Adolescence is a complex stage, in which the transition from childhood to adulthood takes place, that promotes puberty-related body changes, as well as social adaptations(1). The most common indicator to assess the nutritional state of adolescents is the BMI, obtained on dividing the weight by the square of the height, and analysed according to sex and age(2). However, the isolated use of BMI has its limitations, as it cannot differ muscle, bone and fat mass(Reference Krebs, Himes and Jacobson3–Reference Okorodudu, Jumean and Montori5). Thus, more than a quarter of children and adolescents with a high percentage body fat may be misclassified as eutrophic when only BMI is used(Reference Javed, Jumean and Murad6).
Therefore, literature has investigated other obesity phenotypes that affect individuals with normal weight, such as ‘normal-weight obesity’ (NWO). The expression was introduced by De Lorenzo et al. (2006)(Reference De Lorenzo, Martinoli and Vaia7) who adopted it as a criterion for identifying individuals with normal weight, yet with high percentage body fat. Studies carried out in adults indicate positive associations between NWO and cardiometabolic deregulation(Reference Kapoor, Furler and Paul8–Reference Lee, Park and Han10).
Thus, regardless of a normal BMI, NWO presents a low-grade pro-inflammatory state, increased oxidative stress, insulin resistance and dyslipidemia, which lead to an increased risk of metabolic syndrome, CVD and death related to the cardiovascular system, due to the accumulation of body fat(Reference De Lorenzo, Martinoli and Vaia7,Reference De Lorenzo, Del Gobbo and Premrov11–Reference Oliveros, Somers and Sochor18) . Furthermore, modifiable behavioural factors, such as physical inactivity(Reference Männistö, Harald and Kontto19,Reference Olafsdottir, Torfadottir and Arngrimsson20) , smoking and low fibre diet(Reference Männistö, Harald and Kontto19), have been associated with this phenotype.
In adolescents, NWO may go unnoticed for years, due to the young age and normal body weight(Reference Karelis, St-Pierre and Conus21). Hence, the identification of NWO may uncover an unknown risk group, and understanding which factors are related to this phenomenon is key to assist in building effective prevention and intervention strategies. To our knowledge, to date, no systematic review has assessed NWO exclusively in adolescents and the association of this phenotype with cardiometabolic risk remains uncertain, as well as the health behaviours practised by this public. Thus, this systematic review aims to analyse the presence of cardiometabolic risk factors in adolescents with NWO, as well as to investigate the health behaviours associated with the phenotype.
Methods
Data sources and research strategy
The Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA)(Reference Moher, Liberati and Tetzlaff22) guidelines were followed for this systematic review, which was registered in the International prospective register of systematic reviews (PROSPERO: CRD42020161204). The research was based on the components of the PECO acronym, with the following guiding question: “What evidence is available about the relationship between NWO in adolescence and the presence of cardiometabolic risk factors? What are the health behaviours related to the phenotype in adolescents? The literature search was performed through the National Library of Medicine (PubMed), Scientific Electronic Library Online (Scielo) and the ScienceDirect.
The selection of studies was carried out between December 2019 and September 2020, the last search being carried out in September 2020. The references were stored in the Zotero bibliographic software, version 5.0. The steps were carried out in duplicate, without the use of filters, using descriptors from Medical Subject Headings (MeSH), Descritores em Ciências da Saúde (DecS) and other search terms related to the theme, with the following strategy: (adolescent OR adolescence OR teen OR teenager OR youth OR children) AND (‘normal weight obesity’ OR ‘normal weight obese’). The complete search strategy is available in the supplementary file.
Study selection
The steps of inclusion and exclusion of papers (Fig. 1) began with the identification of the papers in the databases. Then, duplicates were excluded. Afterwards, title and abstract screening was performed by two independent reviewers (B. C. C. and L. G. S.) in order to identify studies that met the inclusion criteria. Finally, reading and assessment of the full texts were also performed by the aforementioned reviewers, and divergences were solved through discussions until an agreement was reached. These steps were performed using the Rayyan QCRI software(Reference Ouzzani, Hammady and Fedorowicz23).
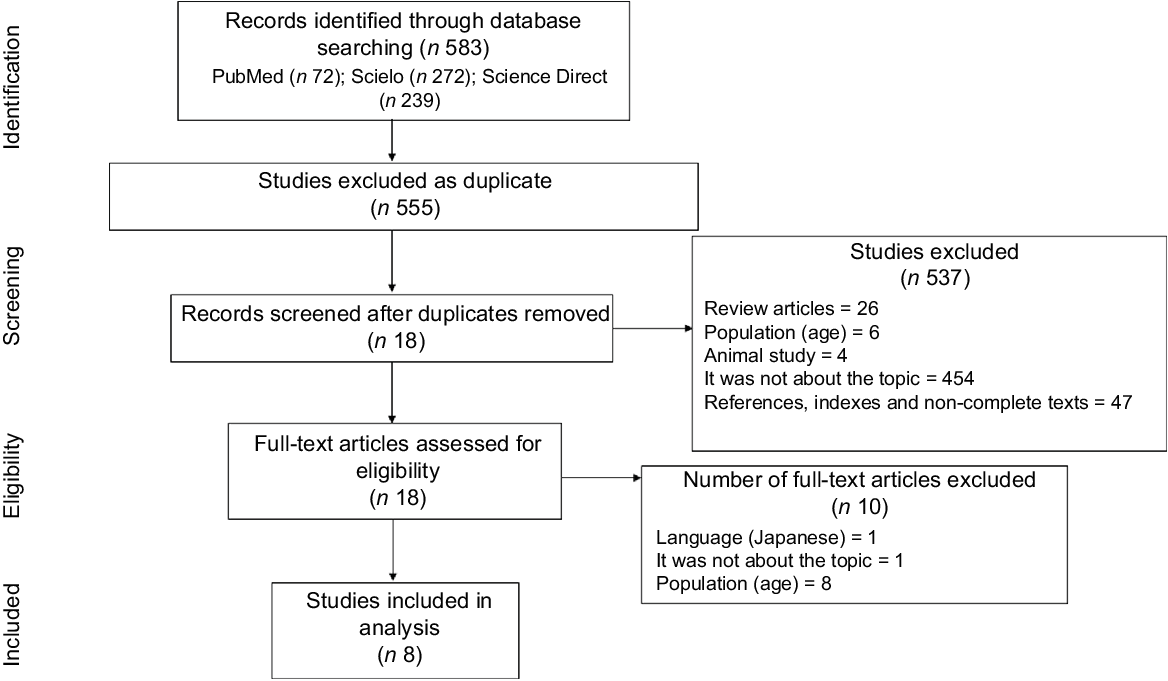
Fig. 1 Flow chart of the literature search and study selection procedures, according to PRISMA recommendation. PRISMA, The Preferred Reporting Items for Systematic reviews and Meta-Analyses
Definition of the normal-weight obese phenotype
Adolescents with adequate weight for height, but with excess body fat, were called NWO. The specific criteria for defining the phenotype, such as the BMI normal range and adiposity considered excessive, were different, according to the authors (Table 1).
Table 1 Studies characteristics that investigated normal-weight obesity in adolescents
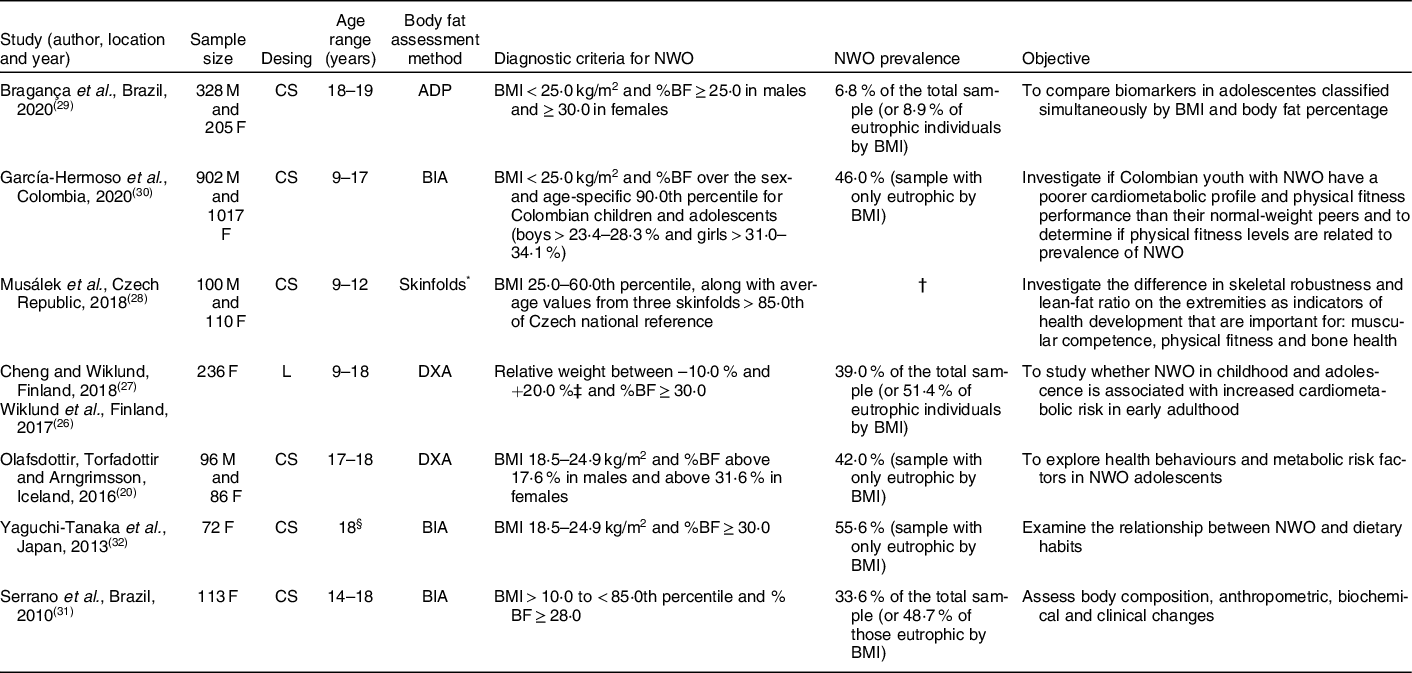
NWO, normal-weight obesity; M, male; F, female; CS, cross-sectional study; ADP, air displacement plethysmography; %BF, body fat percentage; BIA, bioelectrical impedance analysis; L, longitudinal study; DXA, dual-energy X-ray absorptiometry.
* Values of the three skinfolds (over triceps, subscapular and suprailliac) were compared with anthropometric references for Czech children.
† Research with a sample of balanced groups, it is not possible to define actual prevalence of NWO.
‡ Growth charts of each participant were obtained from the Finnish School Health Care System. To be able to compare growth at certain time points, the weight per cent (%) and height z-score were extrapolated from growth charts using a form that was created by the Finnish Paediatric Research Association and accepted by the Finnish National Health Administration (Form No. 7466:92). On the basis of their growth chart data, participants were classified into underweight (relative weight to height from growth chart under –10 %), normal weight (relative weight between –10 and + 20 %) and overweight + obesity (relative weight greater than + 20 %).
§ Age group not informed, but average age of participants was 18 years.
Inclusion and exclusion criteria
The inclusion criteria were based on the selection of original articles carried out with humans published in English, Spanish or Portuguese that assessed the relationship of NWO with the presence of cardiometabolic risk factors or with health behaviours. Participants should be between 10 and 19 years old, that is, be considered a teenager, according to the WHO definition(24). As many papers in the literature are not performed only with the age group of interest, the inclusion criteria were studies in which the majority of participants were between 10 and 19 years old, allowing a variation of +/–1 year of age in sample (9–20 years), as long as the mean/median age is between 10 and 19 years. There was no restriction on the date of publication of the articles. Revisions, book chapters, publications whose full texts were not available, studies with animals, studies with adults and manuscripts that did not meet the inclusion criteria mentioned above were excluded.
Quality assessment
The included studies underwent an analysis, performed in duplicate by independent reviewers (B. C. C. and N. N. L.), using the Quality Assessment Tool for Observational Cohort and Cross-sectional Studies, by National Heart, Lung, and Blood Institute(25). Discrepancies were resolved by consensus.
Data analysis and extraction
Information from each study regarding the author(s), year of publication, study design, country, sample size and according to sex, age, authors’ criteria for defining NWO, method used to evaluate the variables, main objectives, NWO prevalence and main results were extracted.
Results
Included studies
A total of 583 papers were identified following the search through the databases. After removing duplicates, the title and abstract of 555 papers were read. Of these, 537 were excluded, of which 26 were reviews, 6 were not carried out with NWO adolescents, 4 were related to animal study, 454 did not address the topic and 47 were references, indexes or non-complete texts. Thus, 18 were selected for complete reading. After reading the full text, 10 were excluded for not meeting the inclusion criteria, of which 1 was not in an English, Portuguese or Spanish version, 1 did not address the NWO theme and 8 were not conducted with NWO adolescents (Fig. 1) (Supplementary file).
The overall characteristics of the included studies
The overall characteristics of the studies that investigated factors related to NWO are displayed in Table 1. It was found that the articles by Wiklund et al. (2017)(Reference Wiklund, Törmäkangas and Shi26) and Cheng and Wiklund (2018)(Reference Cheng and Wiklund27) come from the same population, that is, from the same study. Thus, for counting the sample size, prevalence of the phenotype, criteria adopted to define NWO, study design and place of performance, they were considered only once, as a study, in order to avoid duplication.
Among the selected studies, three were conducted in Europe(Reference Olafsdottir, Torfadottir and Arngrimsson20,Reference Wiklund, Törmäkangas and Shi26–Reference Musálek, Pařízková and Godina28) , three in South America(Reference Bragança, Oliveira and Fonseca29–Reference Serrano, Carvalho and Pereira31) and one in Asia(Reference Yaguchi-Tanaka, Kawagoshi and Sasaki32). Most articles had a cross-sectional design, except one that was longitudinal(Reference Wiklund, Törmäkangas and Shi26,Reference Cheng and Wiklund27) . The year of publication of the studies varied from 2010(Reference Serrano, Carvalho and Pereira31) to 2020(Reference Bragança, Oliveira and Fonseca29,Reference García-Hermoso, Agostinis-Sobrinho and Camargo-Villalba30) , revealing that it is recent theme (Table 1).
The total sample size was 3265 individuals, mostly females (56·3 %; n 1839). It is valid to consider that total sample is made up of both underweight and overweight, as well as normal-weight individuals. It is important to emphasise that these four studies(Reference Wiklund, Törmäkangas and Shi26–Reference Bragança, Oliveira and Fonseca29,Reference Serrano, Carvalho and Pereira31) were not performed only with eutrophic individuals. From the entire sample (n 3265), 1240 subjects were classified as NWO (40·0 %). And, among those who had adequate weight (n 2864), 43·3 % (n 1240) had NWO, that is, a relevant percentage of people classified as eutrophic according to the BMI had NWO.
The most used method for assessing body fat was bioelectrical impedance analysis(Reference García-Hermoso, Agostinis-Sobrinho and Camargo-Villalba30–Reference Yaguchi-Tanaka, Kawagoshi and Sasaki32), followed by dual-energy X-ray absorptiometry(Reference Olafsdottir, Torfadottir and Arngrimsson20,Reference Wiklund, Törmäkangas and Shi26,Reference Cheng and Wiklund27) . The cut-off points adopted, both for BMI and for excess body fat, in the definition of NWO, were very diverse (Table 1).
Cardiometabolic risk and normal-weight obesity
Most of the studies observed that NWO in adolescents is related to the presence of cardiometabolic risk factors (Table 2). In this context, in the study by Yaguchi-Tanaka et al. (2013)(Reference Yaguchi-Tanaka, Kawagoshi and Sasaki32), individuals with the NWO phenotype displayed higher values of BMI and weight, compared to the eutrophic individuals without the phenotype.
Table 2 Main results of the included studies
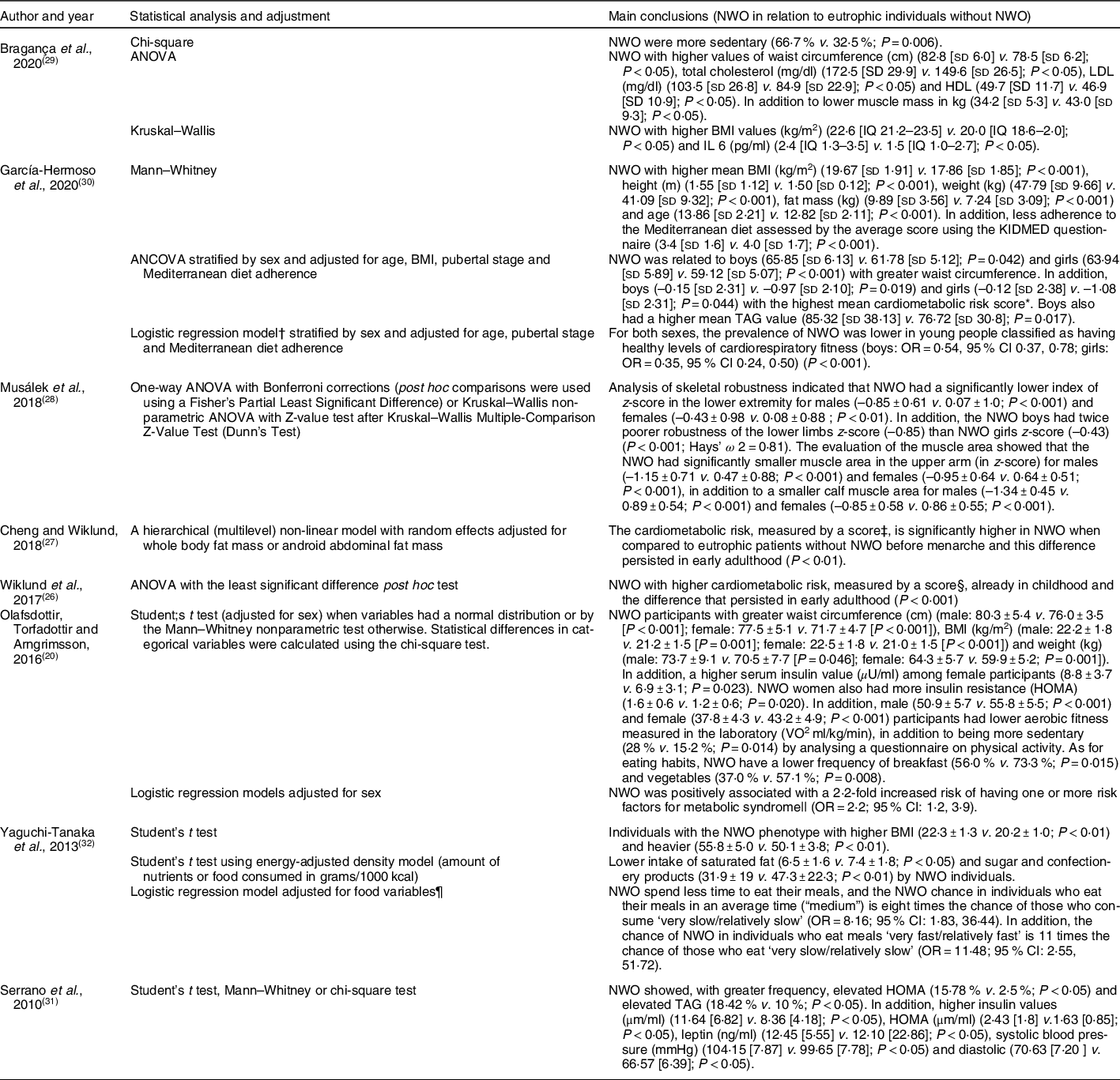
NWO, normal weight obesity; KIMED, Mediterranean Diet Quality Index in Children and Adolescents; HOMA, homoeostasis model assessment.
* A cardiometabolic risk score was created from the sum of the z-scores values of systolic blood pressure, serum TAG, waist circumference, HDL-C (multiplied by –1), and fasting glucose z-score. A higher cardiometabolic risk z-score is indicative of an unhealthier risk profile.
† Logistic regression model was employed to determine the odds of being classified as NWO according to cardiorespiratory fitness categories using unhealthy cardiorespiratory fitness as a reference. Evaluated the cardiorespiratory fitness by the 20-m shuttle run test (20 mSRT) which was grouped into two categories: healthy and unhealthy.
‡ Cardiometabolic risk score (MetS score) was calculated separately for each time point by standardising and then summing the following continuously distributed metabolic traits to create a z-score: mean arterial pressure ([(2 × diastolic blood pressure) + systolic blood pressure]/3); HOMA-IR; serum HDL cholesterol × –1; and fasting serum TAG z-score. A higher score indicates a worse cardiometabolic profile.
§ The risk score was calculated by standardising and then summing the following continuously distributed metabolic traits to create a z-score: mean arterial pressure ([(2 × diastolic blood pressure)+systolic blood pressure]/3); abdominal fat mass; fasting plasma glucose; serum HDL cholesterol × –1; and fasting serum TAG z-score. A higher score indicates a less favourable cardiometabolic risk profile.
|| Metabolic syndrome defined according to the Joint Interim Statement (JIS) of the IDF Task Force on Epidemiology and Prevention, National Heart, Lung and Blood Institute, American Heart Association, World Heart Federation, International Atherosclerosis Society and International Association for the Study of Obesity.
¶ Adjustment for rate of eating, eating breakfast every day, eating afternoon snack every day, eating snack after dinner more than once per week, use of dietary supplements, intentional change of dietary habits, experience of dieting, diet control under direction of physician or dietician, exercise habit. Note: a rate of eating was assessed using a questionnaire in which participants were allowed to mark their meals ‘very slowly’, ‘relatively slowly’, ‘medium’, ‘relatively fast’ and ‘very fast’. For statistical analysis, there was a combination of ‘very slow’ and ‘relatively slow’ into a single category. The same was done for ‘very fast’ and ‘relatively fast’. Thus, there were three categories for assessing the rate of ingestion, with ‘very slow/relatively slow’ being a reference for calculating the OR.
In another study recently published in Brazil(Reference Bragança, Oliveira and Fonseca29), it was found that individuals with NWO displayed higher values of BMI and waist circumference when compared to the eutrophic individuals without the phenotype. Besides, lower muscle mass (in kg) was observed in NWO individuals. When analysing the biomarkers, researchers also observed that NWO individuals had higher values of total cholesterol, LDL, HDL and IL-6.
García-Hermoso et al. (2020)(Reference García-Hermoso, Agostinis-Sobrinho and Camargo-Villalba30) recently published a study in Colombia, comparing NWO individuals and eutrophic individuals without NWO, and reported that NWO individuals displayed higher values of BMI, weight and fat mass. Furthermore, through the ANCOVA, NWO was associated with higher values of TAG for males, and, in both sexes, to higher waist circumference and higher average cardiometabolic risk score. The cardiometabolic risk score was proposed using the sum of the values of the z-scores of the systolic blood pressure, serum TAG, waist circumference, HDL (multiplied by –1) and the z-score of fasting blood glucose, calculated separately according to sex and to each age group (on a yearly basis), whereas a higher z-score of cardiometabolic risk indicates a non-healthy profile.
In a similar fashion, a study conducted in Iceland(Reference Olafsdottir, Torfadottir and Arngrimsson20) showed that NWO participants of both sexes displayed higher waist circumference, BMI and weight compared to eutrophic individuals without the phenotype. In addition, with respect to females, higher values were found for serum insulin and insulin resistance evaluated through the insulin resistance index (homoeostasis model assessment; HOMA). The authors also reported that NWO individuals displayed 2·2 times more likely to manifest one or more metabolic syndrome risk factors (OR = 2·2; CI 95 %: 1·2, 3·9) compared to eutrophic individuals.
In a longitudinal study carried out in Finland, Wiklund et al. (2017)(Reference Wiklund, Törmäkangas and Shi26) and Cheng and Wiklund (2018)(Reference Cheng and Wiklund27) observed that cardiometabolic risk was significantly higher in NWO individuals at the beginning of the study, with younger participants, and that this difference persisted until the early adulthood (P < 0·01). To assess cardiometabolic risk, Wiklund et al. (2017)(Reference Wiklund, Törmäkangas and Shi26) proposed a risk score, which was calculated by standardising and subsequently adding up the following metabolic traits continually distributed to generate a z-score: average blood pressure ([2 × diastolic blood pressure) + systolic blood pressure]/3); abdominal fat; fasting plasma glucose; HDL × –1; and z-score of fasting serum TAG. By their part, Cheng and Wiklund (2018)(Reference Cheng and Wiklund27) calculated the score as follows: average blood pressure ([2 × diastolic blood pressure) + systolic blood pressure]/3); homoeostasis model assessment of insulin resistance (HOMA-IR); HDL × –1; and z-score of fasting serum TAG. In both cases, higher scores implied poorer cardiometabolic profiles.
In the study by Serrano et al. (2010)(Reference Serrano, Carvalho and Pereira31), elevated HOMA and TAG were more frequent in NWO than in eutrophic individuals without excess body fat. Besides, higher values of insulin, HOMA, leptin, and systolic and diastolic blood pressure were found among adolescents with NWO, compared to the eutrophic group without NWO.
Furthermore, Serrano et al. (2010)(Reference Serrano, Carvalho and Pereira31) found that the NWO group displayed similar behaviour compared to overweight group, with respect to blood pressure, HDL fraction and blood glucose. This finding led authors to conclude that excess adiposity in eutrophic adolescents (NWO) may be related to biochemical and clinical changes similar to those found in overweight adolescents.
Health behaviours and normal-weight obesity
With respect to health behaviours, a recently published study(Reference García-Hermoso, Agostinis-Sobrinho and Camargo-Villalba30) assessed the adherence to the Mediterranean diet, characterised by the rich consumption of plant foods, olive oil, fresh, unprocessed products and fish(Reference Graça, Mateus and Lima33). The assessment was carried out through the Mediterranean Diet Quality Index in Children and Adolescents (KIDMED) questionnaire, which is highly (α = 0·79) consistent to determine this adherence. KIDMED includes 16 questions based on the assessment of food habits, according to the principles that sustain and weaken the food patterns of this diet. The sum of all the values of the administered questionnaire was categorised into two levels: (1) 0–7, low/moderate adherence and (2) 8–12, good adherence. By resorting to this instrument, García-Hermoso et al. (2020)(Reference García-Hermoso, Agostinis-Sobrinho and Camargo-Villalba30) showed a low adherence to the Mediterranean diet among the NWO individuals, in comparison to eutrophic individuals without NWO.
Furthermore, García-Hermoso et al. (2020)(Reference García-Hermoso, Agostinis-Sobrinho and Camargo-Villalba30) assessed the cardiorespiratory fitness through 20-m running test, in which the peak oxygen consumption was estimated through the equation proposed by Barnett et al. (1993)(Reference Barnett, Chan and Bruce34) that enables the classification of cardiometabolic risk into ‘healthy’ and ‘non-healthy’ in Colombian children and adolescents, according to sex and age. In both sexes, the authors observed that the NWO prevalence was lower in young individuals with healthy levels of cardiorespiratory fitness (boys: OR = 0·54, CI 95 % = 0·37, 0·78; girls: OR = 0·35, CI95 % = 0·24, 0·50) (P < 0·001).
Likewise, another study(Reference Olafsdottir, Torfadottir and Arngrimsson20) identified that NWO individuals of both sexes displayed lower aerobic fitness measured in laboratory (maximum oxygen uptake was assessed through open-circuit spirometry with a treadmill exercise test protocol), also being more sedentary, according to a questionnaire about lifestyle and health behaviours that included physical activity. Besides, the assessment of food habits through a 24-h recall and a FFQ showed that NWO individuals displayed lower frequency of breakfast and vegetables intake than eutrophic individuals without NWO.
Bragança et al. (2020)(Reference Bragança, Oliveira and Fonseca29), through the 24-h Physical Activity Recall, which was developed based on an adaptation of the Self-Administered Physical Activity Checklist(Reference Sallis, Strikmiller and Harsha35), measured the physical activity level, obtained by the calculation of the weekly number of metabolic equivalents of task. The metabolic equivalents of task for each activity were obtained in the Compendium of Physical Activities(Reference Ainsworth, Haskell and Leon36). To categorise the physical activity levels, the following cut-off values of the International Physical Activity Questionnaires in metabolic equivalents of task/week were applied: sedentary (0), low (1 to < 600), moderate (600 to < 3000) and high (≥ 3000)(Reference Benedetti, Antunes and Rodriguez-Añez37), whereas the authors found that NWO individuals were more sedentary than eutrophic individuals (66·7 % v. 32·5 %; P = 0·006).
Musálek et al. (2018)(Reference Musálek, Pařízková and Godina28) investigated the skeletal robustness and muscle areas on the upper arm and calf as indicators of muscle strength, physical fitness and bone age, taking into account that the higher levels of physical activity positively affects lean mass and bone development. To perform the skeletal breadth measurements, measures of humeral and femoral epicondyle were taken using a T520 thoracometer. After obtaining these measures, the researchers calculated the skeletal robustness indices according to the formula proposed by Frisancho (1990)(Reference Frisancho38), from humerus and femur breadth epicondyles. Musálek et al. (2018)(Reference Musálek, Pařízková and Godina28) concluded that NWO individuals displayed significantly lower skeletal robustness in the lower extremities, according to the Frame index (z-score) from the femur. Furthermore, NWO individuals displayed lower z-scores for the muscle areas on the upper arm and calf.
In the study by Yaguchi-Tanaka et al. (2013)(Reference Yaguchi-Tanaka, Kawagoshi and Sasaki32) that investigated food habits, NWO individuals displayed lower intake of saturated fat, sugar and bakery products, assessed through the brief self-administered diet history questionnaire. Yaguchi-Tanaka et al. (2013)(Reference Yaguchi-Tanaka, Kawagoshi and Sasaki32) also assessed the speed of ingestion of foods through a questionnaire to which participants should point out whether they eat their meals ‘very slowly’, ‘relatively slowly’, ‘moderate’, ‘relatively quickly’ and ‘very quickly’. For statistical analysis, the ‘very slowly’ and ‘relatively slowly’ classifications were combined into a single category. The same was done for the ‘very quickly’ and ‘relatively quickly’ classifications. The authors observed that the likelihood of NWO in individuals who eat their meals in a moderate time is eight times greater than in those who eat very slowly or relatively slowly (OR = 8·16; CI 95 %: 1·83, 36·44). Also, the likelihood of NWO in individuals who eat their meals very quickly or relatively quickly is 11 times greater than in those who eat very slowly or relatively slowly (OR = 11·48; CI 95 %: 2·55, 51·72).
Risk of bias
All the selected studies underwent duplicate evaluation using a critical appraisal tool from the National Heart, Lung, and Blood Institute (2014)(25). The main limitations, according to what was assessed in the Checklist items, include the fact that the exposure was not assessed prior to outcome measurement and there was not sufficient time frame to see an effect, in six studies. However, this can be justified by the cross-sectional design of most studies included in this review. Four studies were not clear with respect to the criteria for identification and definition of confounding factors. One study did not provide enough details on the sample and resorted to a self-referred measure for assessment of a variable but did not establish objective criteria (Table 3). Despite these limitations, the papers selected allowed to raise important questions regarding NWO and also showed good quality.
Table 3 Quality assessment of observational cohort and cross-sectional studies
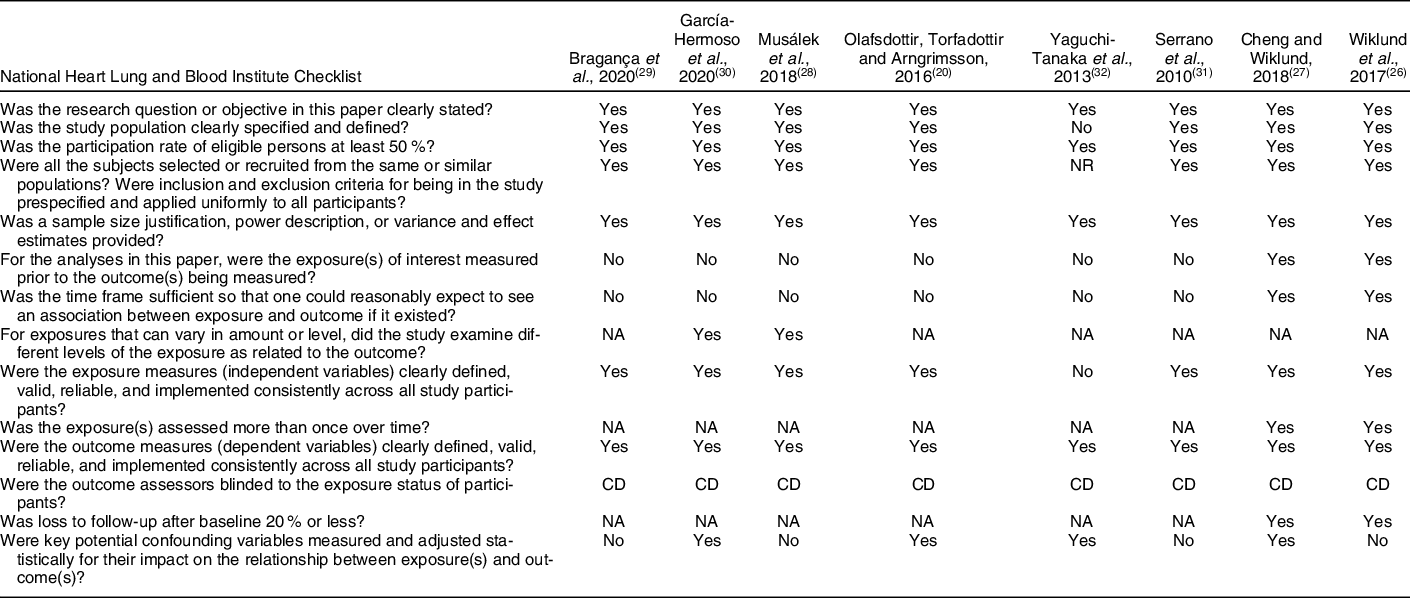
NR, not reported; NA, not applicable; CD, cannot determine.
Discussion
NWO has been regarded as an important risk factor for metabolic dysregulation and CVD, especially in adults(Reference Jean, Somers and Sochor39). This is, to our knowledge, the first systematic review that addresses the presence of cardiometabolic risk factors in NWO adolescents, as well as the health behaviours related to the phenotype. The available evidence has shown that, although NWO adolescents are within the normal range of BMI or have an appropriate weight, they have higher values of anthropometric measurements, such as weight, BMI and waist circumference. Furthermore, they have more changes in the biochemical markers of cardiometabolic risk, such as insulin resistance and hypertriglyceridemia, besides being more sedentary and having less physical fitness, than eutrophic individuals without NWO.
The prevalence of the NWO phenotype is not uncommon, ranging from 5 % to 45 %, due to ethnic differences in populations, the use of different cut-off points for excess body fat and the lack of consensus on the diagnostic criteria(Reference Olafsdottir, Torfadottir and Arngrimsson20,Reference Conus, Rabasa-Lhoret and Péronnet40,Reference Marques-Vidal, Chiolero and Paccaud41) . In the present study, the prevalence of NWO in adolescents also varied between studies, from 6·8 % to 55·6 %, with high values, revealing the importance of adopting measures to prevent and control this phenotype. Anthropometric markers of cardiometabolic risk, such as weight and waist circumference, are associated with insulin resistance, type 2 diabetes mellitus, CVD and premature death(Reference Katzmarzyk, Srinivasan and Chen42–Reference Balagopal, de Ferranti and Cook44). In addition, biochemical markers, such as plasma cholesterol and TAG levels, directly correlate with the chance of CVD(Reference Schulte, Cullen and Assmann45,Reference Kannel46) .
In this sense, considering the young age group in question, the identification of cadiometabolic risk factors in NWO adolescents in this study is worrisome because, in addition to influencing the occurrence of metabolic syndrome and CVD in the future, these changes tend to persist into adulthood(Reference Lauer, Clarke and Mahoney47–Reference Weihrauch-Blüher, Schwarz and Klusmann53).
As for health behaviours, some factors, such as physical activity and eating habits, are fundamental for health promotion and quality of life(54,55) . Thus, sedentary lifestyle and inappropriate eating habits contribute to the accumulation of adipose tissue and are also related to chronic degenerative diseases(Reference Barnes56–Reference Barroso, Marins and Alves58).
In this review, it was evidenced that NWO are more sedentary and have less physical fitness. Sedentary behaviour is related to body fat accumulation(Reference Mac Ananey, McLoughlin and Leonard59,Reference Garcia-Pastor, Salinero and Sanz-Frias60) , metabolic syndrome(Reference Edwardson, Gorely and Davies61) and diabetes(Reference Hu, Li and Colditz62), thus influencing the onset of NWO. In contrast, regular physical activity generates numerous health benefits, such as cardiovascular strengthening and integrity, increased insulin sensitivity and additional energetic expenditure(Reference Mahan and Raymond63). Besides these positive physiological effects, participation in physical activity can improve physical self-perceptions and enhance self-esteem in young people(Reference Lubans, Richards and Hillman64).
Although conclusive results on NWO eating habits have not been reached in this review, since studies have evaluated different parameters and by different methods, it is valid to consider that the quality of the diet is an important factor for health promotion(Reference de Souza, Ferreira and Barbosa65). Therefore, the appropriate consumption of fibres may be associated to a decrease in glucose levels, blood pressure, serum lipids(Reference Gallagher66) and inflammatory markers, thus contributing to reducing noncommunicable chronic diseases(Reference Bernaud and Rodrigues67). García-Hermoso et al. (2020)(Reference García-Hermoso, Agostinis-Sobrinho and Camargo-Villalba30) observed that NWO adolescents have less adherence to the Mediterranean diet, which is characterised by high consumption of plant foods(Reference Graça, Mateus and Lima33) and, in the study by Olafsdottir, Torfadottir and Arngrimsson (2016)(Reference Olafsdottir, Torfadottir and Arngrimsson20), NWO presented less frequency of consumption of vegetables.
Regarding the risk of bias in individuals studies, it was observed that six studies did not evaluate the exposure prior to outcome measurement and there was not sufficient time frame to see an effect; four studies were not clear with respect to the criteria for identification and definition of confounding factors; one study did not provide enough details on the sample and resorted to a self-referred measure for the assessment of a variable. Despite these limitations, the papers selected allowed to raise important questions regarding NWO and also showed good quality.
Some limitations need to be taken into consideration in the present review. Different procedures for the assessment of body composition were used. In addition, there was no standardisation regarding the NWO definition criteria, with different cut-off points considered to be adequate for BMI and body fat. The papers were restricted to those published in Portuguese, English or Spanish, and there was a heterogeneity of the data, which ruled out the possibility of conducting a meta-analysis. Most papers, except one, were transversal, which makes an inference about the causal relation between the factors and NWO infeasible. More studies are necessary to clarify these associations and potential mechanisms involved with NWO.
The strengths of this review included its systematic approach based on the PRISMA(Reference Moher, Liberati and Tetzlaff22) guidelines, peer-reviewed studies and evaluation of risk of bias in articles by the National Heart, Lung, and Blood Institute(25). The studies exhibit good quality and all had comparison groups – the healthy eutrophics – for data analysis. Furthermore, the studies were conducted with different populations, in seven countries across three continents, emphasising the consistency of the findings. Besides, it is worth to highlight that the present study provides the literature with innovative knowledge and stresses the need for further epidemiological studies.
Conclusions
In conclusion, the available evidences suggest that NWO may be identified, with high prevalence, in adolescents. Furthermore, the phenotype is related to the early development of cardiometabolic risk factors, sedentary lifestyle and lower physical fitness. Further investigations are still needed to better clarify these relations and support the implementation of prevention and control measures regarding the phenotype.
Acknowledgements
Acknowledgements: Coordination for the Improvement of Higher Education Personnel (CAPES, Brazil). Financial support: This research received no specific grant from any funding agency, commercial or not-for-profit sectors. Conflict of interest: None. Authorship: BCC participated in the research, selection and evaluation process of the included articles, wrote the paper and analysed data. In addition, it was ultimately responsible for the content. LGS participated in the research and selection process of the included articles, as well as the critical review of the paper. NNL participated in the evaluation of the included articles, as well as the critical review of the paper. PFP, SAVR and SCCF participated in the critical review of the paper and discussions about the included articles. All authors have read and approved the final manuscript. Ethics of human subject participation: Not applicable (ethical approval was not required for the study).
Supplementary material
For supplementary material accompanying this paper visit https://doi.org/10.1017/S1368980020004863









