree with. These pre-existing relationship dynamics are an additional challenge for doctors when communicating the diagnosis.Reference Dooley, Bailey and McCabe29
Dementia diagnosis as a process
Although this study reports a microanalysis of diagnosis delivery, it reflects wider discussions about what people want from a dementia diagnosis. Patients and companions prefer honesty but want to maintain hope.Reference Mastwyk, Ames, Ellis, Chiu and Dow42 Providing this balance is a complex task, combining practical and moral dilemmas.Reference Pinner43 Preferences for how, when and what information should be shared vary greatly. In general, doctors receive little training in diagnosis delivery beyond basic breaking bad news training, with most not receiving training specifically in psychiatry or dementia.Reference Bailey, Dooley and McCabe9 Doctors report wanting to communicate information that is tailored to the individual, but find this difficult when meeting the patient for the first time, which applied to 75% of meetings.Reference Bailey, Dooley and McCabe9 Additionally, provision of support and advice as the illness progresses is also extremely important.Reference Guss27 Conceptualising assessment and communication of a dementia diagnosis as a process, rather than a single event, is therefore integral.
Strengths and limitations
The strengths of this study come from a rigorous qualitative analysis of a large data-set, with a variety of different doctors, in specialist memory clinics in two different geographical areas. However, the sample did not extend to primary care or other settings where a diagnosis may be delivered. Additionally, all the clinicians in the study were medical doctors, and different healthcare professionals may approach the diagnosis differently. The consent rate was 51% and the 49% who declined may differ from those who participated, which may affect generalisability. Not all types of dementia or different ethnic and cultural groups were represented. The presence of cameras may have altered doctor communication. Finally, it was beyond the scope of this paper to analyse how patients responded to the diagnosis, or the role of the companions.
Future directions for research
In conclusion, doctors are clearly naming dementia but are more variable in discussing prognosis. Further work is needed to explore the ethical issues involved in communicating the degenerative nature of dementia in the diagnostic feedback meeting, as well as what information at this stage will facilitate planning for the future while also preserving hope.
Supplementary material
Supplementary material is available online at https://doi.org/10.1192/bjp.2017.64.
Funding
This paper presents independent research funded by the National Institute for Health Research (NIHR) under its Research for Patient Benefit (RfPB) Programme (Grant Reference Number PB-PG-1111-26063), and supported by the NIHR Collaboration for Leadership in Applied Health Research and Care South West Peninsula. The views expressed are those of the authors and not necessarily those of the NHS, the NIHR or the Department of Health.
Acknowledgements
We thank all the patients, companions and healthcare professionals who allowed us to film the consultations, as well as Professor Gill Livingston and the ShareD project team.







eLetters
No eLetters have been published for this article.