Introduction
The diversity of marine fish during the latest Mississippian (Serpukhovian) and its subsequent transition to the Pennsylvanian (Bashkirian) is at present poorly understood. This in turn has had a major influence on what we know of fish extinction and diversification rates on a global scale (Friedman and Sallan, Reference Friedman and Sallan2012; Sallan, Reference Sallan2014; Friedman, Reference Friedman2015). Part of our poor understanding of this transition is due to the disjunct between the better quality and better known specimens from the latest Mississippian versus the few isolated specimens collected from the Early Pennsylvanian because Carboniferous faunas are highly biased by the small number of lagerstätte, and partly also a lack of collections covering both macro and micro data from sites so that the total assemblage can be analysed. However, most of what we know from the latest Mississippian is from the Bear Gulch Limestone and Bearsden Lagerstätten localities that have produced relatively complete fish specimens (Stahl, Reference Stahl and Schultze1999; Coates and Sequeira, Reference Coates and Sequeira2001; Ginter et al., Reference Ginter, Hampe, Duffin and Schultze2010; Lund et al., Reference Lund, Greenfest-Allen and Grogan2012, Reference Lund, Greenfest-Allen and Grogan2015). Thus, new localities and specimens are needed to help balance the data disparity for ancient fish between the Late Mississippian and the Early Pennsylvanian.
In the early 1980s, a U.S. Geological Survey of the western Grand Canyon (Billingsley and McKee, Reference Billingsley, McKee and McKee1982) took place to help define a stratigraphic unit of Late Mississippian age within the canyon, the Surprise Canyon Formation (Billingsley and Beus, Reference Billingsley and Beus1985). During this survey, a few macrovertebrate fossils (namely chondrichthyan teeth and spines) were collected along with bulk sediment samples for conodont assays within the Surprise Canyon Formation and the overlying Watahgomigi Formation. The macrofossils were identified and housed in the Smithsonian Paleobiology collections (R. Lund, personal communication, Reference Lund, Greenfest-Allen and Grogan2015). The conodont assay was conducted by Martin (Reference Martin1992), then refined by Martin and Barrick (Reference Martin, Barrick, Billingsley and Beus1999), and the conodonts noted for stratigraphic provenance in the stratigraphic sections made by Billingsley and Beus (Reference Billingsley, Beus, Billingsley and Beus1999b). The fortuitous discovery of the remaining conodont residues from Martin's (Reference Martin1992) study that had been overlooked and forgotten, but containing an extensive vertebrate fauna, occurred in 2013 and the material was passed to one of us (DKE) for study. Because the localities are extremely difficult to access, there was no prospect of being able to visit them to collect additional samples. This forms the main part of the present study together with chondrichthyan teeth and large dermal spines originally collected by the 1980s survey of the Surprise Canyon and Watahgomigi formations. This allows comments on their stratigraphic context and their bearing on the transition of the latest Mississippian to Early Pennsylvanian fish assemblages.
Geologic setting and age
The Surprise Canyon Formation is the name given to a series of channel fills and karstic cave deposits of Late Mississippian (Serpukhovian) age in Grand Canyon (Billingsley and Beus, Reference Billingsley and Beus1985). These represent a considerable hiatus between the Redwall Limestone and the overlying Supai Group, which was originally recognized by McKee and Gutschick (Reference McKee and Gutschick1969) who gave several examples and descriptions of these deposits, although at that time considering them to be part of the basal Supai Group. The channels were originally described by Billingsley (Reference Billingsley1978) and later interpreted as paleo-valleys by Billingsley and McKee (Reference Billingsley, McKee and McKee1982). The formation is nowhere continuous and consists of isolated, lens-shaped outcrops scattered over Grand Canyon and Marble Canyon to the east. Outcrops are generally up to 45 m thick in central Grand Canyon close to the presumed headwaters of the paleochannels, but reach 122 m in the west in proximity to the estuary (Billingsley and Beus, Reference Billingsley and Beus1985). Description of the extensive fossil invertebrate assemblages was carried out by Beus (Reference Beus1985, Reference Beus1986, Reference Beus, Billingsley and Beus1999), who showed that the lower beds are fluvial while the upper part of the succession is marine.
The Surprise Canyon Formation was originally divided into a lower unit consisting of fluvial clastics and an upper marine unit composed of siltstones and limestones (Billingsley and Beus, Reference Billingsley and Beus1985). Subsequent studies indicated the presence of three units: a lower fluvial chert pebble conglomerate interbedded with coarse- to fine-grained, red-brown sandstone and siltstone mainly of terrestrial origin; a middle marine unit of gray-yellow or reddish-brown, coarsely crystalline, thin-bedded limestone separated from the lower unit by an erosional unconformity; and an upper marine unit of reddish-brown, calcareous siltstone, with minor limestone. Some of the lower fluvial rocks in western Grand Canyon include interbedded limestone and shale, suggesting brief marine incursions into paleoriver drainages (Billingsley and Beus, Reference Billingsley, Beus, Billingsley and Beus1999a).
The sequence in the paleovalley fills is interpreted as representing deposition in channels developed in the Redwall Limestone. These were shallow deltaic and tidal drainage channels formed during the westward retreat of the sea in which the Redwall Limestone had accumulated. Development of karst and entrenchment of the channels probably occurred in late Meramecian or early Chesterian times (Billingsley and Beus, Reference Billingsley, Beus, Billingsley and Beus1999a) and led to the development of a network of drainages. Highlands to the east became a source area for detrital material that was incorporated into deposits of the Surprise Canyon Formation. In the late Chesterian/Serpukhovian a period of subsidence allowed marine waters to gradually flood the eroded valleys forming local estuaries (Billingsley and Beus, Reference Billingsley, Beus, Billingsley and Beus1999a). As the sea transgressed, the estuaries also moved eastwards, their deposits forming the marine middle and upper units of the Surprise Canyon Formation. A minor unconformity between the Surprise Canyon Formation and the overlying Watahomigi Formation (the basal formation of the Supai Group) suggests a regional interval of uplift and erosion (Billingsley and Beus, Reference Billingsley, Beus, Billingsley and Beus1999a).
The initial age determination of the Redwall and Watahomigi formations was based on calcareous foraminifera (Skipp, Reference Skipp, McKee and Gutschick1969) and corals (Sando, Reference Sando, McKee and Gutschick1969). Based on these studies, the youngest Redwall strata were thought to be Osagean or early Meramecian and the lowest Watahomigi beds were thought to be Middle Pennsylvanian. This meant that the erosional unconformity between them represented the Late Mississippian and the Early Pennsylvanian. The subsequent discovery of an erosional remnant of Redwall containing late Meramecian or early Chesterian foraminifera and corals (Skipp, Reference Skipp, McKee and Gutschick1969; Sando, Reference Sando, McKee and Gutschick1969) reduced the gap between the Redwall and the Watahomigi formations to the Chesterian and part of the Morowan. This gap was further narrowed by the determination of a mid-Morrowan (Bashkirian) age for the lowermost units of the Watahomigi Formation based on brachiopods (Gordon, Reference Gordon and McKee1982).
Conodonts were first used to determine an age for the Redwall Limestone and these indicated that in east-central Arizona the youngest Redwall strata were late Meramecian in age (Racey, Reference Racey1974). Later work by Grover (Reference Grover1989) recovered conodonts from several limestones within the Surprise Canyon Formation and demonstrated the presence of the late Chesterian unicornis zone and the Early Pennsylvanian primus zone, suggesting that the Mississippian-Pennsylvanian boundary was encompassed by the Surprise Canyon Formation. A conodont study was undertaken to verify dates through the Surprise Canyon Formation and into the Watahomigi Formation (Martin and Barrick, Reference Martin, Barrick, Billingsley and Beus1999). Based on a sparse, low-diversity conodont fauna, Martin and Barrick (Reference Martin, Barrick, Billingsley and Beus1999) determined that the Surprise Canyon Formation is latest Chesterian (Late Mississippian) in age and that the Mississippian-Pennsylvanian boundary occurs in the lower part of the Watahomigi Formation, 17 m above the Surprise Canyon-Watahomigi contact. These determinations are supported by the invertebrate, palynomorph, and foraminiferal data (Beus and Martin, Reference Beus, Martin, Billingsley and Beus1999). Although ‘shark teeth’ were noted in the residues from several of the localities (Martin and Barrick, Reference Martin, Barrick, Billingsley and Beus1999), no attempt was made to have these identified. Dermal denticles are also present in these residues, but are not dealt with here.
Localities
At present eight localities within the Grand Canyon have produced identifiable vertebrate fossils from the Surprise Canyon and Watahomigi formations (Fig. 1). However, of these eight localities, only four show significant fish assemblages from the transitional lower/middle, middle, and upper members of the Surprise Canyon Formation and the lower member of the Watahomigi Formation, while the other localities had a few taxa respectively (Fig. 2). Precise coordinates for the localities are on file at the Museum of Northern Arizona.
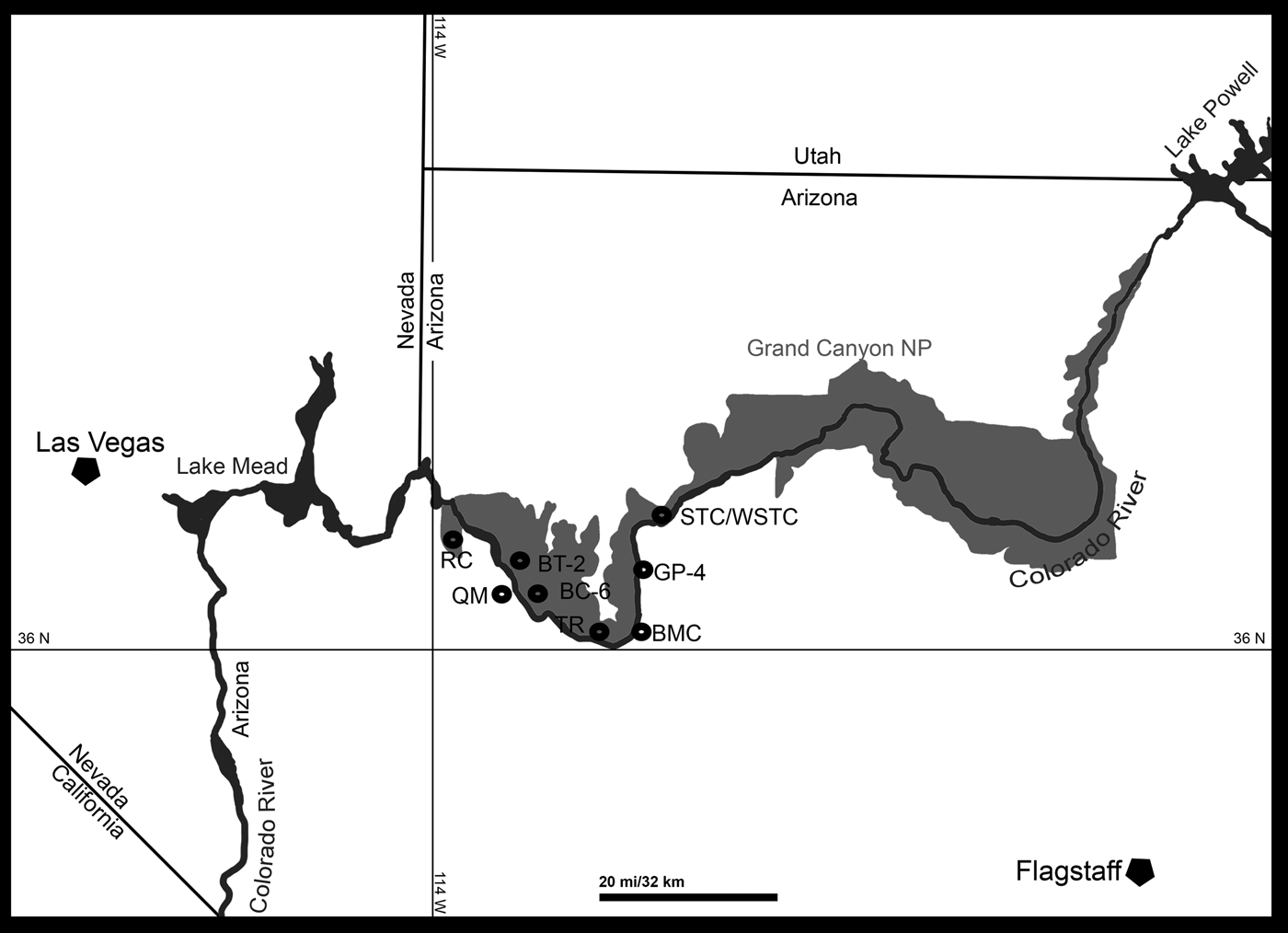
Figure 1. Map of the sampled localities within the Grand Canyon National Park. BC-6, Burnt Springs Canyon section 6; BMC, Blue Mountain Canyon locality; BT-2, Bat Tower locality 2; GP-4, Granite Park section 4; QM, Quartermaster Canyon locality; RC, Rampart Cave Section; STC/WSTC, Stairway Canyon/Western Stairway Canyon; TR, Travertine Canyon locality; (Billingsley and Beus, Reference Billingsley, Beus, Billingsley and Beus1999b).
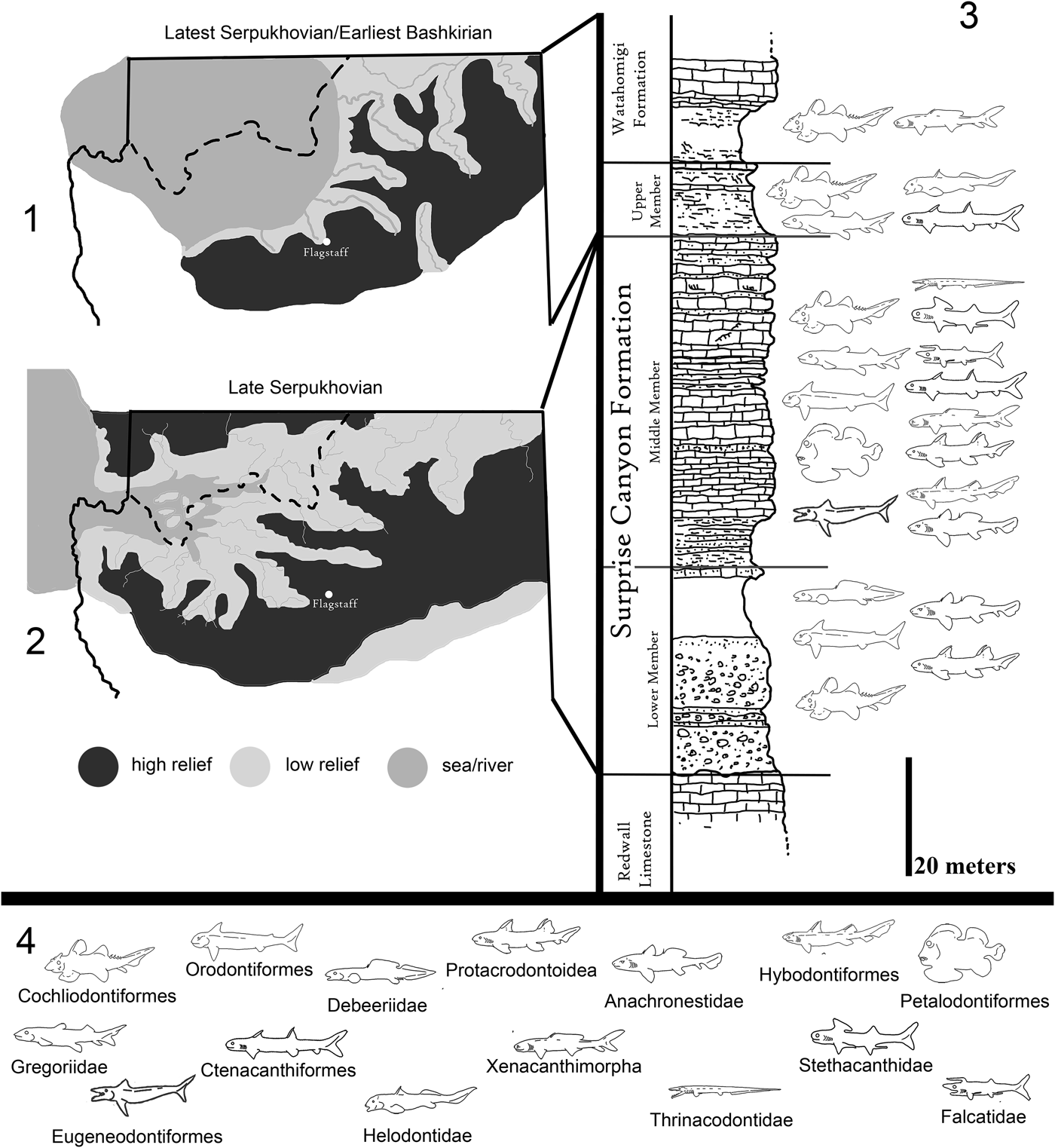
Figure 2. Paleogeographic maps of the Serpukhovian and Bashkirian of the Grand Canyon and generalized stratigraphic distribution of chondrichthyan taxa within the Surprise Canyon and Watahomigi formations. (1) Paleogeographic map of the open marine phase of the upper member of the Surprise Canyon Formation and the lower Watahomigi Formation; (2) paleogeographic map of the shallow marine and estuarine phase of the lower and middle members of the Surprise Canyon Formation; (3) type section, Bat Tower locality 2, showing the general distribution of chondrichthyan taxa within the Surprise Canyon and Watahomigi formations; (4) the major chondrichthyan taxonomic groups presented in this study.
Surprise Canyon Formation, lower member
The majority of shark fossils collected from the lower member of the Surprise Canyon Formation consist of large isolated dental and dermal elements in fluvial or estuary deposits, with lack of abrasion suggesting that they had not been significantly transported. Isolated cochliodont tooth plates were collected from unit 6 of the Rampart Cave (RC) section of the western Grand Canyon, which is a thin-bedded, yellowish-orange and grayish-green siltstone and sandstone that contained crossbedding, ripple marks, and abundant plant fragments and carbonized wood (Billingsley and Beus, Reference Billingsley, Beus, Billingsley and Beus1999b). The Rampart Cave Canyon paleovalley had a width of 396 m and a depth of 51.2 m (Billingsley and Beus, Reference Billingsley, Beus, Billingsley and Beus1999b). A single isolated fragmentary fin spine was collected from unit 4 of Quartermaster Canyon (QM) locality 2 section in the western Grand Canyon, from a dark purplish-brown, medium- to coarse-grained sandstone and light-gray, thin-bedded, siltstone, which was noted for numerous bone fragments (Billingsley and Beus, Reference Billingsley, Beus, Billingsley and Beus1999b). The Quartermaster Canyon paleovalley had an estimated width of ~305 m and a depth of 60.4 m (Billingsley and Beus, Reference Billingsley, Beus, Billingsley and Beus1999b). A single cochliodont dental plate was collected from unit 1 of the Travertine Canyon (TR) section of the western Grand Canyon, which consists of sandstone and conglomerate of angular white and gray chert pebbles, in dark reddish-brown to black, medium- to very coarse-grained sandstone that was noted for large vertebrate bone fragments (Billingsley et al., Reference Billingsley, Beus, Grover, Billingsley and Beus1999; Billingsley and Beus, Reference Billingsley, Beus, Billingsley and Beus1999b). The Travertine Canyon paleovalley has an estimated width of 92 m and a depth of 25.5 m (Billingsley and Beus, Reference Billingsley, Beus, Billingsley and Beus1999b). A cochliodont dental plate and isolated fin spine were collected from the Granite Park Wash (GP-4) section 5, which is a massive conglomerate bed composed of subrounded red chert pebbles in a dark-gray limestone that also contained solitary corals and spiriferid brachiopods (Billingsley and Beus, Reference Billingsley, Beus, Billingsley and Beus1999b). The Granite Park paleovalley is estimated to be 350 m wide and 64.9–68.9 m deep (Billingsley and Beus, Reference Billingsley, Beus, Billingsley and Beus1999b).
Surprise Canyon Formation, transitional lower/middle member
A small but diverse assemblage of microvertebrate fossils was collected from conodont residues from the Burnt Springs Canyon (BC) locality from section 10, which is a calcareous light orange-brown, fine- to medium-grained, thinly laminated to thin-bedded sandstone that was treated by Billingsley and Beus (Reference Billingsley, Beus, Billingsley and Beus1999b) as the lowermost section of the middle member. However, the lithology is also similar to some of the upper sections of the lower member elsewhere in the Surprise Canyon Formation and may represent either the uppermost lower member or the lowermost middle member of the Surprise Canyon Formation. The paleovalley of this section of the Burnt Springs Canyon is estimated to be 425 m wide and 89.2 m deep (Billingsley and Beus, Reference Billingsley, Beus, Billingsley and Beus1999b).
Surprise Canyon Formation, middle member
The majority of the diversity seen in the sharks from the Surprise Canyon Formation occurs in the middle member. Bat Tower Overlook (BT-2) is the type section for the Surprise Canyon Formation and a diverse microvertebrate assemblage was collected from sections 5 and 6 of the middle member from conodont residues. Section 5 of the type section consists of a thinly bedded, dark reddish-brown to yellowish-gray marine limestone with brachiopods, small gastropods, crinoids, and abundant shell fragments (Billingsley and Beus, Reference Billingsley, Beus, Billingsley and Beus1999b). Section 6 consists of a thin dark purplish-gray limestone with separations of thin silty limesone beds and contains brachiopods (Billingsely and Beus, Reference Beus, Billingsley and Beus1999). The paleovalley at this section of the Bat Tower Overlook is estimated to be 305 m wide and 91.1 m deep.
A diverse micro-vertebrate assemblage was collected from conodont residues from the Blue Mountain Canyon (BMC) section 3 and section 4 from the top of unit 2. Unit 2 consists of a medium yellowish-gray skeletal limestone with abundant brachiopods near the base (Billingsley and Beus, Reference Billingsley, Beus, Billingsley and Beus1999b). The Blue Mountain Canyon paleovalley is estimated to be 140 m wide with a depth of 22–47.9 m (Billingsley and Beus, Reference Billingsley, Beus, Billingsley and Beus1999b). Macro-vertebrate fossils were collected from unit 3 of the West Stairway Canyon section (WSTC), which consists of a conglomerate of pale-red to yellow chert pebbles in a light yellowish-gray limestone matrix with brachiopods and crinoid plates (Billingsley and Beus, Reference Billingsley, Beus, Billingsley and Beus1999b). The paleovalley at West Stairway Canyon is estimated to be 140 m wide and 19.5 m deep. The West Stairway Canyon section is presently the most easterly locality of the middle member of the Surprise Canyon Formation to produce vertebrate fossils within the Grand Canyon.
Surprise Canyon Formation, upper member
Vertebrate fossils from the upper member are primarily known from isolated macro-vertebrates, with the exception of a small assemblage from the Bat Tower 2 type section. This assemblage was collected from unit 7 of the Bat Tower 2 type section, which consists of a red shale or siltstone and gray limestone beds. The Stairway Canyon (STC) section has produced macro-vertebrate fossils from two units within the upper member. Unit 3 produced a few cochliodont dental plates and marks the lowest unit within the upper member at Stairway Canyon, which consists of a dark reddish-brown to purple mudstone with brachiopod, coral, and bryozoan debris. A single large cladodont tooth was collected from unit 5 of the Stairway Canyon section, which consists of a medium-gray sandy to silty limestone and pale red siltstone. The paleovalley at Stairway Canyon is estimated to be 122 m wide and 39 m deep (Billingsley and Beus, Reference Billingsley, Beus, Billingsley and Beus1999b). Both unit 3 and unit 5 of the Stairway Canyon section mark the easternmost vertebrate localities within the upper member of the Surprise Canyon Formation in the Grand Canyon.
Watahomigi Formation
The fossils decribed here were collected primarily from the lower Watahomigi Formation of the western Grand Canyon. A small microvertebrate assemblage is identified from Quartermaster Canyon (QM) in unit 12 of section 4, which consists of lavender to purplish-gray limestone and shale (Martin, Reference Martin1992). An isolated cochliodont tooth was collected from the lower Watahomigi Formation, “10 meters above the Surprise Canyon Formation” from an undisclosed locality close to Three Springs Canyon in the western Grand Canyon by George Billingsley in 1984 (Smithsonian Paleobiology records). At this phase of the transgression, all paleovalleys were filled.
Materials and methods
All samples were collected as part of the initial surveys of the Surprise Canyon and Watahomigi formations (Martin, Reference Martin1992; Billingsley and Beus, Reference Billingsley, Beus, Billingsley and Beus1999a). The macrofossils were mechanically prepared in some cases, and all specimens were photographed after coating with ammonium chloride. Microfossils were collected from conodont residues that were processed by crushing bulk samples and then breaking them down with acetic acid and screening the residue (see Martin, Reference Martin1992 for details of methods used). Microfossils were illustrated using SEM imagery at the Northern Arizona University Imaging and Histology Core Facility using a Zeiss Supra 40VP field emission scanning electron microscope. Because acid etching caused extreme fragility, some specimens were illustrated in a limited number of views.
Repositories and institutional abbreviations
AMNH, American Museum of Natural History, New York; BGS, British Geological Survey, Keyworth, England; BYU, Bringham Young University, Provo, UT; CM, Carnegie Museum of Natural History, Section of Vertebrate Paleontology, Pittsburgh, PA; IGPUW, Institute of Geology, University of Warsaw, Poland; KUVP, University of Kansas Vertebrate Paleontology Collection, Lawrence, KS; MCZ, Harvard University Museum of Comparative Zoology, Cambridge, MA; MNA, Museum of Northern Arizona, Flagstaff, AZ; MV, University of Montana Vertebrate Collections, Missoula, MT; NHM-P, Natural History Museum, London; TMM, Texas Memorial Museum, Austin, TX; USNM PAL, Department of Paleobiology, Smithsonian Institution, National Museum of Natural History, Washington, DC; ZPAL, Institute of Palaeobiology, Polish Academy of Sciences, Warsaw, Poland.
Systematic paleontology
Class Chondrichthyes Huxley, Reference Huxley1880
Subclass Elasmobranchii Bonaparte, Reference Bonaparte1838
Order Phoebodontiformes Ginter, Hairapetian, and Klug, Reference Ginter, Hairapetian and Klug2002
Family Thrinacodontidae Grogan and Lund, Reference Grogan and Lund2008
Genus Thrinacodus St. John and Worthen, Reference St. John and Worthen1875
Thrinacodus gracia Grogan and Lund, Reference Grogan and Lund2008

Figure 3. Teeth of Thrinacodus gracia. (1) MNA V11260, tooth in occlusal view; (2) MNA V11256, tooth in oblique labial view; (3) MNA V11255; (4) MNA V11261, tooth in occlusal view; (5) MNA V11254, tooth in occlusal view. Scale bars = 200 µm.
Holotype
Complete female specimen (CM 62724) from Bear Gulch Limestone lens, Bear Gulch Member of the Heath Formation, SW of Becket, Fergus County, Montana, USA.
Occurrence
Surprise Canyon Formation; middle member; latest Mississippian (Serpukhovian); Bat Tower 2, sections 13 and 15; Blue Mountain Canyon locality 4, section 2; Blue Mountain Canyon locality 84-9, section 3.
Description
Six small tricuspid teeth, in various degrees of preservation. The distal cusp is larger than the median and lateral cusps. The median cusp is positioned near the labial border of the tooth. The labial surfaces of the cusps are smooth with coarse cristae ornamenting the lingual surface. Orientation of the cusp row varies from being nearly in line to slightly angled mesially. The lingual torsus of the tooth base is broad mesiodistally and moderately elongated lingually. The lingual torsus is bulbous, tall, and not flattened. A large lingual foramen is present, just adjacent to the midline of the tooth.
Material
MNA V11254, MNA V11255, MNA V11256, MNA V11257, MNA V11258, MNA V11259, MNA V11260, MNA V11261.
Remarks
The majority of the thrinacodont teeth are of the morphology seen in the eel-like chondrichthyan Thrinacodus gracia. Teeth of T. gracia are characterized by conical cusps with coarse cristae on the lingual margin, which are moderately distally displaced with the mesial cusp the largest (Grogan and Lund, Reference Grogan and Lund2008; Ginter et al., Reference Ginter, Hampe, Duffin and Schultze2010). The lingual torsus in T. gracia is not oro-aborally flattened and spatulate in form, but relatively thick and bulbous (Grogan and Lund, Reference Grogan and Lund2008; Ginter and Turner, Reference Ginter and Turner2010). The taxonomic nomenclature for T. gracia has been the subject of much debate because previous work on thrinacodont dentitions was based primarily on isolated teeth and T. gracia is the only species known from multiple complete endoskeletons (Turner, Reference Turner1983; Long, Reference Long1990; Duncan, Reference Duncan2003; Grogan and Lund, Reference Grogan and Lund2008; Ginter et al., Reference Ginter, Hampe, Duffin and Schultze2010; Ginter and Turner, Reference Ginter and Turner2010).
Two teeth, MNA V11254 and MNA V11261 (Fig. 3.2, 3.5), have some resemblance to other thrinacodont taxa. MNA V11254 is similar to Thrinacodus dziki Ginter et al., Reference Ginter, Duffin, Dean and Korn2015 based on the lingual placement of the median cusp and the presence of a labial bulge with a labial foramen (Fig. 3.5; Ginter et al., Reference Ginter, Duffin, Dean and Korn2015). However, the Surprise Canyon Formation specimen differs from the type specimens in having overall a more robust and shorter lingual torsus than in the type material from the Visean of northern Europe (Ginter et al., Reference Ginter, Duffin, Dean and Korn2015; Smith et al., Reference Smith, Martill and Duffin2017), which is more lingually elongated and slender mesiodistally. MNA V11261 is similar to Thrinacodus ferox Turner, Reference Turner1982. This specimen is the only example of a thrinacodont tooth with an enlarged distal cusp and reduced median and mesial cusps from the Surprise Canyon samples (Fig. 3.4). Turner (Reference Turner1982) first described thrinacodont teeth with these features from the Late Devonian of Australia. She placed them in a new taxon Harpago ferox, which was later designated Harpagodens ferox because the previous genus name was preoccupied (Turner, Reference Turner1983). Later, it was determined that this taxon should be placed in the preexisting Thrinacodus (St. John and Worthen, Reference St. John and Worthen1875) as T. ferox (Long, Reference Long1990). Thrinacodus ferox is presently the only thrinacodont taxon to have the feature of an enlarged and recurved distal cusp with the medial and mesial cusps reduced in size on the lateral teeth (Ginter and Turner, Reference Ginter and Turner2010; Ginter et al., Reference Ginter, Hampe, Duffin and Schultze2010). These two specimens are most likely rare variants belonging to a single species, T. gracia, within the Surprise Canyon assemblage.
Superorder Xenacanthimorpha Nelson, Reference Nelson1976
Order Bransonelliformes Hampe and Ivanov, Reference Hampe and Ivanov2007
Genus Bransonella Harlton, Reference Harlton1933
Bransonella nebraskensis Johnson, Reference Johnson1984

Figure 4. Teeth of Xenacanthomorpha. (1–4) Bransonella nebraskensis; (1, 2) MNA V11262, (1) labial view, (2) occlusal view; (3, 4) MNA V11263, (3) labial view, (4) occlusal view. (5–7) Hokomata parva n. gen. n. sp., MNA V11264, holotype, (5) labial view, (6) occlusal view, (7) oblique aboral view. Scale bars = 200 µm.
Holotype
Tooth (TMM 41647-328) from Peru locality, Sites 2 and 3, south of Peru, Nemaha County Nebraska, USA.
Occurrence
Surprise Canyon Formation; middle member; latest Mississippian (Serpukhovian); Blue Mountain Canyon locality 3, section 2; Blue Mountain Canyon locality 4, section 2.
Description
Two small tricuspid teeth with broken cusps and complete tooth bases. The cusps are mostly broken, with MNA V11262 (Fig. 4.1, 4.2) having a worn median cusp still present. The lateral cusps have larger diameters than the median cusp with large pulp cavities. The labial surface of MNA V11263 (Fig. 4.3) shows a series of cristae were present. The basolabial projection is mesiodistally broad and slightly recurved aborally. Two labial foramina are present on either side of the basolabial projection. The oral-lingual button is ovate in shape, broad mesiodistally, and does not merge with the lingual margin of the tooth. A large foramen is present on the lingual margin of the oral-lingual button.
Material
MNA V11262, MNA V11263.
Remarks
Bransonella is known from four species and ranges from the Middle Mississippian to the middle Permian worldwide (Elliott and Hodnett, Reference Elliott and Hodnett2013). Of these four, B. lingulata Ivanov and Ginter, Reference Ivanov and Ginter1996 and B. nebraskensis occur in the latest Mississippian. Although the Surprise Canyon specimens are fragmentary, features of the orolingual button identify which species of Bransonella they belong to. In B. lingulata the lingual margin of the orolingual button is merged with the lingual rim of the tooth base (Ivanov and Ginter, Reference Ivanov and Ginter1996). In B. nebraskensis the orolingual button is forward of the lingual rim and has a basal canal between the orolingual button and the lingual rim (Johnson, Reference Johnson1984; Ivanov and Ginter, Reference Ivanov and Ginter1996). The orolingual button in the Surprise Canyon specimens does not merge with the lingual rim and has a basal canal between the orolingual button and the lingual rim, indicating that they are B. nebraskensis.
Order Xenacanthiformes Berg, Reference Berg1937
Family Diplodoselachidae Dick, Reference Dick1981
Genus Hokomata new genus
Type species
Hokomata parva n. gen. n. sp.
Diagnosis
As for species, by monotypy.
Etymology
Hokomata (Yuman dialect), a mischievous Hualapai and Havasupai tribal deity who brought forth a great flood that would eventually carve the Grand Canyon.
Holotype
MNA V11264.
Diagnosis
A small diplodoselachian xenacanth with tall, near erect cusps. All cusps are smooth, lacking verticle cristae, with a slight mesial incline. Lateral and distal cusps labiolingually compressed with un-serrated carina. Median cusp evenly spaced from the lateral cusps, labiolingually rounded, and half the height of the lateral and distal cusps. Tooth base ovate; oral-aborally compressed, orolingual button near circular with a lingual shaft. Basolabial projection small and elliptical. Kidney-shaped aboral depression with four large foramina.
Occurrence
Watahomigi Formation; lower member; Early Pennsylvanian (Bashkirian); Quartermaster Canyon, locality 4, section 9.
Description
Single complete tricuspid tooth ~500 µm wide mesiodistally, 550 µm long labiolingually, and 500 µm in height (Fig. 4.5–4.7). Cusps elongated, nearly erect, with slight mesial orientation. Median cusp about half the height of distal and lateral cusps, which are labiolingually compressed with a smooth carina on mesiodistal margins of crown. Surface of cusps is smooth and lacks verticle cristae. The tooth base is approximately ovate and oral-aborally compressed. The tooth base is longer labiolingually than mesiodistally. A median foramen is present on the oral surface between the lingual side of the median cusp and the labial border of the orolingual button (Fig. 4.6). The orolingual button is near the center of the tooth base with a narrow lingually projecting shaft that terminates at the midline of the lingual margin. A few small foramina are present along the mesiodistal margins of this shaft. The orolingual button and the mesiodistal margins of the tooth base have a fine sponge-like texture. The basolabial projection is mesiodistally narrow and elliptical in shape with a small lingually directed wedge-like projection. A well-defined kidney-shaped depression is present medially on the aboral surface between the lingual margin of the basolabial projection and a third of the distance from the lingual margin. Four large foramina are present on the mesiodistal margins of this aboral depression, with the most labially positioned foramen being the largest.
Etymology
Latin parva, little.
Remarks
Hokomata parva n. gen. n. sp. posesses characters common to three other xenacanth genera: Orthacanthus, Triodus, and Hagenoselache. All three taxa are known from nearly complete specimens and occur in the Early Pennsylvanian (Bashkirian) of Europe and North America. Johnson and Thayer (Reference Johnson and Thayer2009) described the teeth of two xenacanth taxa, Orthacanthus donnelljohnsi Johnson and Thayer, Reference Johnson and Thayer2009 and Triodus elpia Johnson and Thayer, Reference Johnson and Thayer2009, from the Black Prince Limestone (Bashkirian) in the Swisshelm Mountains of southern Arizona. Additional Early Pennsylvanian Orthacanthus and Triodus taxa include O. gibbosus Binney, Reference Binney1841 and T. serratus Davis, Reference Davis1892 from England (Ginter et al., Reference Ginter, Hampe, Duffin and Schultze2010). Hagenoselache sippeli Hampe and Heidtke, Reference Hampe and Heidtke1997 is known from a single nearly complete individual from the Serpukovian/Bashkirian Hagen-Vorhalle of Germany (Hampe and Heidtke, Reference Hampe and Heidtke1997; Ginter et al., Reference Ginter, Hampe, Duffin and Schultze2010).
The lateral cusps of Hokomata parva n. gen. n. sp. are similar to those in Triodus and Hagenoselache in having a near-erect orientation. In Orthacanthus, the lateral cusps, particularly the mesially positioned cusp, angle away from the median cusp. The lateral cusps of H. parva differ from Triodus and Hagenoselache in being labiolingually compressed and lacking cristae on the distal tips. The lateral cusps of Orthacanthus are also labiolingually compressed, but those of H. parva differ in being less expanded mesiodistally and lacking serrations on the carina. The median cusp in Orthacanthus is labiolingually compressed and is positioned forward of and close to the lateral cusps. In H. parva n. gen. n. sp., Triodus, and Hagenoselache the median cusp is labiolingually rounded, but in H. parva it lacks distal cristae. There is a well-developed gap between the lateral cusps and the median cusp, and they are all nearly in line in H. parva n. gen. n. sp. In Triodus, the median cusp is positioned just forward of the lateral cusps with only a slight gap between the cusps. The median cusp in Hagenoselache is rounded (Hampe and Heidtke, Reference Hampe and Heidtke1997) and seems to be positioned nearly in line with the lateral cusps (Hampe and Heidtke, Reference Hampe and Heidtke1997; Fig. 4) but this is difficult to discern from the illustrations.
The tooth base of Hokomata parva n. gen. n. sp. is more oral-aborally compressed than in Triodus, Hagenoselache, and Orthacanthus. In H. parva, the mesiodistal width is proportionally broader than in Triodus and Hagenoselache, and it is also less elongated labiolingually, similar to the proportions seen in Orthacanthus. The orolingual button has a lingually directed shaft, a feature that is also seen in Triodus, Orthacanthus, and on some of the teeth of Hagenoselache (Hampe and Heidtke, Reference Hampe and Heidtke1997; Ginter et al., Reference Ginter, Hampe, Duffin and Schultze2010). The mesiodistal width of the basolabial projection in H. parva n. gen. n. sp. is narrower and not as broad mesiodistally as in Triodus. The basolabial projection has a small lingually oriented wedge-shaped projection, which is similar to that seen in Orthacanthus and Hagenoselache. There is a single median nutrient foramen orally between the median cusp and the orolingual button, a feature seen in Hagenoselache and some teeth of Triodus and Orthacanthus (Ginter et al., Reference Ginter, Hampe, Duffin and Schultze2010). A few small nutrient foramina also occur along the lingual shaft of the orolingual button in H. parva, which is similar to the condition seen in Triodus and Orthacanthus (Johnson and Thayer, Reference Johnson and Thayer2009; Ginter et al., Reference Ginter, Hampe, Duffin and Schultze2010). On the aboral surface of the tooth base in H. parva n. gen. n. sp., four large nutrient foramina are present, while Triodus and Orthacanthus typically have no more than two or three prominent foramina, with some smaller foramina (Ginter et al., Reference Ginter, Hampe, Duffin and Schultze2010). Hagenoselache can have up to seven nutrient foramina on the aboral surface of the tooth base (Ginter et al., Reference Ginter, Hampe, Duffin and Schultze2010).
Most xenacanths, excluding Bransonella, were associated with brackish and fresh water environments during the Mississippian/Pennsylvanian transition (Ginter et al., Reference Ginter, Hampe, Duffin and Schultze2010). The oldest xenacanthids are Diplodoselache woodi Dick, Reference Dick1981 from the Visean of the Oil Shale groups of Scotland, which comes from a lagoonal deposit that had both marine and brackish influences (Dick, Reference Dick1981), and Reginaselache morrisi Turner and Burrow, Reference Turner and Burrow2011 from the mid-Visean Ducabrook Formation of central Queensland, which comes from estuarine deposits (Turner and Burrow, Reference Turner and Burrow2011). Bransonella itself is primarily known from marine environments (Ivanov and Ginter, Reference Ivanov and Ginter1996; Elliott and Hodnett, Reference Elliott and Hodnett2013), but was also recovered from the estuarine Lower Pennsylvanian Black Prince Limestone in the Swisshelm Mountains in Southern Arizona, together with the xenacanths Triodus and Orthacanthus and lepospondyl amphibians (Thayer, Reference Thayer1985; Johnson and Thayer, Reference Johnson and Thayer2009). The Watahomigi Formation has no indication of brackish or fresh water influences and so we consider Hokomata parva n. gen. n. sp. to be a marine xenacanth.
Order Symmoriiformes Zangerl, Reference Zangerl and Schultze1981
Family Stethacanthidae Lund, Reference Lund1974
Stethacanthid indeterminate
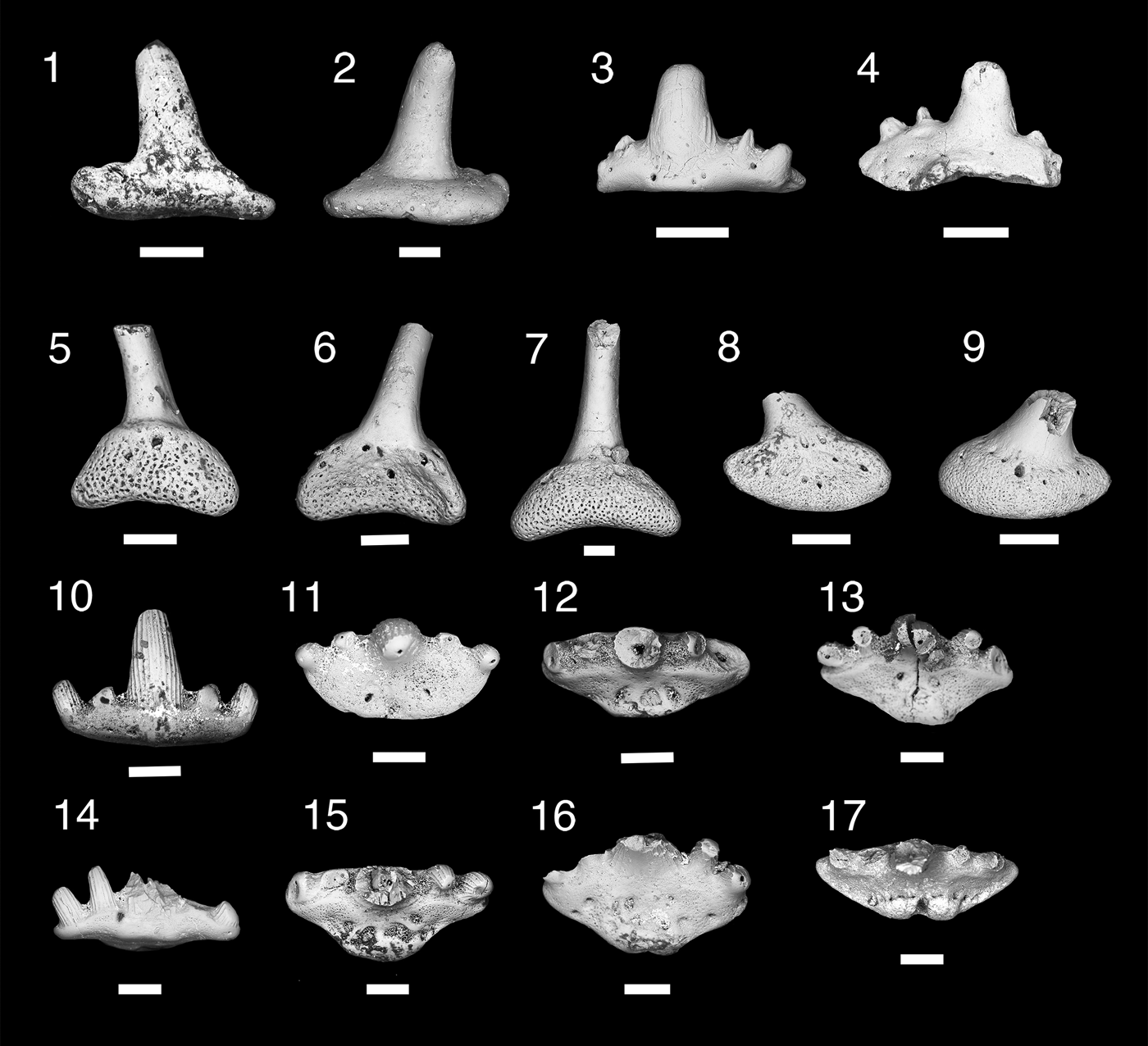
Figure 5. Teeth and denticles of Symmoriiformes. (1–9) Stethacanthid indeterminate; (1, 2) MNA V11265, tooth, (1) labial view, (2) lingual view; (3, 4) MNA V11266, tooth, (3) labial view, (4) lingual view; (5, 6) MNA V11268, denticle, (5) anterior view, (6) posterior view; (7) MNA V11267, denticle, posterior view; (8, 9) MNA V11269, denticle (8) anterior, (9) posterior. (10–16) Falcatid indeterminate 1; (10, 11) MNA V11270, (10) labial view, (11) occlusal view; (12) MNA V11272, occlusal view; (13) MNA V11274, occlusal view; (14, 15) MNA V11271, (14) labial view, (15) occlusal view; (16) MNA 11273, occlusal view. (17) Falcatid indeterminate 2, MNA V11275 occlusal view. Scale bars = 200 µm.
Occurrence
Surprise Canyon Formation; middle member; latest Mississippian (Serpukhovian); Bat Tower 2, section 13; Blue Mountain Canyon locality 3, section 2 and 3; Blue Mountain Canyon locality 4, section 2.
Description
Two small worn cladodont teeth (Fig. 5.1–5.4) and three denticles (Fig. 5.5–5.9). Teeth are ~600 µm wide mesiodistally, median cusp tall, narrow, and circular in cross section. Lateral and intermediate denticles worn, but were small relative to median cusp. Surfaces of cusps are smooth in MNA V11265 (Fig. 5.1, 5.2), possibly due to abrasion. MNA V11266 (Fig. 5.3, 5.4) shows coarse cristae on lateral surface of median cusp. Tooth base oval, wide mesiodistally, and compressed labiolingually in both specimens. Slight lingual boss present on oral-lingual margin of MNA V11265 (Fig. 5.2). Faint basolabial projection present on both specimens. Denticles (Fig. 5.5–5.9) monocuspid with the cusp long, narrow, and circular in cross section. Surface of cusp is smooth. Bases of denticles are elliptical with three to four lateral foramina and single ventral and dorsal foramina.
Material
MNA V11265, tooth; MNA V11266, tooth; MNA V11267, MNA V11268, MNA V11269, denticles.
Remarks
The poor preservation of the two teeth make a generic designation difficult, but these teeth can be classified as stethacanthid from the proportion of the cusps and position of the lingual boss. Stethacanthid dentitions are cladodont, but tend to have smaller lateral cusps in relation to the median cusp than in falcatid sharks, which have much larger lateral cusps (Ginter et al., Reference Ginter, Hampe, Duffin and Schultze2010). The lingual boss in stethacanthids is not positioned near the lingual margin as it is in the falcatid Denaea williamsi Ginter and Hansen, Reference Ginter, Hansen and Nowakowski2010, and tends to be positioned forward as a short longitudinal ridge or square boss that is approximately the width of the median cusp (Zidek, Reference Zidek1993; Coates and Sequeria, Reference Coates and Sequeira2001; Ginter and Hansen, Reference Ginter, Hansen and Nowakowski2010; Ginter et al., Reference Ginter, Hampe, Duffin and Schultze2010). Larger species, such as Stethacanthus altonensis St. John and Worthen, Reference St. John and Worthen1875 and Akmonistion zangerli Coates and Sequeria, Reference Coates and Sequeira2001, have coarse longitudinal cristae (Zidek, Reference Zidek1993; Coates and Sequeria, Reference Coates and Sequeira2001), similar to those seen in MNA V11266 but the Surprise Canyon specimen is much smaller. The teeth of the Surprise Canyon specimens fit the expected size range of dentition for the small stethacanthid Orestiacanthus fergusi Lund, Reference Lund1984, however at present the dentition of Orestiacanthus is not well known (Lund, Reference Lund1984; Ginter, et al., Reference Ginter, Hampe, Duffin and Schultze2010). Lund (Reference Lund1985a) described additional stethacanthids from Bear Gulch, which would fit the expected dental range for the Surprise Canyon specimens, but presently the dentition of these specimens is poorly understood. At present, the Surprise Canyon specimens are best regarded as stethacanthid indeterminate.
Family Falcatidae Zangerl, Reference Zangerl1990
Falcatid indeterminate 1
Occurrence
Surprise Canyon Formation, middle member, latest Mississippian (Serpukhovian); Bat Tower 2 overlook section 15.
Description
Five small cladodont teeth, ~600 µm wide mesiodistally. Cusps with longitudinal cristae and arranged in a slight arc on labial margin of the base. Median cusp tall, narrow, and circular in cross section with lateral and intermediate cusps angling away from it. In some specimens (MNA V11270, MNA V11271, and MNA V11274; Fig. 5.10, 5.11, 5.13–5.15), a lingual ridge present. In all specimens, lingual torsus of tooth base short, with two flanking oral foramina, and no lingual foramen or articulating orolingual boss. Some specimens have two or three slight oral depressions flanking midline of tooth base. Basolabial projection absent or forms slight narrow bulge.
Material
Five teeth: MNA V11270; MNA V11271; MNA V11272, MNA V11273, MNA V11274.
Remarks
These five teeth are characterized as being from a small falcatid shark based on their slender median cusp, subparallel cristae on both labial and lingual sides of the cusps, and narrow lingual torus of the tooth base (Lund, Reference Lund1985b; Ginter et al., Reference Ginter, Hampe, Duffin and Schultze2010). The lack of an orolingual boss on the lingual margin of the tooth eliminates placement of these teeth in Denaea, which bears this feature in varying degrees depending on species (Ginter and Hansen, Reference Ginter, Hansen and Nowakowski2010; Ginter et al., Reference Ginter, Hampe, Duffin and Schultze2010). The teeth of Stethacanthulus are characterized by the absence of the orolingual boss, but differ from the Surprise Canyon specimens in having a single median foramen piercing the lingual torus and a labiolingual groove on the oral surface (Zangerl, Reference Zangerl1990; Ginter et al., Reference Ginter, Hampe, Duffin and Schultze2010). Lund (Reference Lund1985b) described the teeth of Falcatus falcatus Lund, Reference Lund1985b as having a maximum base length of 0.3 mm and a narrow lingual torus with five cusps, but also noted the teeth were too small to determine if cristae were present. Lund (Reference Lund1986) described the teeth of Damocles serratus Lund, Reference Lund1986 as being five cusped, the median cusp the largest, and cusps ornamented with fine cristae. Ginter et al. (Reference Ginter, Hampe, Duffin and Schultze2010) noted that the lateral cusps were half the height of the median cusp. The lingual torus is relatively narrow with a vaguely defined orolingual button and has a very narrow, rounded basolabial projection (Lund, Reference Lund1986; Ginter et al., Reference Ginter, Hampe, Duffin and Schultze2010). Another possible identification for these specimens is Ozarcus mapesae Pradel et al., Reference Pradel, Maisey, Tafforeau, Mapes and Mallatt2014 from the Fayetteville Formation in Arkansas (Pradel et al., Reference Pradel, Maisey, Tafforeau, Mapes and Mallatt2014). Although the description by Pradel et al. (Reference Pradel, Maisey, Tafforeau, Mapes and Mallatt2014) focused primarily on the visceral skeleton, they did present images of teeth from computed tomography scanning. These teeth are ~1 mm wide mesiodistally with a very tall slender median cusp and lateral cusps almost half the height of the median cusp. In labial and lingual views, no well-developed orolingual or basolabial articulating processes appear to be present (Pradel et al., Reference Pradel, Maisey, Tafforeau, Mapes and Mallatt2014, extended data fig. 2C). At this stage, however, it is difficult to determine which taxon the teeth described here belong to, although superficially they look most similar to Falcatus falcatus, Damocles serratus, or Ozarcus mapesae. It is presently best to keep the designation to falcatid indeterminate 1 until new data and specimens became available. This taxon is known only from the type locality Bat Tower 2, section 15 and has not yet been identified elsewhere. Bat Tower 2 is located in the western more open water environment of the Surprise Canyon embayment.
Falcatid indeterminate 2
Occurrence
Surprise Canyon Formation; middle member; latest Mississippian (Serpukhovian); Blue Mountain Canyon locality 84-9, section 3.
Description
Single tooth of small falcatid shark with mesiodistal width of ~1 mm (Fig. 5.17). Cusps missing, but base of cusps suggests crown was circular with coarse cristae on labial and lingual sides. Cusps nearly in line and inclining lingually. Tooth base labiolingually compressed with a shallow elliptical shape. Lingual torus very slightly extended. Single well-developed foramen present on lingual margin, dividing two rounded orolingual projections that are merged with lingual rim and have five evenly spaced foramina labial to them.
Material
MNA V11275.
Remarks
The morphology of the orolingual projections in this specimen is fairly rare among the falcatids. The only other cladodont sharks with divided orolingual projections are the ctenacanths Glikmanius, Heslerodus, “Ctenacanthus” costellatus Traquair, Reference Traquair1884, Kaibabvenator, and Nanoskalme (Ginter et al., Reference Ginter, Hampe, Duffin and Schultze2010; Hodnett et al., Reference Hodnett, Elliott, Olson and Wittke2012). However, the placement of these structures in the ctenacanths is forward of the lingual rim and they do not have a complex of evenly spaced foramina along the labial side of the projections. Both Denaea saltsmani Ginter and Hansen, Reference Ginter, Hansen and Nowakowski2010 and D. williamsi have single orolingual projections on the lingual rim of the tooth base, which bears a single large nutrient foramen, and few nutrient foramina are present just labial of the orolingual projection (Ginter and Hansen, Reference Ginter, Hansen and Nowakowski2010; Ginter et al., Reference Ginter, Hampe, Duffin and Schultze2010). However, we feel that the unique morphology of the divided orolingual projections and the arrangement of the labially positioned oral nutrient foramen suggest that this is a unique taxon with a close relationship to Denaea.
Genus Denaea Pruvost, Reference Pruvost1922
Denaea williamsi Ginter and Hansen, Reference Ginter, Hansen and Nowakowski2010
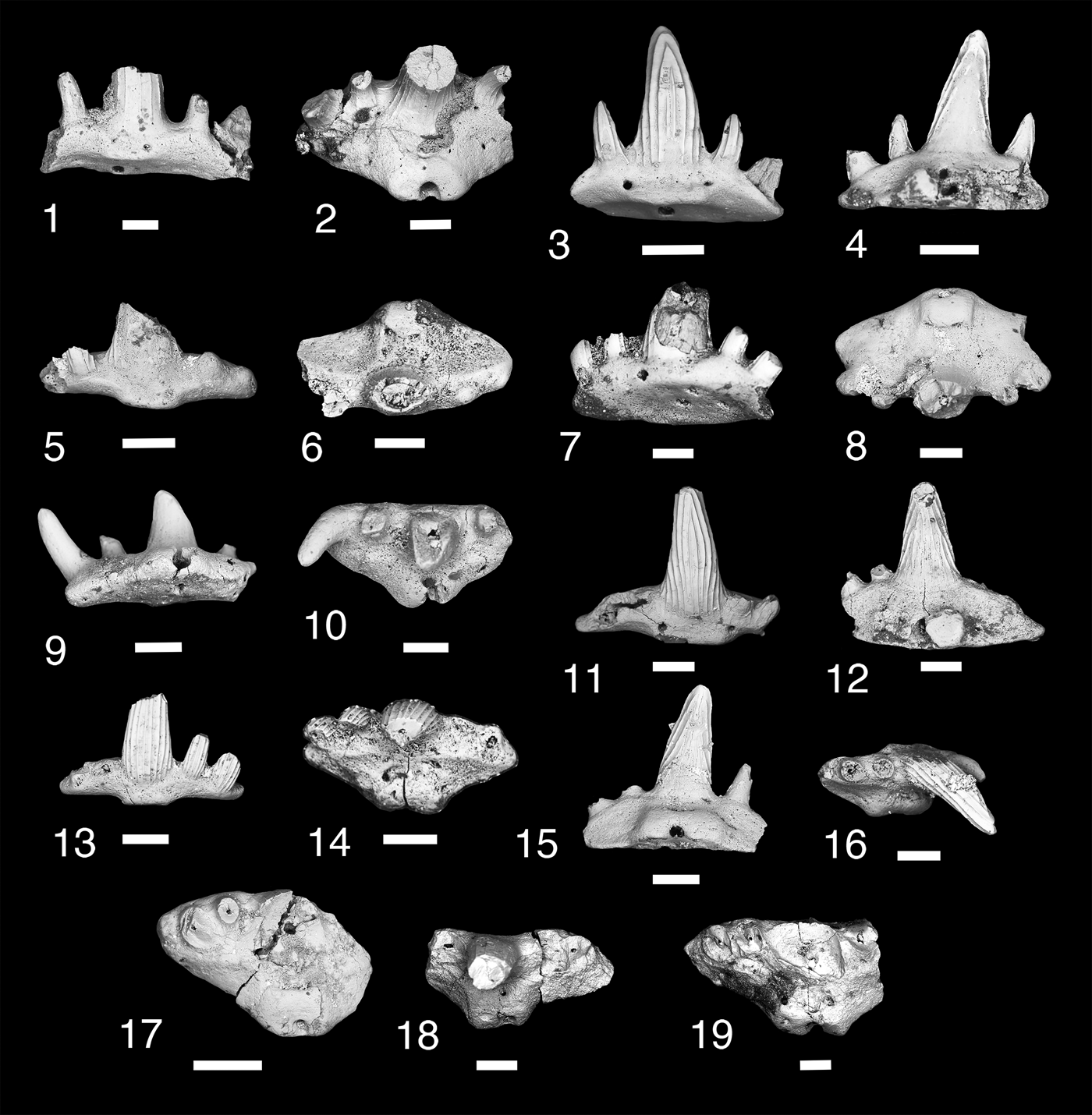
Figure 6. Teeth of Denaea williamsi. (1, 2) MNA V11277, (1) labial view, (2) occlusal view; (3, 4) MNA V11278, (3) labial view, (4) lingual view; (5, 6) MNA V11276, (5) labial view, (6) occlusal view; (7, 8) MNA V 11286, (7) oblique labial view, (8) occlusal view; (9, 10) MNA V11285, (9) lingual view, (10) occlusal view; (11, 12) MNA V11279, (11) labial view, (l2) lingual view; (13, 14) MNA V11280, (13) labial view, (14) occlusal view; (15, 16) MNA V11283, (15) lingual view, (16) occlusal view; (17) MNA V11281, occlusal view; (18) MNA V11284, occlusal view; (19) MNA V11287, occlusal view. Scale bars = 200 µm.
Holotype
Denaea williamsi Ginter and Hansen, Reference Ginter, Hansen and Nowakowski2010, p. 34, fig. 3. Tooth (IGPUW/Ps/8/1) from the Grove Creek Shale, unit 12, Cedar Grove Church, Johnson County Illinois, USA.
Occurrence
Surprise Canyon Formation; middle member; latest Mississippian (Serpukhovian); Bat Tower 2, section 13; Blue Mountain Canyon locality 4, section 2; Blue Mountain Canyon locality 84-9, section 3.
Description
Small cladodont teeth with five cusps recurving lingually. Labial surface of cusp with three to five coarse cristae. Lingual cusp surface has a few cristae that terminate a third of the distance from base of crown leaving distal part of lingual surface smooth. Median cusp tall, broad near base, circular in cross section, and tapers to a narrow point. Lateral and intermediate cusps splay mesiodistally with lateral cusp larger than intermediate cusp and about half the height of the median cusp. Tooth base trapezoidal. Square oral-lingual button present on median lingual margin, which has a large foramen in center. Basolabial projection thin and shallow, with labial aboral margin nearly flat. Single labial foramen present on either side of basolabial projection. Single median aboral foramen present.
Material
12 teeth: MNA V11276, MNA V11277, MNA V11278, MNA V11279, MNA V11280, MNA V11281, MNA V11282, MNA V11283, MNA V11284, MNA V11285, MNA V11286, MNA V11287.
Remarks
Ginter and Hansen (Reference Ginter, Hansen and Nowakowski2010) described this taxon from a series of teeth from the Serpukhovian of Illinois. It is also known from the late Visean of the Holy Cross Mountains, Poland (Ginter et al., Reference Ginter, Duffin, Dean and Korn2015), the Visean–Serpukhovian of Derbyshire, England, Scotland, and the Moscow Syneclise, Russia (Ginter et al., Reference Ginter, Hampe, Duffin and Schultze2010), and tentatively Denaea cf. D. williamsi from the late Serpukhovian fauna from the Gissar Mountains, Uzbekistan (Ivanov, Reference Ivanov, Lucas, DiMichelle, Barrick, Schneider and Spielmann2013). Denaea williamsi is known from the type section of the Surprise Canyon Formation (Bat Tower 2) and from the eastern near-shore Blue Mountain localities.
Order Ctenacanthiformes Glikman, Reference Glikman1964
Family Ctenacanthidae Dean, Reference Dean1909
Genus Cladodus Agassiz, Reference Agassiz1843
Cladodus cf. C. marginatus Agassiz, Reference Agassiz1843
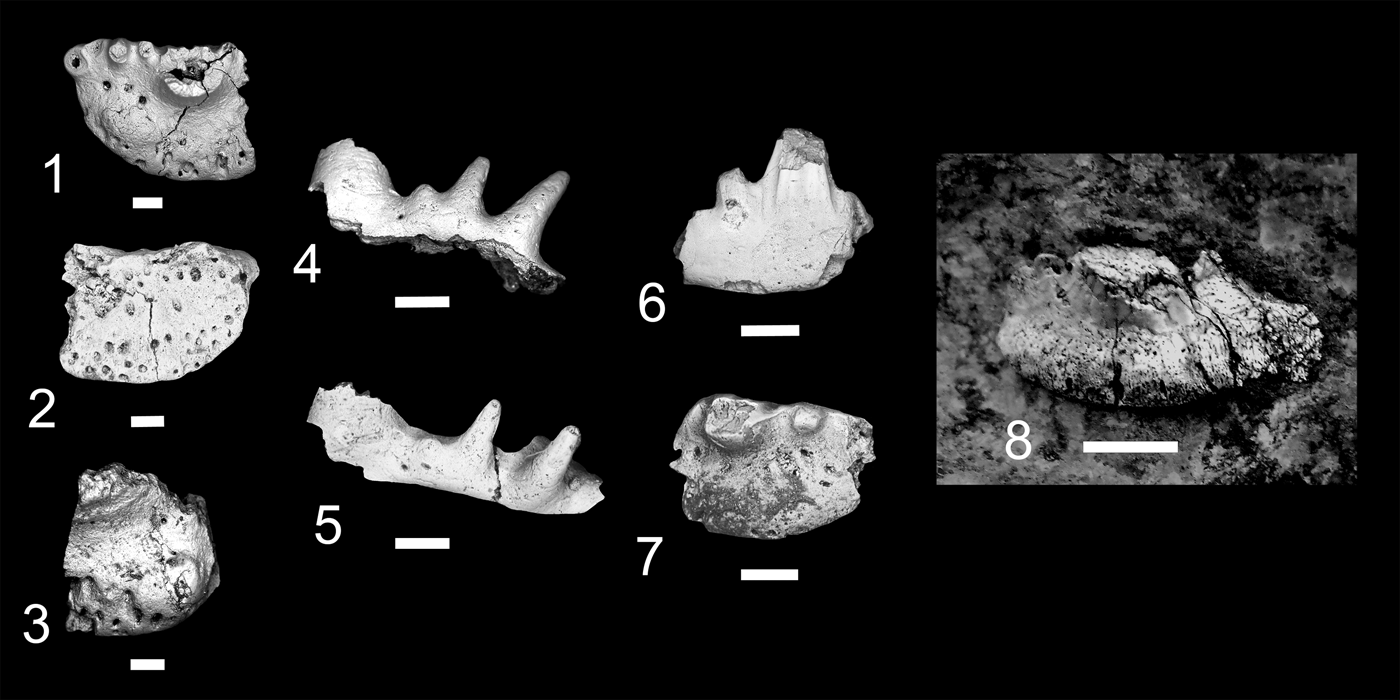
Figure 7. Teeth of Ctenacanthiformes. (1–5) cf. “Ctenacanthus” costellatus; (1, 2) MNA V11288, (1) occlusal view, (2) aboral view; (3) MNA V11289, occlusal view; (4, 5) MNA V11290, (4) labial view, (5) oblique occlusal view. (6, 7) ctenacanth indeterminate, MNA V11291, (6) labial view, (7) occlusal view. (8) Cladodus cf. C. marginatus, USNM PAL 412169, lingual view. Scale bars = 200 µm (1–7); 1 cm (8).
Occurrence
Surprise Canyon Formation, upper member, Late Mississippian (Serpukhovian), Stairway Canyon, unit 5.
Description
Tooth base, ~160 mm wide, exposed lingually in a sandy limestone nodule (Fig. 7.8). Cusps broken or worn. Cross section of median cusp indicates it was labiolingually compressed. Lateral cusps closely spaced and originate together on labial margin of tooth base, which is reniforme or “D-shaped.” Oral-lingual ridge forms near lingual margin of tooth, terminating approximately at intermediate cusps, and has two lingual foramina.
Material
USNM PAL 412169, tooth.
Remarks
This specimen is the largest of the “cladodont” tooth morphs from the Surprise Canyon Formation. Its placement as a ctenacanthiform is supported by remnants of a prominent median cusp, convex lingually and labially flattened, the cusps connected by enamel, and a lingually deep base with a wide orolingual ridge that is wider than the median cusp (Duffin and Ginter, Reference Duffin and Ginter2006; Ginter et al., Reference Ginter, Hampe, Duffin and Schultze2010). Identification as Cladodus is based on its mesiodistally wide median cusp, wide tooth base with wide orolingual ridge, and its relatively large size. The tentative placement of this specimen as Cladodus cf. C. marginatus is based on the relatively close position of the lateral and intermediate cusps to the median cusp and the labiolingual compression of all the cusps (Duffin and Ginter, Reference Duffin and Ginter2006; Ginter et al., Reference Ginter, Hampe, Duffin and Schultze2010). The fragmentary nature of USNM PAL 412169 means this identification is tentative. This represents the youngest occurrence of C. marginatus and the first record of this taxon outside the vicinity of Armagh, Northern Ireland (Duffin and Ginter, Reference Duffin and Ginter2006; Ginter et al., Reference Ginter, Hampe, Duffin and Schultze2010). This specimen was collected from the easternmost Stairway Canyon locality from the upper member of the Surprise Canyon Formation, which was formed during the open water phase of deposition. The paleogeographic position of the locality suggests that the tooth was deposited in open water, some distance from the paleo-shoreline.
Family uncertain
cf. “Ctenacanthus” costellatus Traquair, Reference Traquair1884
Holotype
Ctenacanthus costellatus Traquair, Reference Traquair1884, p. 3, pl. 2, figs. 1–7. Nearly complete specimen (NHM-P 5900) from Eskdale, Dumphreshire, Scotland.
Occurrence
Surprise Canyon Formation; middle member; latest Mississippian (Serpukhovian); Blue Mountain Canyon locality 4, section 2.
Description
Three small fragmentary teeth. A small fragment with no tooth base has worn median cusp, lateral cusp, and two intermediate cusps inclined mesially (Fig. 7.4, 7.5). Two fragmentary tooth bases have rounded oral-lingual buttons and elliptical basolabial projections.
Material
Three teeth: MNA V11288, MNA V11289, MNA V11290.
Remarks
These teeth are tentatively identified as cf. “Ctenacanthus” costellatus based on having two intermediate cusps and two separate button-like orolingual and basolabial projections, as also seen in the specimens referred to by Ginter (Reference Ginter2002) from the type locality of Eskdale, Dumphrieshire, Scotland (Traquair, Reference Traquair1884; Moy-Thomas, Reference Moy-Thomas1936; Ginter, Reference Ginter2002; Ginter et al., Reference Ginter, Hampe, Duffin and Schultze2010). The Surprise Canyon specimens differ from the Eskdale specimens in that the orolingual projections are spaced farther apart and are found closer to the lingual margin of the tooth base. The two separate orolingual and basolabial projections are also found in the Pennsylvanian and Permian genera Glikmanius, Heslerodus, Kaibabvenator, and Nanoskalme; although these taxa have variable cusp morphologies (although typically a single or no intermediate cusp) and basolabial depressions (Ginter, Reference Ginter2002; Ginter et al., Reference Ginter, Ivanov and Lebedev2005, Reference Ginter, Hampe, Duffin and Schultze2010; Hodnett et al., Reference Hodnett, Elliott, Olson and Wittke2012). The generic status of “C.” costellatus needs review as Ginter et al. (Reference Ginter, Hampe, Duffin and Schultze2010) has shown that the teeth of the Devonian Ctenacanthus differ from those from the teeth of “C.” costellatus in having a single ridge for the orolingual and basolabial projections. Along with the specimens from Glencartholm, Scotland (Visean) (Traquair, Reference Traquair1884; Moy-Thomas, Reference Moy-Thomas1936), “C.” costellatus has also been recognized from the late Visean of Ticknall, Derbyshire, England, and the Holy Cross Mountains, Poland (Ginter et al., Reference Ginter, Duffin, Dean and Korn2015). The Surprise Canyon species is only known from the near shore deposit of Blue Mountain Canyon locality 4, in the lower second section. This may be the youngest and the first Western Hemisphere example of “C.” costellatus.
Ctenacanthidae indeterminate
Occurrence
Surprise Canyon Formation; middle member; latest Mississippian (Serpukhovian); Blue Mountain Canyon locality 4, section 2.
Description
Tooth fragment. Median cusp narrow with coarse cristae. Intermediate cusp small, closely associated with median cusp. Tooth base reniform with low basolabial ridge, wider than median cusp and thick dorsoventrally. Orolingual ridge weakly developed along lingual rim and wider than median cusp. Basolabial depression is absent.
Material
Tooth fragment: MNA V11291.
Remarks
An indeterminate ctenacanth is present in the Surprise Canyon Formation, which differs from the “Ctenacanthus” costellatus and Cladodus marginatus morphs. The tooth base fragment is similar to Cladodus in being reniform in shape and having elongated orolingual and basolabial ridges, although rather weakly developed. The basolabial depression, which is moderately developed in Cladodus, is absent in this taxon. The enamel on the preserved crowns does connect between the cusps, which is a ctenacanth trait (Ginter et al., Reference Ginter, Hampe, Duffin and Schultze2010). This tooth is similar to two indeterminate ctenacanth taxa from the Mississippian. Behan et al. (Reference Behan, Walken and Cuny2012) described a series of teeth, designated Ctenacanthiformes indet. A, from the Tournasian karst residues of Cromhall Quarry, Gloucestershire, England. This tooth morph has a prominent median cusp with coarse cristae, a mesiodistally wide tooth base with very little indication of a basolabial depression, and a slight orolingual ridge along the lingual rim (Behan et al., Reference Behan, Walken and Cuny2012). However, the Surprise Canyon taxon differs from the Cromhall Quarry taxon in the position of the intermediate cusp, which is closer in association to the median cusp and has a thicker tooth base. Ginter et al. (Reference Ginter, Duffin, Dean and Korn2015) described two teeth from the late Visean of the Holy Cross Mountains of Poland, which are also similar to the Surprise Canyon and Cromhall Quarry taxa in having a median cusp with a few coarse labial cristae and seemingly having an underdeveloped basolabial depression. The Holy Cross taxon differs from the Surprise Canyon and Cromhall taxa in having an orolingual ridge that is more well developed, and less wide mesiodistally than the other two taxa. Ginter et al. (Reference Ginter, Duffin, Dean and Korn2015) considered that the Holy Cross taxon represented juvenile specimens; in contrast we feel that the Surprise Canyon taxon may represent a small adult form.
Primitive Elasmobranchii incertae sedis
Genus Clairina Ginter, Reference Ginter1999
Clairina sp.
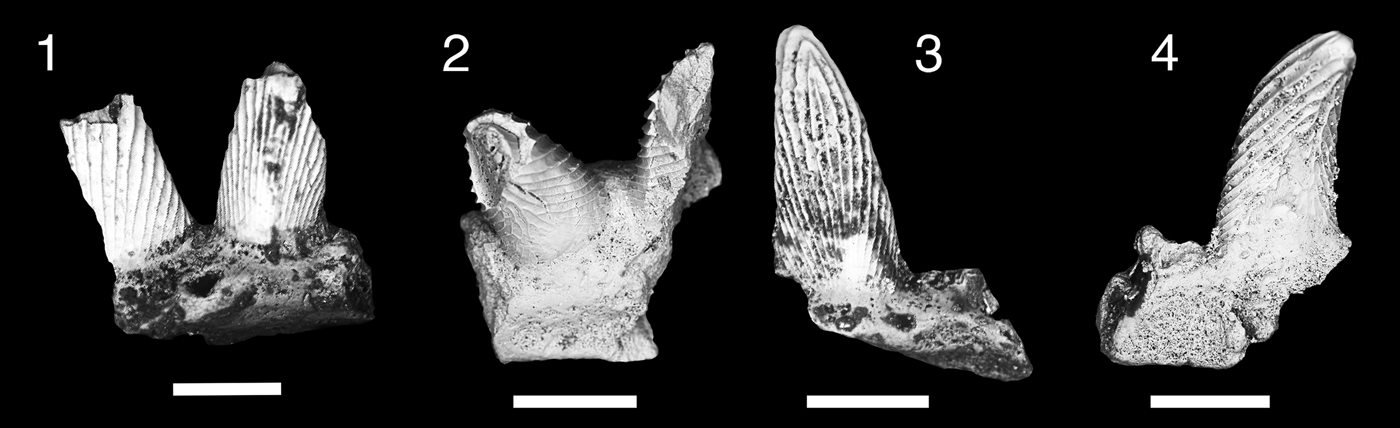
Figure 8. Teeth of Clairina sp. (1, 2) MNA V11292, (1) labial view, (2) lingual view; (3, 4) MNA V11293, (3) labial view, (4) lingual view. Scale bars = 200 µm.
Occurrence
Surprise Canyon Formation, middle member, latest Mississippian (Serpukhovian), Bat Tower Locality 2, sections 13 and 15.
Description
Two small fragmentary multicuspid teeth. Cusps long and slender, recurving lingually. Labial surface of cusps covered by elongated, overlapping, leaf-shaped cristae that narrow towards the base. Lingual surface of the cusp covered by short v-shaped cristae that diverge towards mesiodistal edge of the cusp. One to two vein-like cristae also occur on the midline of lingual side of cusp. The base is moderately flattened and plate-like, with little evidence of a well-developed lingual torus.
Material
Two teeth: MNA V11292, MNA V11293.
Remarks
The Surprise Canyon specimens are the first record of the genus Clairina extending into the Carboniferous. The two fragments from the Surprise Canyon Formation were collected from the type section, Bat Tower 2, but at two separate intervals, sections 13 and 15 of the middle member. These teeth are not Denaea williamsi because we see no evidence of a lingual torus of the tooth base. MNA V11292 (Fig. 8.1, 8.2) shows an enlarged intermediate cusp compared to the median cusp, and the median cusp of MNA V11293 (Fig. 8.3, 8.4) shows the cristae on the lingual side are extending mediodistally from the midline of the tooth, with no indication of vertical cristae as seen on in D. williamsi. Clairina was previously recognized from the Late Devonian, Famenian, of the Anti-Atlas region of Morocco (Derycke, Reference Derycke1992), Thuringia of Germany (Ginter, Reference Ginter1999), and Holy Cross Mountains of Poland (Ginter, Reference Ginter1995). The morphology of Clairina teeth, consisting of five lingually recurved cusps that reduce in height laterally from the tall median cusp, and with fluted cristae and a tooth base that is slightly extended lingually, suggests a primitive placement within the Elasmobranchii (Ginter et al., Reference Ginter, Hampe, Duffin and Schultze2010).
Cohort Euselachii Hay, Reference Hay1902
Superfamily Protacrodontoidea Zangerl, Reference Zangerl and Schultze1981
Family Protacrodontidae Cappetta, Duffin, and Zidek, Reference Capetta, Duffin, Zidek and Benton1993
Genus Microklomax new genus
Type species
Microklomax carrieae n. gen. n. sp.
Diagnosis
As for species, by monotypy.
Etymology
Greek mikros, little; Greek klomax, heap of stones; in recognition of its small size and its multiple round cusps.
Holotype
MNA V11294, mediolateral tooth.
Diagnosis
Small heterodont durophagous protacrodont shark with multicuspid teeth ~1 mm long mesiodistally, 400 µm wide labiolingually, and 300–400 µm tall (Fig. 9.3, 9.4 holotype MNA V11294). Tooth base shallow, curved aborally or moderately flattened, mesiodistally elongated, and lingually offset from crown. Enlarged foramina in a single row on lingual and labial margins of tooth base. Tooth crown with prominent median cusp that is either compressed labiolingually or rounded, one to two lateral cusps near equal in height, labial and lingual horizontal ridges forming cingulum, and labial and lingual vertical ridges on cusps.
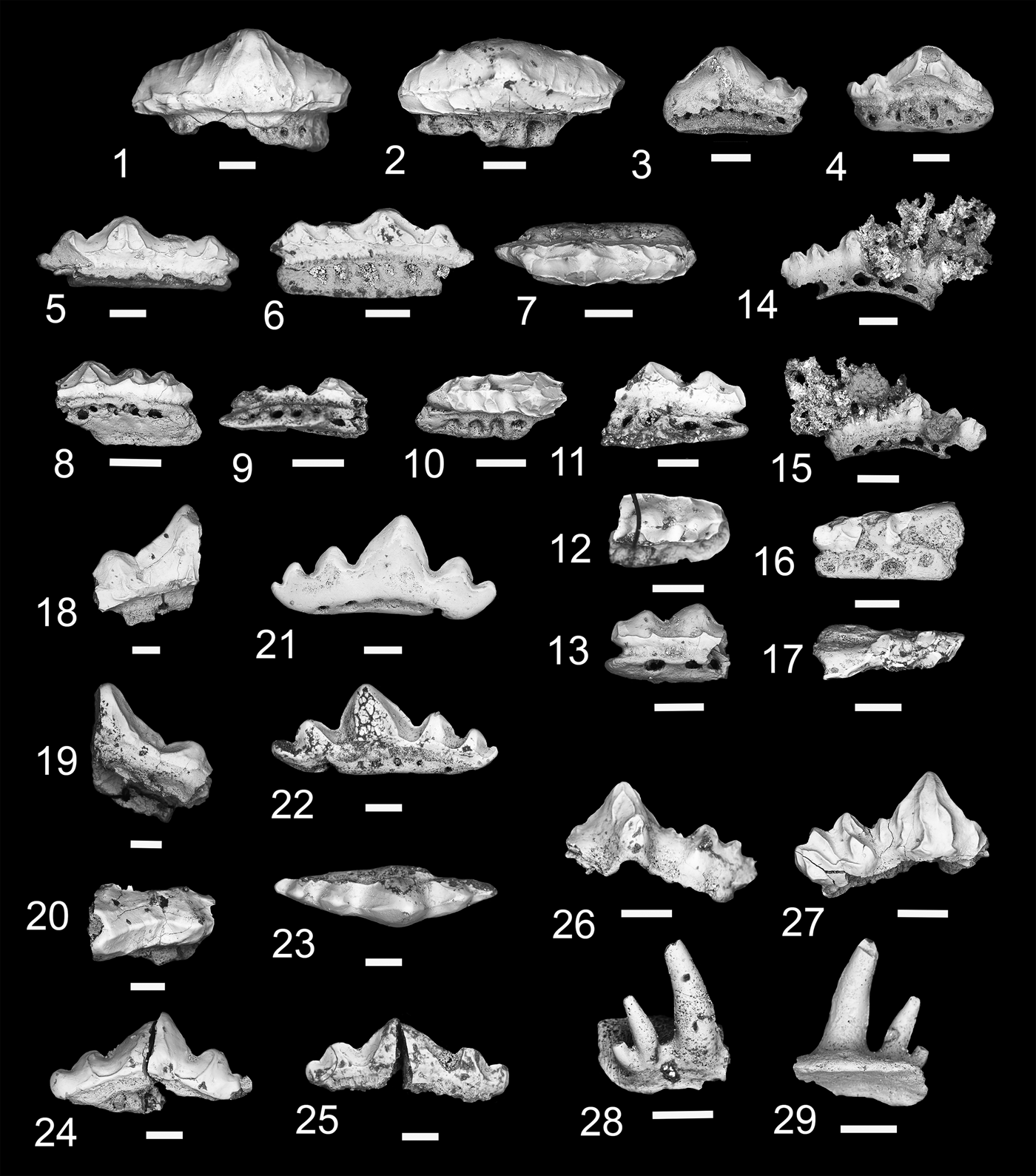
Figure 9. Teeth and denticles of Protacrodontiformes and Hybodontiformes. (1–17) Microklomax carriea n. gen. n. sp.; (1, 2) MNA V11297, anterior tooth, (1) labial view, (2) occlusal view; (3, 4) MNA V11294, holotype, anterior tooth, (3) labial view, (4) lingual view; (5–7) MNA V11298, mediolateral tooth, (5) labial view, (6) lingual view, (7) occlusal view; (8–10) MNA V11296, posterior tooth, (8) labial view, (9) lingual view, (10) occlusal view; (11–13) MNA V11300, posterior tooth, (11) lingual view, (12) occlusal view, (13) labial view; (14, 15) MNA V11295, posterior tooth, (14) lingual view, (15) labial view; (16, 17) MNA V1299, posterior tooth, (16) lingual view, (17) occlusal view. (18–25) Novaculodus billingsleyi n. gen. n. sp.; (18–20) MNA V11303, anterior tooth, (18) lingual view, (19) labial view, (20) occlusal view; (21–23) MNA V11301, holotype, lateral tooth, (21) labial view, (22) lingual view, (23) occlusal view; (24, 25) MNA V11302, (24) labial view, (25) lingual view. (26, 27) cf. Mesodmodus sp., MNA V11304, (26) labial view, (27) lingual view; (28, 29) cf. Hamiltonichthyes sp., MNA V11305, (28) anterior view, (29) posterior view. Scale bars = 200 µm.
Occurrence
Bat Tower locality 2, sections 13 and 18; Blue Mountain Locality 4, section 2; Burnt Springs Canyon locality 6, section 1, Surprise Canyon Formation, lower and middle member, Serpukhovian, Grand Canyon, Mohave County Arizona.
Description
The tooth base is plate-like and rectangular, concave aborally, and offset to the crown lingually. Height of base varies with tooth position; thicker in anterior and mediolateral teeth and thin in posterolateral teeth. Large nutrient foramina are present on labial and lingual margins and are positioned in line with one another. An aboral vascular channel is directly positioned under the crown with a smooth lingual margin on the aboral side. Crown morphology varies with tooth position. All tooth crowns share the traits of a blunt ridge on either side of each cusp extending mesiodistally, a labial and/or lingual ridge forming a cingulum, and one to three coarse vertical cristae on each cusp. Anterior and mediolateral teeth are labiolingually compressed with median cusp very large in comparison to lateral cusps. Median cusps for anterior and mediolateral teeth are triangular with mediolateral median cusp being proportionately thicker than in more anteriorly positioned teeth. Apex of median cusp is blunt. Anterior tooth has a single small lateral cusp and mediolateral tooth has two small lateral cusps, descending slightly in height. Lateral and posterolateral teeth have a lower cusp height than anterior and mediolateral teeth. Median cusp subtriangular or almost circular with a rounded blunt apex. Two lateral cusps are present on either side of median cusp. Height of lateral cusps in lateral and posterolateral teeth are nearly equal and they have rounded, blunt apices.
Etymology
In honor of Carrie Brugger-Schorr of Northern Arizona University, who brought to our attention the microvertebrate material used in this study.
Material
Teeth: MNA V11295, MNA V11296, MNA V11297, MNA V11298, MNA V11299, MNA V11300.
Remarks
The teeth of Microklomax carrieae n. gen. n. sp. are placed in the Protacrodontidae based on the vascular canal morphology of the tooth base, which consists of a single row of large oval foramina. This feature is also seen in the Late Devonian (Frasnian–Famenian) Protacrodus vetustus Jaekel, Reference Jaekel1925, which also has a single row of oval foramina together with a large median cusp (Gross, Reference Gross1938; Ginter et al., Reference Ginter, Hampe, Duffin and Schultze2010). Other Protacrodus species, P. serra Ginter, Hairapetian, and Klug, Reference Ginter, Hairapetian and Klug2002 and P. aequalis Ivanov, Reference Ivanov1996 have proportionately smaller vascular foramina compared to M. carrieae n. gen. n. sp. and P. vetustus. Deihim mansureae Ginter, Hairapetian, and Klug, Reference Ginter, Hairapetian and Klug2002, which is placed tentatively within the Protacrodontidae, has a more elongated lingual torsus for the tooth base than Protacrodus and Microklomax n. gen. (Ginter et al., Reference Ginter, Hairapetian and Klug2002). In addition to a more elongated lingual torus, the vascular foramina are distributed more irregularly lingually, in a more hybodont-like anascularized fashion.
The crown of Microklomax carrieae n. gen. n. sp. shows a typical protacrodont design, as seen in Protacrodus and Deihim, in which there is a tall median cusp flanked by two or three lateral cusps that are near to or equal in height to one another (Ginter et al., Reference Ginter, Hampe, Duffin and Schultze2010). Typically, Protacrodus and Deihim have three lateral cusps on the post-anterior tooth families, although Ginter and Sun (Reference Ginter and Sun2007) described a set of protacrodont teeth as “Protacrodus sp.” from the early Mississippian of Muhua, Southern China that had two lateral cusps. The crown of M. carrieae n. gen. n. sp. differs from Protacrodus and Deihim in having low, robust, blunt, median, and lateral cusps that are expanded labiolingually. Microklomax n. gen. is similar to both Protacrodus and Deihim in having cusps ornamented with longitudinal coarse ridges that can extend along the rim of the crown and to the apex of the cusps. Deihim was described as being a possibly durophagous protacrodont shark based on having cusps that are rounded as compared to Protacrodus (Ginter et al., Reference Ginter, Hairapetian and Klug2002, Reference Ginter, Hampe, Duffin and Schultze2010). However, the teeth of Microklomax n. gen. are far more developed for durophagous feeding than either Protacrodus or Deihim, and it is considered here to be the first true durophagous protacrodont shark.
Genus Novaculodus new genus
Type species
Novaculodus billingsleyi n. gen. n. sp.
Diagnosis
As for species by monotypy.
Etymology
Latin novacula, razor; Latin odus, tooth; in recognition of its tall, well developed labiolingually compressed cusps.
Holotype
MNA V11301, a lateral tooth.
Diagnosis
Small multicuspid heterodont protacrodont shark with laterally compressed dentition, teeth 1–1.5 mm long mesiodistally, 400 µm wide labiolingually, and ~600 µm tall (Fig. 9.21–9.23 holotype MNA V11301). Tooth base shallow, aborally recurved or moderately flattened, and slightly extended lingually. Four to six nutrient foramina present in a single row on labiolingual margin of tooth base. Crown with prominent labiolingually compressed triangular median cusp, one to three laterally compressed lateral cusps with broad longitudinal crests present on each cusp.
Occurrence
Surprise Canyon Formation, middle member, latest Mississippian (Serpukhovian), Bat Tower Locality 2, sections 13 and 1; Blue Mountain Locality 4, section 2.
Description
Taxon represented by three teeth comprising an anterior or mediolateral tooth and two lateral teeth. Tooth bases are thin with ovate nutrient foramina that extend through labial margin to lingual margin of base. Shallow aboral longitudinal groove present labially under the crown. Lingual torus reduced in width. In all teeth cusps are labiolingually compressed with smooth cutting carina, triangular median cusp, and lateral cusps about a quarter the height of median cusp and only slightly reducing in height mesiodistally. Broad longitudinal lingual crest extends between each cusp, a similar, though less well developed, longitudinal crest is present labially. A smaller longitudinal crest can be present along the labial and lingual rim of crown. Specimen representing a possible anterior or mediolateral tooth (MNA V11303) shows that the median crown was proportionately broader mesiodistally than in the lateral teeth, and more triangular. A single lateral cusp is present on a mesiodistally shorter base. Lateral teeth elongated mesiodistally with prominent median and lateral cusps.
Etymology
In honor of George H. Billingsley for his contributions to the study of the Surprise Canyon Formation in the Grand Canyon.
Material
Two teeth: MNA V11302, lateral tooth; MNA V11303, anterior or mediolateral tooth.
Remarks
Novaculodus billingsleyi n. gen. n. sp. is placed in the Protacrodontidae based on the vascularization pattern of its tooth base, which consists of a single row of oval nutrient foramina that extends labiolingually through the tooth base. Novaculodus billingsleyi n. gen. n. sp. differs from Microklomax n. gen., Protacrodus, and Deihim in having a reduced width of the lingual torus. The crown of Novaculodus n. gen. differs from Microklomax n. gen., Protacrodus, and Deihim in being more labiolingually compressed with more blade-like cusps. The teeth of Novaculodus n. gen. bear a superficial resemblance to Sphenacanthus because both taxa have tall triangular median cusps (Dick, Reference Dick1998; Ginter et al., Reference Ginter, Hampe, Duffin and Schultze2010). However, Sphenacanthus differs from Novaculodus n. gen. in having a more developed lingual torus, with numerous vascular foramina that do not form a single row labiolingually, and the cusps of Sphenacanthus are ornamented with numerous thin longitudinal cristae (Dick, Reference Dick1998; Ginter et al., Reference Ginter, Hampe, Duffin and Schultze2010). Microklomax carrieae n. gen. n. sp. and Novaculodus billingsleyi n. gen. n. sp. represent a further temporal extension and diversification of the protacrodontids into the latest Mississippian (Serpukhovian), as previous records only extended into the earliest Mississippian (Tournaisian) and only referred to Protacrodus (Ginter and Sun, Reference Ginter and Sun2007; Ginter et al., Reference Ginter, Hampe, Duffin and Schultze2010). These two taxa also show two new extremes in dental morphology for the protacrodontids—a durophagous morphotype and a hyper-carnivorous morphotype. Taxa like Protacrodus and Deihim had a more generalized crown morphology for grasping and some crushing.
Order Hybodontiformes Maisey, Reference Maisey1975
Family Indeterminate
Genus Mesodmodus St. John and Worthen, Reference St. John and Worthen1875
cf. Mesodmodus sp.
Occurrence
Surprise Canyon Formation, middle member, latest Mississippian (Serpukhovian), Blue Mountain locality 4, section 2.
Description
Tooth crown without base. Median cusp is triangular with a thick carina on the mesiodistal margins (Fig. 9.26, 9.27). Four lateral cusps are present with that closest to the median cusp greatly reduced in size. All cusps have one to three thick sinuous cristae. A small peg-like labial node is present.
Material
MNA V11304, tooth.
Remarks
This tooth is tentatively identified as Mesodmodus, an enigmatic hybodont taxon from the early Mississippian (Tournaisian) of North America and Belgium (Ginter et al., Reference Ginter, Hampe, Duffin and Schultze2010). The crown of Mesodmodus consists of a prominent median cusp and a series of smaller lateral cusps ornamented by vertical striations that descend from the crown apex to the junction of the base, and which commonly bifurcate (Ginter et al., Reference Ginter, Hampe, Duffin and Schultze2010). Additionally, the Surprise Canyon specimen also has labial nodes near the midline of the median cusp, a feature that is present in most species of Mesodmodus except M. khabenji Derycke-Khatir, Reference Derycke-Khatir2005 (Ginter et al., Reference Ginter, Hampe, Duffin and Schultze2010). The Surprise Canyon specimen is morphologically similar to M. khabenji in having more prominent lateral cusps and robust vertical striations, but overall it is more robust than M. khabenji (Derycke-Khatir, Reference Derycke-Khatir2005) and less mesiodistally elongated than M. exsculptus St. John and Worthen, Reference St. John and Worthen1875, M. explanatus St. John and Worthen, Reference St. John and Worthen1875, and M. ornatus St. John and Worthen, Reference St. John and Worthen1875 (St. John and Worthen, Reference St. John and Worthen1875).
Genus Hamiltonichthys Maisey, Reference Maisey1989
cf. Hamiltonichthys sp.
Occurrence
Surprise Canyon Formation; middle member; latest Mississippian (Serpukhovian); Blue Mountain Canyon locality 84-9, section 3.
Description
Partial cephalic spine from a small hybodont shark with a mediolateral width of ~400 µm, an anteroposterior length of ~300 µm, and an approximate height of 500 µm. Spine base is rectangular with two posterior foramina; dorsal surface of the spine base slightly rugose and thickens slightly mediolingually. Ventral surface of spine base smooth with no indication of foramina. Denticles consist of a tall narrow median cusp with two smaller and narrow lateral cusps. Cusps are smooth, slightly inclined posteriorly, and circular in cross section.
Material
MNA V11305, cephalic spine.
Remarks
A single multicuspid cephalic spine from the vicinity of Blue Mountain Canyon suggests the presence of a small male hybodont in the Surprise Canyon embayment. In the extinct Elasmobranchii, only male hybodont sharks had cephalic spines (Maisey, Reference Maisey1982a). There are two Paleozoic hybodont taxa that had multicuspid cephalic spines: Onychoselache traquairi Dick, Reference Dick1978 and Hamiltonichthys mapesi Maisey, Reference Maisey1989. Onychoselache is from the late Mississippian of Scotland, but can be excluded here in that the cephalic spine base is anteroposteriorly elongated with three cusps of equal height, one anterior cusp and paired posterior cusps (Coates and Gess, Reference Coates and Gess2007). The cephalic spines of Hamiltonichthys are multicuspid, positioned on the anterior end of an anteroposteriorly compressed plate in a transverse row, with the cusps connected by enamel (Maisey, Reference Maisey1989). The median cusp of the cephalic spine of Hamiltonichthys is strongly recurved posteriorly with the three lateral cusps less recurved (Maisey, Reference Maisey1989), features shared by the Surprise Canyon taxon. The Surprise Canyon taxon shares also with Hamiltonichthys cusps that are circular in cross-section and a thick central area of the basal plate (Maisey, Reference Maisey1989). However, the Surprise Canyon spine differs from the type material of Hamiltonichthys in having only two lateral cusps and being approximately a quarter of the dimensions of Hamiltonichthys spines.
Subcohort Neoselachii Compagno, Reference Compagno1977
Family Anachronistidae Duffin and Ward, Reference Duffin and Ward1983
Genus Coolyella Gunnell, Reference Gunnell1933
Cooleyella fordi Duffin and Ward, Reference Duffin and Ward1983

Figure 10. Teeth of Anachronistidae. (1–12), Cooleyella platera n. sp.; (1–3), MNA V11308, tooth, (1) labial view, (2) occlusal view, (3) aboral view; (4–6), MNA V11307, holotype, tooth, (4) labial view, (5) occlusal view, (6) aboral view; (7–9), MNA V11311, tooth, (7) labial view, (8) occlusal view, (9) lingual view; (10–12), MNA V11310, tooth, (10) labial view, (11) occlusal view, (12) mesial view. (13–15), Cooleyella fordi; MNA V11306, tooth, (13) labial view, (14) occlusal view, (15) mesial view. Scale bars= 200 µm.
Holotype
Coolyella peculiaris Gunnell, Reference Gunnell1933, p. 290. Tooth (NHM-P60670) from Steeplehouse Quarry, Derbyshire England.
Occurrence
Surprise Canyon Formation, middle member, latest Mississippian (Serpukhovian), Blue Mountain locality 4, section 2.
Description
Single tooth ~1 mm mesiodistally, 600 µm labiolingually, and 600 µm tall (Fig. 10.13–10.15). Crown consists of a lingually directed central cusp and two lateral blades with cutting carinae directed orolingually. Left lateral blade mesiodistally longer than right lateral blade. Central cusp somewhat prominent and triangular. Labial face of crown extends downwards forming rounded basal flange that just overlaps basolabial projection. Basal flange has slight ridge on right labial margin of crown. Tooth base consists of single basolabial peg-like projection and a wide transverse lingual basal ridge. Central pit with aboral foramen between the basolabial projection and transverse lingual basal ridge. Small median orolingual foramen present on lingual basal ridge.
Material
Tooth: MNA V11306.
Remarks
The teeth of Cooleyella fordi are known primarily from the Visean (Late Mississippian) of the Steeplehouse Quarry near Matlock, Derbyshire, England, but also from a tentative report of Cooleyella cf. C. fordi from the Cisuralian (early Permian) of the middle and south Urals of Russia (Duffin and Ward, Reference Duffin and Ward1983; Ivanov, Reference Ivanov2005). The Surprise Canyon specimen is the first record of C. fordi for the Serpukhovian of North America.
Cooleyella platera new species
Holotype
Tooth, MNA V11307.
Diagnosis
Small anachronistid neoselachian shark with monocuspid teeth 400–600 µm long mesiodistally, 400–700 µm wide labiolingually, and 300–400 µm tall. Crown smooth and ovate, overlapping basolabial projection, no discernable median cusp, lingually inclined. Tooth base composed of single short basalolabial projection and tall and mesiodistally wide lingual ridge, both structures separated by a deep and broad central pit. Lingual foramen or longitudinal groove present on lingual ridge.
Occurrence
Surprise Canyon Formation, lower and middle members, latest Mississippian (Serpukhovian), Burnt Springs Canyon location 6, concentration 1(MNA V11308 and MNA V11309); Blue Mountain Locality 3, section 2 (MNA V11307 and MNA V11310); Blue Mountain Locality 4, section 2 (MNA V11311).
Description
Crown of Cooleyella platera n. sp. lacks definitive median cusp and is primarily circular or ovate with cutting edge present on lingual margin. Crown inclined labially, wide mesiodistally, moderately tall labiolingually. Labial surface of crown smooth, lacking marginal crests or ridges, and overlaps basolabial projection. Ventral margin of crown rounded mesiodistally. Tooth base comprises a single basolabial peg-like projection and wide transverse lingual basal ridge. Basolabial projection small, round, and positioned just linguad of the ventral labial margin of crown. Lingually projecting basal ridge less wide or as wide as crown and a single median labiolingual foramen or open median canal is present with the basal ridge. Deep central pit present between basolabial projection and lingual basal ridge.
Etymology
Latin platera, a flat dish; in recognition of the crown's smooth, nearly circular, plate-like shape.
Material
MNA V11308, tooth; MNA V11309, tooth; MNA V11310, tooth; MNA V11311, tooth.
Remarks
The tooth base of C. platera n. sp. is similar to the other Cooleyella species, C. peculiaris, C. fordi, and C. amazonensis Duffin, Richter, and Neis, Reference Duffin, Richter and Neis1996, and consists of a small basolabial projection that is present near the ventrolabial margin of the of the crown and a lingually projecting, mesiodistally wide, basal ridge with a single median foramen or canal present (Gunnel, Reference Gunnell1933; Duffin and Ward, Reference Duffin and Ward1983; Duffin et al., Reference Duffin, Richter and Neis1996). Variability in the shape and size of the basolabial projection does occur in the known taxa because it is more triangular in C. fordi and rounded in C. amazonensis, C. peculiaris, and C. platera n. sp. The rounded basolabial projection in C. peculiaris is proportionately larger than that seen in C. amazonensis and C. platera n. sp. (Hanson, Reference Hansen1986; Duffin et al., Reference Duffin, Richter and Neis1996; Ivanov, Reference Ivanov2011). The lingual basal ridge is less broad mesiodistally in C. peculiaris, but is more mesiodistally expanded in C. fordi, C. amazonensis, and C. platera n. sp. (Duffin and Ward, Reference Duffin and Ward1983; Hansen, Reference Hansen1986; Duffin et al., Reference Duffin, Richter and Neis1996; Ivanov, Reference Ivanov2011). The central pit in C. platera n. sp. is relatively deep and enlarged in contrast to C. peculiaris, C. fordi, and C. amazonensis in which it is relatively shallow and small (Duffin and Ward, Reference Duffin and Ward1983; Hansen, Reference Hansen1986; Duffin et al., Reference Duffin, Richter and Neis1996; Ivanov, Reference Ivanov2011). The crown of C. platera n. sp. is the least developed of the Cooleyella species. In C. peculiaris, C. fordi, and C. amazonensis the crown has a median lingually directed cusp and lateral blade like cusps that are directed horizontally (C. peculiaris and C. amazonensis) or angled downward (C. fordi) mesiodistally (Duffin and Ward, Reference Duffin and Ward1983; Hansen, Reference Hansen1986; Duffin et al., Reference Duffin, Richter and Neis1996; Ivanov, Reference Ivanov2011). Cooleyella platera n. sp. is unique in lacking median and lateral cusps, but consists of a single circular or ovate cusp with a broad convex lingual cutting edge. The ventrolabial rim of the crown in C. platera n. sp. is broad and rounded mesiodistally, similar to that seen in C. peculiaris and C. amazonensis, and not narrow and pointed as in C. fordi (Duffin and Ward, Reference Duffin and Ward1983; Hansen, Reference Hansen1986; Duffin et al., Reference Duffin, Richter and Neis1996; Ivanov, Reference Ivanov2011).
The distribution and diversity of the Anachronistidae show that this group is presently restricted to the Late Mississippian to late Permian (Ginter et al., Reference Ginter, Hampe, Duffin and Schultze2010; Ivanov, Reference Ivanov2011). At least five species of Cooleyella are presently recognized. The type species, C. peculiaris, is known from the Pennsylvanian (Missourian), Kansas City Group Winterset Limestone and Cherryvale Shale at Kansas City (Gunnel, Reference Gunnell1933; Ginter et al., Reference Ginter, Hampe, Duffin and Schultze2010); from the Pennsylvanian (Virgilian) Levenworth Limestone of Iowa, Kansas, Nebraska, and Oklahoma (Tway, Reference Tway1982); the Virgilian Heebner Shale from Iowa, Kansas, Missiouri, Nebraska, and Oklahoma (Tway, Reference Tway1982); the Virgilian Plattsmouth Limestone from Iowa, Kansas, Missiouri, Nebraska, and Oklahoma (Tway, Reference Tway1982); and the Pennsylvanian Appalachian Group of Ohio (Hansen, Reference Hansen1986). Cooleyella fordi is one of the oldest Cooleyella species, known from the Visean of the Steeplehouse Quarry Derbyshire U.K. (Duffin and Ward, Reference Duffin and Ward1983) and the Serpukhovian Surprise Canyon Formation of the western Grand Canyon (this study). Although seemingly widely distributed in central North America, C. peculiaris has been little studied since the work by Gunnel (Reference Gunnell1933) and Hansen (Reference Hansen1986) and needs review. Cooleyella amazonensis is known from the Early Pennsylvanian (Bashkirian/Moscovian) of the Itaituba Formation of the Amazon Basin, Brazil and the early Permian in the Urals of Russia (Ivanov, Reference Ivanov2011). Ivanov (Reference Ivanov2011) recognized an undescribed new species from the middle Permian of western Texas previously referred to as Cooleyella sp. in Duffin and Ward (Reference Duffin and Ward1983), which Ivanov et al. (Reference Ivanov, Nestell and Nestell2015) later named Cooleyella duffini Ivanov, Nestell, and Nestell, Reference Ivanov, Nestell and Nestell2015. Cooleyella duffini differs from all other Cooleyella species in having a central cusp and two to four lateral cusplets on the occlusal crest and aserrated labial edge of the crown (Ivanov et al., Reference Ivanov, Nestell and Nestell2015). Cooleyella platera n. sp. is presently only known from the lower and middle members of the Serpukhovian Surprise Canyon of the western Grand Canyon.
Genus Amaradontus new genus
Type species
Amaradontus santuccii n. gen. n. sp.
Diagnosis
As for species, by monotypy.
Etymology
Greek Amara, a ditch or channel, Greek odontos, tooth, in recognition of its occurrence in the ancient paleo-valleys of the Surprise Canyon Formation within the Grand Canyon.
Remarks
See remarks for species.
Amaradontus santuccii new species
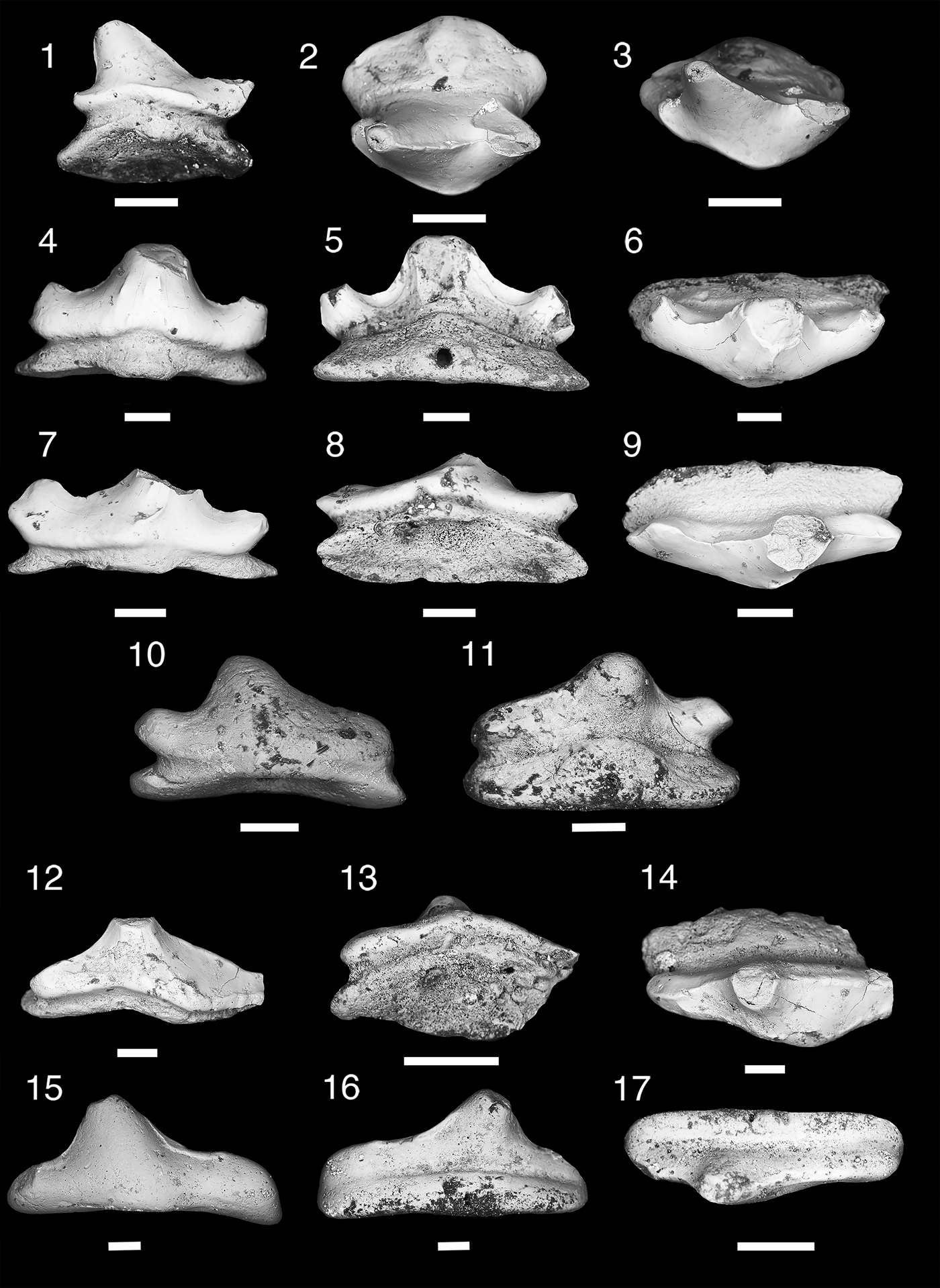
Figure 11. Teeth of Amaradontus santuccii n. gen. n. sp; (1–17), (1–3) MNA V11316, anterior tooth, (1) labial view, (2) occlusal-lingual view, (3) occlusal view; (4–6) MNA V11312, holotype, mediolateral tooth, (4) labial view, (5) lingual view, (6) occlusal view; (7–9) MNA V11317, lateral tooth, (7) labial view, (8) aboral view, (9) occlusal-lingual view; (10, 11) MNA V11315, lateral tooth, (10) labial, (11) lingual; (12–14) MNA V11313, posterior tooth, (12) labial view, (13) aboral view, (14) occlusal view; (15–17) MNA V11314, (15) labial view, (16) lingual view, (17) occlusal view. Scale bars = 200 µm.
Holotype
Mediolateral tooth, MNA V11312.
Diagnosis
Small anachronistid shark with heterodont teeth ranging from ~600 μm–1 mm mesiodistally to ~400–500 µm labiolingually, and an approximate height of 300–400 µm. Crown asymmetric. Median cusp broad mesiodistally, inclined distally, and with rounded apex. Lateral cusps low or absent. Cusps connected by sharp carinae. One to two vertical ridges can be present on median cusp. A faint labial cingulum present. Tooth base comprises an elliptical mesiodistally broad basolabial projection and lingually shallow mesiodistally wide basal ridge. Basolabial projection positioned under median cusp. Central pit shallow and wide mesiodistally. A single large circular foramen present on lingual margin of base exits labially.
Occurrence
Surprise Canyon Formation, lower and middle members, latest Mississippian (Serpukhovian), Grand Canyon, Mohave County, Arizona, Burnt Springs Canyon location 6, concentration 1; Blue Mountain Locality 4, section 2, Bat Tower 2, sections 13 and 18.
Description
All teeth of Amaradontus santuccii n. gen. n. sp. exhibit asymmetric crowns. Median cusp broad mesiodistally, with rounded nearly blunt apex, and smooth carina on mesial and distal margins (Fig. 11.1–11.3). Median and lateral cusps inclined lingually over tooth base (Fig. 11.1–11.3). Mesial lateral cusp more elongated than distal lateral cusp. Mesial and distal lateral cusps of more anterior tooth families low but oriented vertically. Lateral cusps become steadily reduced in height posteriorly until completely lost and replaced by continuous mesiodistal carina in most posterior tooth families. Slight labial cingulum and faint vertical ridge can be present on crown. Lateral basal margin of crown does not extend below basolabial projection, but instead ends just above tooth base with nearly straight basal margin to crown. Tooth base lingually extended in anterior tooth families forming rounded lingual margin, which becomes progressively more compressed labiolingually and straight in more posterior tooth families (Fig. 11.2, 11.11). Single mesiodistally broad basolabial projection present and in line with midline of median cusp and labial margin of tooth base. Basolabial projection elliptical aborally, narrow labiolingually. Lingual basal ridge wider mesiodistally than crown and typically has a nearly straight lingual margin (with a few exceptions). Single large foramen present at midline of median cusp on lingual border of lingual basal ridge. Shallow, mesiodistally long, and labiolingually narrow aboral groove present with single median foramen. Anterior teeth overall tend to be less elongated mesiodistally and wider labiolingually. Lateral and posterior teeth more elongated mesiodistally and wider labiolingually.
Etymology
In honor of Vincent Santucci in recognition of his services to the National Park Service and to NPS paleontology.
Material
MNA V11313, lateral tooth; MNA V11314, lateral tooth; MNA V11315; lateral tooth; MNA V11316, anterior tooth; MNA V11317, mediolateral tooth.
Remarks
Ivanov et al. (Reference Ivanov, Liapin and Bolshijanov2014) were the first to report a new, but unnamed, anachronistid neoselachian shark from the Serpukhovian of the Moscow region. This taxon, like Amaradontus santuccii n. gen. n. sp. differs from Cooleyella in having a crown that is more compressed labiolingually, with well-developed median and lateral cusps. The basal labial rim does not extend over the basolabial projection, the tooth base is elongated mesiodistally and wider than the crown, and a more reduced lingual basal ridge is present. Amaradontus santuccii n. gen. n. sp., however, is not the same genus as the taxon from the Moscow syneclise, differing in having a more asymmetric crown in which the mesial lateral cusp is longer than the distal lateral cusp and the median and lateral cusps are broader mesiodistally and blunter. The lateral cusps are less tall and well developed and posteriorly become reduced until finally being lost in Amaradontus n. gen., while in the Moscow taxon all teeth have well-developed lateral cusps for each tooth family. In Amaradontus n. gen. the basolabial projection is in line with the labial margin of the tooth base, although in the Moscow taxon the basolabial projection is positioned forward of the labial margin of the tooth base. Clearly the Moscow taxon and A. santuccii n. gen. n. sp. represent a new subfamily within the Anachronistidae (A. Ivanov, personal communication, 2015) with A. santuccii n. gen. n. sp. being the most heterodont of the two taxa. However, defining this new subfamily and its significance is beyond the scope of this paper. A more thorough review of the Anachronistidae is needed in light of the new taxa described here, together with other recently described forms (Duffin and Ward, Reference Duffin and Ward1983; Duffin et al., Reference Duffin, Richter and Neis1996; Duffin and Ivanov, Reference Duffin and Ivanov2008; Ivanov, Reference Ivanov2011; Ivanov et al., Reference Ivanov, Liapin and Bolshijanov2014).
Subclass Euchondrocephali Lund and Grogan, Reference Lund and Grogan1997
Infraclass Paraselachii Grogan and Lund, Reference Grogan and Lund2000
Family Gregoriidae Lund and Grogan, Reference Lund, Grogan, Arratia, Wilson and Cloutier2004
Genus Srianta Lund and Grogan, Reference Lund, Grogan, Arratia, Wilson and Cloutier2004
Srianta cf. S. srianta Lund and Grogan, Reference Lund, Grogan, Arratia, Wilson and Cloutier2004
Occurrence
Surprise Canyon Formation, middle and upper member, latest Mississippian (Serpukhovian), Bat Tower Locality 2, sections 13 and 26.
Description
Two teeth missing distal portion of tooth base. Labiolingually compressed crowns consist of a low central cusp with three minute lateral cusps in a mesiodistally directed arch (Fig. 12.1, 12.2). Prominent basal, labially projecting boss present, positioned on midline of central cusp with thick longitudinal crest extending towards central cusp apex (Fig. 12.1, 12.4). Approximately 13 or 14 additional thin longitudinal crests present on lingual surface of crown. Well-developed cingulum present along lower margin of labial and lingual sides of crown, just above tooth base. Tooth base in both specimens incomplete, but indicates it was elongated, labiolingually compressed, and directed downwards. Thin longitudinal elliptical foramina present on labiolingual surface of tooth base.
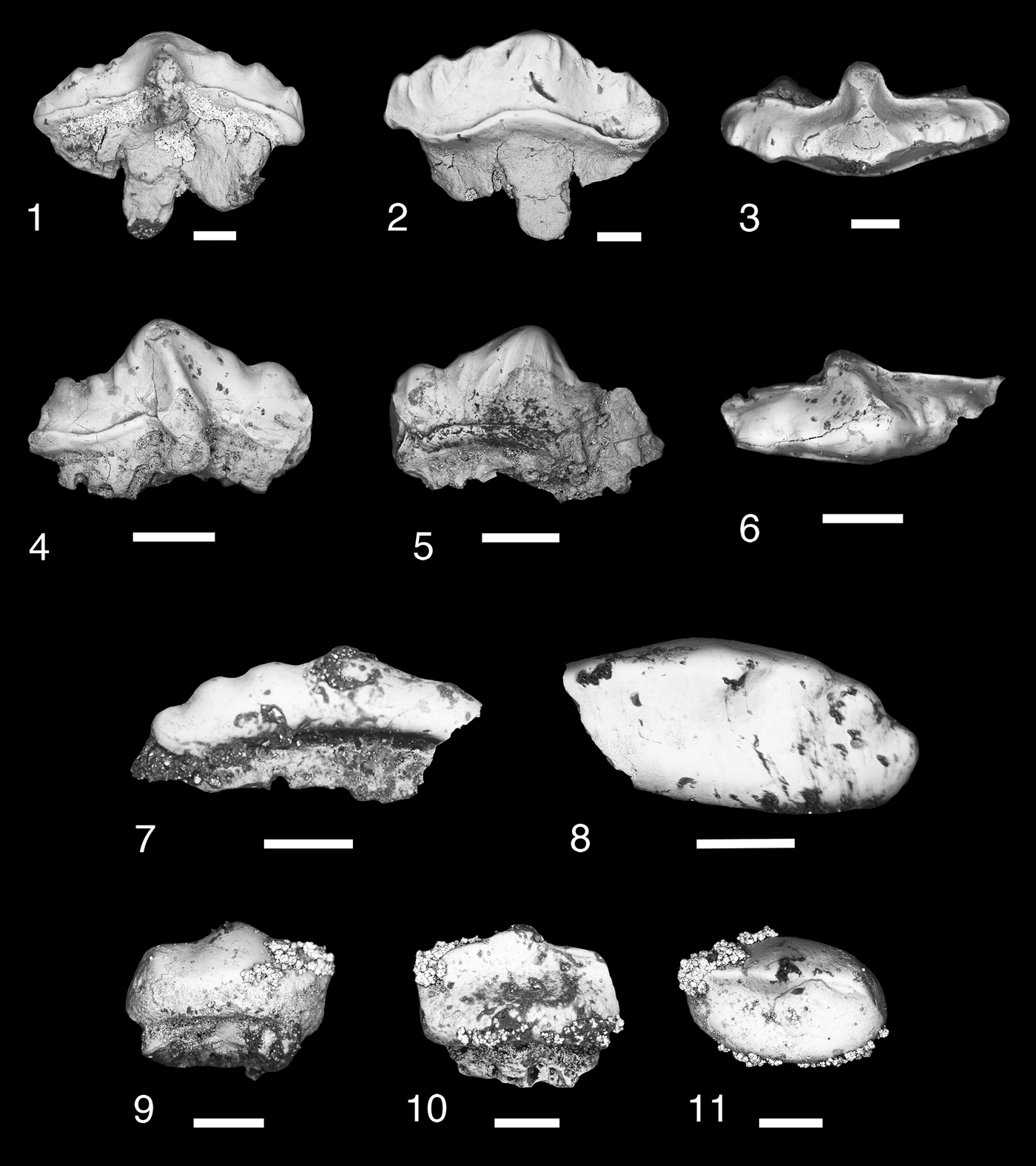
Figure 12. Teeth of Euchondrocephali; Paraselachii. (1–6) Srianta cf. S. srianta; (1–3), MNA V11318, tooth, (1) lingual view, (2) labial view, (3) occlusal view; (4–6), MNA V11319, tooth, (4) lingual view, (5) labial view, (6) occlusal view. (7–11) Heteropetalus sp.; (7, 8) MNA V11320, tooth, (7) labial view, (8) occlusal view; (9–11) MNA V11321, tooth, (9) labial view, (10) lingual view, (11) occlusal view. Scale bars = 500 µm.
Material
Teeth: MNA V11318, MNA V11319.
Remarks
The teeth described here are the first evidence of a gregoriid chondrichthyan, specifically the genus Srianta, outside the Bear Gulch fauna. Lund and Grogan (Reference Lund, Grogan, Arratia, Wilson and Cloutier2004) established this taxon based on three genera (Gregorius, Bealbonn, and Srianta), which share autodiastylic suspensoria, a protacrodont-like squamation, teeth with fluted crowns with a single basal ridge in the upper and lower jaws, presence of a symphysial and parasymphysial dentition composed of single conical cusps lacking basal ridges and having a remote narrow anal fin. The Surprise Canyon specimens’ best match Srianta, the teeth of which have low central cusps with three to four lateral cusps that bear transverse crests, and a prominent basal ridge on the labiolingual margins of the crown (Lund and Grogan, Reference Lund, Grogan, Arratia, Wilson and Cloutier2004). We tentatively place these teeth as Srianta cf. S. srianta, based on a labially projecting boss present on the lower teeth, a feature seemingly lacking in S. iarlais Lund and Grogan, Reference Lund, Grogan, Arratia, Wilson and Cloutier2004 and S. dawsoni Lund and Grogan, Reference Lund, Grogan, Arratia, Wilson and Cloutier2004 (Lund and Grogan, Reference Lund, Grogan, Arratia, Wilson and Cloutier2004). These teeth were collected from the upper section of the middle member and the lower section of the upper member of the Surprise Canyon Formation at the Bat Tower locality 2 type section.
Family Debeeriidae Grogan and Lund, Reference Grogan and Lund2000
Genus Heteropetalus Lund, Reference Lund1977
Heteropetalus sp.
Occurrence
Surprise Canyon Formation, lower/middle member, latest Mississippian (Serpukhovian), Burnt Springs Canyon locality 6, concentration 1.
Description
Two teeth with distal tooth base missing and representing anterior and lateral positions. Crowns of both teeth share slightly taller median than lateral cusps, basal portion of crown slightly concave and expanded into basin-like lingual margin, labial margin short and convex. Basolingual margins in both teeth U-shaped, narrow and slightly arched. Anterior tooth has single median cusp with single horizontal chisel-like lateral cusp on either side. Lateral tooth missing a third of the crown, but enough remains to show that it had a low median cusp and at least three low and blunt lateral cusps (Fig. 12.7). Tooth base, though mostly missing, compressed labiolingually and projected aborally.
Material
Teeth: MNA V11320, MNA V11321.
Remarks
These teeth represent the first record of Heteropetalus outside the Bear Gulch fauna of Montana. However, due to the incomplete nature of the dentition we cannot confidently place these teeth above the generic level until more specimens are available for comparison. The teeth of Heteropetalus differ from the other debeerid chondrichthyan, Debeerius ellefseni Grogan and Lund, Reference Grogan and Lund2000 in having crowns with more well defined and numerous cusps and a U-shaped lingual basal margin, while in D. ellefseni, the crown either lacks defined cusps or they are slight and have a V-shaped basal lingual margin (Lund, Reference Lund1977; Grogan and Lund, Reference Grogan and Lund2000). Superficially, debeerid teeth look similar to gregoriid teeth but differ in the presence of a well-developed basal cingulum around the labial and lingual margins of the crown, which gregoriids have and debeerids lack (Lund, Reference Lund1977; Grogan and Lund, Reference Grogan and Lund2000; Lund and Grogan, Reference Lund, Grogan, Arratia, Wilson and Cloutier2004). The Surprise Canyon specimens of Heteropetalus have thus far only been collected from the lower member of the Burnt Springs Canyon Locality 6.
Order Orodontiformes Zangerl, Reference Zangerl and Schultze1981
Family Orodontidae De Konnick Reference De Koninck1878
Genus and species indeterminate
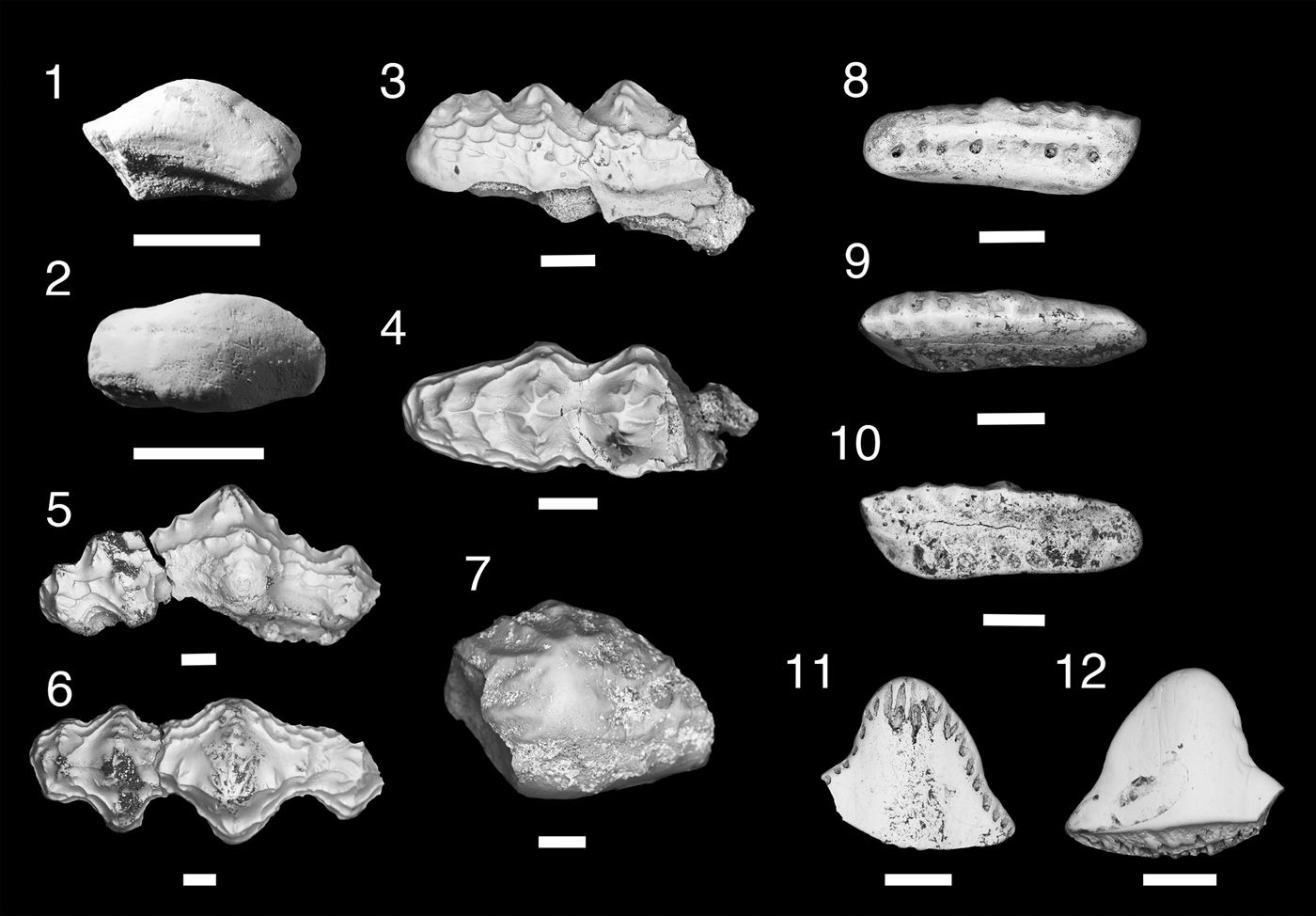
Figure 13. Teeth of Euchondrocephali; Helododontiformes, Eugenodontiformes, Orodontiformes, and Petalodontiformes. (1, 2) Helodus? sp., USNM PAL 603799 tooth, (1) labial view, (2) occlusal view. (3–7) Eugeneodontiformes genus and species indeterminate, (3, 4) MNA V11325, tooth, (3) lingual view, (4) occlusal view; (5, 6) MNA V11324, tooth, (5) labial view, (6) occlusal view; MNA V11323, tooth fragment, (7) occlusal view. (8–10) Orodontiformes genus and species indeterminate, MNA V11322, tooth, (8) labial-aboral view, (9) occlusal view, (10) lingual view. (11, 12) Petalodontiformes genus and species indeterminate, MNA V11326, tooth, (11) lingual view, (12) labial view. Scale bars = 1 cm (1, 2); 500 µm (3–12).
Occurrence
Surprise Canyon Formation, lower/middle member, latest Mississippian (Serpukhovian), Burnt Springs Canyon locality 6, concentration 1.
Description
A single complete lateral tooth ~2 mm mesiodistally, 500 µm labiolingually, and 600 µm tall. Mesiodistally elongated and narrows labiolingually. Crown is low with small median cusp, slightly higher than lateral cusps. Median cusp narrow labiolingually forming a small domed peak. Approximately five very low lateral cusps with transverse ridges extending to basal rim of crown. Slight median mesiodistal crest present on crown. Tooth base taller than crown and directed aborally. Nine labial foramina present in a shallow mesiodistal groove. Lingual margin of tooth base flat with nine foramina present.
Material
Tooth: MNA V11322.
Remarks
The orodont specimen from the Surprise Canyon Formation is nearly identical to “Orodus sp.” specimens described by Elliott et al. (Reference Elliott, Irmis, Hansen and Olson2004) from the Middle Pennsylvanian (Desmoinesian) Naco Formation of central Arizona. The Surprise Canyon taxon and the Naco taxon both share a mesiodistally elongated crown that is narrow labiolingually, a tall median cusp with five to seven lateral cusps with transverse ridges, the tooth base taller than the crown, and a labial mesiodistal basal groove with numerous foramina in a single row. Elliott et al. (Reference Elliott, Irmis, Hansen and Olson2004) suggested the Naco teeth resembled the teeth of Orodus greggi Zangerl, Reference Zangerl and Schultze1981, which is known from a large partial but articulated specimen from the Middle Pennsylvanian (Desmoinesian) Logan Quarry shale of Indiana (Zangerl, Reference Zangerl and Schultze1981). The Orodontiformes has been argued to be an artificial grouping that needs revision (Ginter et al., Reference Ginter, Hampe, Duffin and Schultze2010). The Surprise Canyon and Naco specimens differ from the type material of Orodus and as such we feel these specimens are probably not Orodus sensu lato. However, along with “O.” greggi, these specimens most likely represent an orodontid group closely related to Orodus.
Order Eugeneodontiformes Zangerl, Reference Zangerl and Schultze1981
Superfamily Caseodontoidea Zangerl, Reference Zangerl and Schultze1981
Family uncertain
Genus and species indeterminate
Occurrence
Surprise Canyon Formation, middle member; latest Mississippian (Serpukhovian); Blue Mountain Canyon locality 3, section 2; Bat Tower Locality 2, section 13.
Description
Three partial lateral pavement teeth lacking tooth bases, measuring between 800 µm and 1 mm mesiodistally. Crown slightly arched orally, mesiodistally elongated, and narrow labiolingually. Median cusp conical with slightly crenulated labiolingual crest on apex of cusp. Lateral cusps small relative to median cusp, and have labiolingual crests with crenulated ornamentation (Fig. 13.5, 13.6). Two to three cingulum-like crenulated ridges present on the labial and lingual margins of crown. Labial and lingual bosses present in line with median and lateral cusps with labial bosses more prominent than lingual bosses.
Material
Teeth: MNA V11323, MNA V11324, MNA V11325.
Remarks
These teeth are superficially similar to some orodont dentitions, like that seen in the Orodus mammillaris Newberry and Worthen, Reference Newberry and Worthen1866 group (Ginter et al., Reference Ginter, Hampe, Duffin and Schultze2010), which have enlarged circular median cusps and can have less prominent lateral cusps or cusplets. However, the Surprise Canyon taxon has well-developed labial and lingual projections and labiolingually directed crests that extend over the apices of each cusp, a feature lacking in orodont dentitions but prominent in the lateral pavement teeth of caseodontid eugenodonts (Ginter et al., Reference Ginter, Hampe, Duffin and Schultze2010). Ginter et al. (Reference Ginter, Hampe, Duffin and Schultze2010) did remark that the distinction between orodont and eugenodont dentitions is a matter of arbitrary and intuitive opinion. We feel that these teeth fall within the parameters of a caseodontid eugeneodont due to the presence of sharply defined transverse (labiolingual) ridges, which have secondary crenulations that are separated by deep excavations (Ginter et al., Reference Ginter, Hampe, Duffin and Schultze2010).
The record of eugeneodont sharks primarily extends through the Pennsylvanian and Permian (Zangerl, Reference Zangerl and Schultze1981; Ginter et al., Reference Ginter, Hampe, Duffin and Schultze2010). The earliest record of a eugeneodont shark is based on the lateral pavement dentition of Campodus agassizianus De Koninck, Reference De Koninck1844, a caseodontid eugeneodont, that has been recognized from the latest Mississippian (Serpukhovian) of Belgium and Missouri, USA (Zangerl, Reference Zangerl and Schultze1981; Ginter et al., Reference Ginter, Hampe, Duffin and Schultze2010). A second slightly younger taxon, Chiastodus obvallatus Trautschold, Reference Trautschold1879, from the Mississippian/Pennsylvanian of Moscow is based on a single symphysial tooth (Ginter et al., Reference Ginter, Hampe, Duffin and Schultze2010), and therefore not valuable for comparison here. Zangerl (Reference Zangerl and Schultze1981) had suggested, however, that Chiastodus might be a synonym of Campodus. The Surprise Canyon taxon has a few similarities to Campodus including the presence of labial and lingual bosses and an orally arched crown. However, the Surprise Canyon taxon differs from Campodus in having the labiolingual crests not extending to the basal margins of the crown, more conical median and lateral cusps, and the presence of two to three labial and lingual crenulations. We suggest that the Surprise Canyon taxon represents the newest example of a small Late Mississippian caseodontid eugeneodont shark.
Order Petalodontiformes Zangerl, Reference Zangerl and Schultze1981
Family uncertain
Genus and species indeterminate
Occurrence
Surprise Canyon Formation; middle member; latest Mississippian (Serpukhovian); Bat Tower Locality 2, sections 13
Description
A single tooth fragment consisting of a labiolingually compressed asymmetric triangular cusp. The mesiodistal carina is smooth. The labial edge of the carina has a series of vertical elliptical openings. The tooth base is missing.
Material
Tooth fragment: MNA V11326.
Remarks
This is the only record of a petalodont from the Surprise Canyon Formation. The tooth fragment consists of a labiolingually flattened cusp with an asymmetric, triangular tip. The asymmetric nature of this tooth fragment eliminates petalodont taxa that tend to have symmetrical crowns in all tooth families such as Petalodus, Belantsea, and Polyrhizodus (Lund, Reference Lund1983, Reference Lund1989; Ginter et al., Reference Ginter, Hampe, Duffin and Schultze2010), or symmetric upper symphysial teeth as in Petalorhynchus, Siksika, Orbuchevodus, Fissodopsis, and Netsepoye (Lund, Reference Lund1989; Grogan et al., Reference Grogan, Lund and Fath2014; Lund et al., Reference Lund, Grogan and Fath2014). This fragment could represent the lower Fissodus-like symphysial tooth morph, recently recognized in a majority of petalodonts from Bear Gulch, which consists of a bifurcated and mesiodistally expanded crown (Lund et al., Reference Lund, Grogan and Fath2014). The lack of crenulations on the crown, a feature that is present in Siksika and Orbuchevodus, suggests a closer relationship to Fissodopsis, Netsepoye, or Petalorhynchus (Lund et al., Reference Lund, Grogan and Fath2014). However, the fragmentary nature of the specimen leaves a generic assignment ambiguous at best.
Superorder Holocephali Bonaparte, Reference Bonaparte1832–1841
Order Helodontiformes Patterson, Reference Patterson1965
Family Helodontidae Patterson, Reference Patterson1965
Genus Helodus Agassiz, 1838
Helodus? sp.
Occurrence
Surprise Canyon Formation, upper member, latest Mississippian (Serpukhovian); West Stairway Canyon, (Unit 3).
Description
A single tooth, weathered free of matrix and missing a small portion at one end. Small (length as preserved 8.8 mm; width 4.7 mm; height 4.7 mm), and consists of a tumid crown and narrow base. Tooth elongated mesiodistally, crown in oral view has somewhat convex lateral margins, a truncated termination, and is surmounted at the midpoint by a rounded crown. Crown has a smooth surface and numerous small vascular channels visible penetrating the worn surface and producing a characteristic punctate pattern.
Root is narrow (2.0 mm wide) and projects slightly at undamaged end of tooth where it terminates in a rounded point. Consists of thin basal plate with smooth, slightly concave, base separated from the crown by a narrow groove on both sides. Root slightly worn but shows a series of small, evenly spaced foramina along its length on both sides.
Material
Tooth: USNM PAL 603799.
Remarks
This genus is known from several incomplete skeletons and many isolated teeth (Stahl, Reference Stahl and Schultze1999). Moy-Thomas (Reference Moy-Thomas1936) described and reconstructed the type species (H. simplex), and the dentition is only known in its entirety from this species. As reconstructed it consists of a series of eight or nine tooth families along each jaw ramus with four or five tooth crowns visible in each family. The teeth tend to be smaller at the mesial and distal ends of the series than in the center where the teeth are fused into plates (Stahl, Reference Stahl and Schultze1999). Many Helodus species are based on isolated teeth and because the dentition is composed of diverse elements, as shown by the presence of several different species of Helodus tooth in an associated dentition of Psephodus (Traquair, Reference Traquair1885), it is probable that the number of species is exaggerated.
The genus is known worldwide from the Late Devonian to the early Permian, but is particularly common in the upper Carboniferous of the British Isles (McCoy, Reference McCoy1855; Morris and Roberts, Reference Morris and Roberts1862; Ward, Reference Ward1875), the lower Carboniferous of Belgium (De Koninck, Reference De Koninck1878) and northern France (Pruvost, Reference Pruvost1919), and the lower Carboniferous of Midwestern USA (Newberry and Worthen, Reference Newberry and Worthen1866; St. John and Worthen, Reference St. John and Worthen1875; Newberry, Reference Newberry1879).
Order Cochliodontiformes Obruchev, Reference Obruchev1953
Family Cochliodontidae Owen, Reference Owen1867
Genus Cochliodus Agassiz, 1838
Cochliodus cf. C. contortus Newberry and Worthen, Reference Newberry and Worthen1870
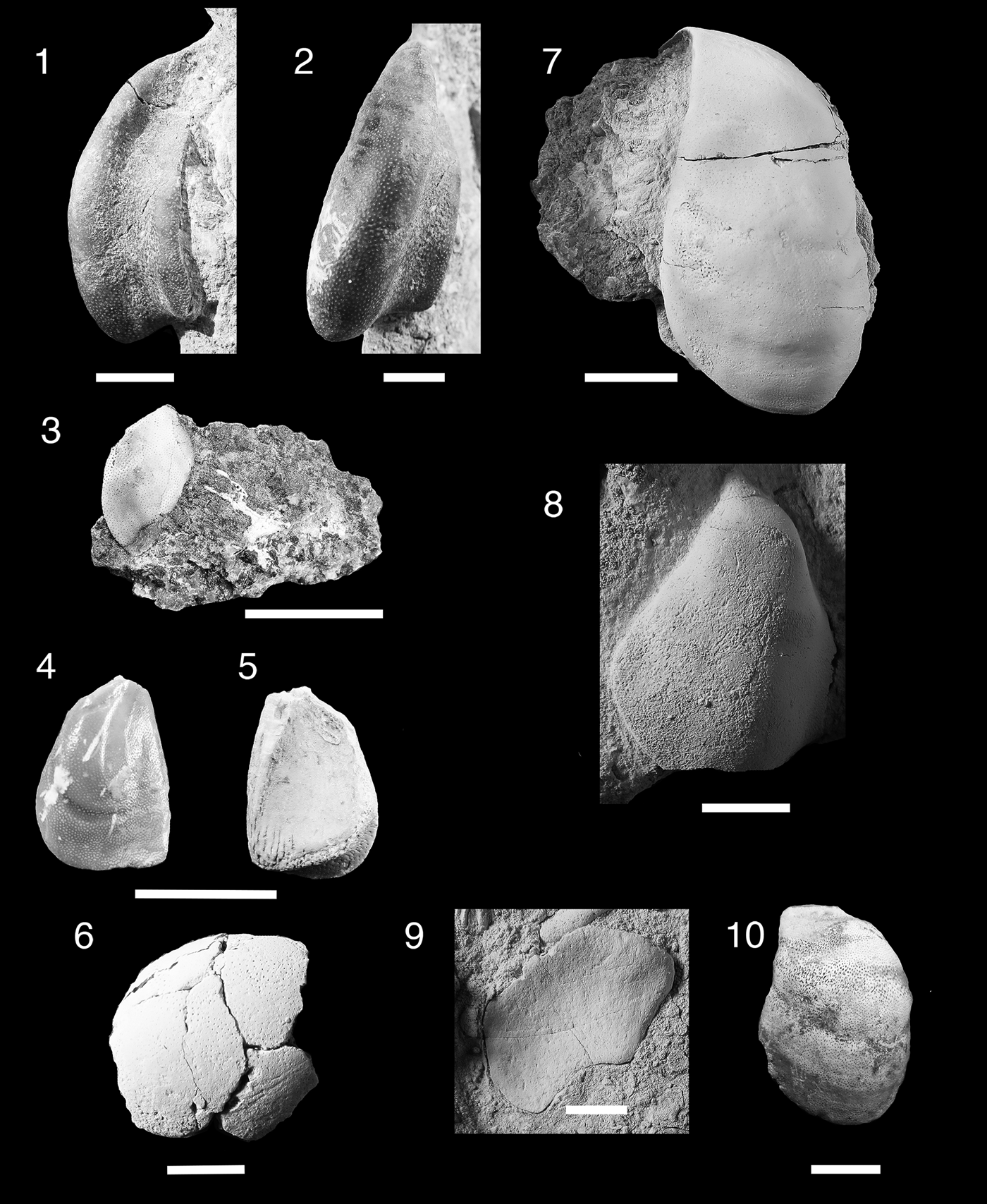
Figure 14. Teeth of Euchondrocephali; Holocephali. (1, 2) Cochliodus cf. C. contortus; (1, 2) USNM PAL 412147 anterior right dental plate, (1) labial view, (2) occlusal view. (3–6) Deltodus cf. D. angularis; (3) USNM PAL 412168 anterior mandibular dental plate in occlusial view, (4, 5) USNM PAL 603798 posterior mandibular dental plate, (4) occlusal view, (5) aboral view; (6) USNM PAL 412170 upper jaw dental plate in occlusial view. (7, 8) Deltodus cf. D. cingulatus; (7) USNM PAL 412146 right mandibular dental plates in occlusal view, (8) USNM PAL 412148 right mandibular dental plate in occlusal view. (9, 10) Deltodus sp.; (9) USNM PAL 4121773 upper dental plate in aboral view, (10) USNM PAL 412145 upper dental plate in occlusal view. Scale bars = 1 cm.
Occurrence
Surprise Canyon Formation, middle unit; latest Mississippian (Serpukhovian); Rampart Cave Section.
Description
Single large right anterior dental plate (32.0 mm along the radial axis), strongly curved, convex on occlusal surface and with a high, narrow, ridge along the radial axis. Distal to the ridge is a deep groove, bordered on the distal margin by a low, rounded, ridge. Mesial margin slightly broken and junction between the labial and mesial edges is incomplete.
Material
Tooth plate: USNM PAL 412147.
Remarks
Cochliodontid holocephalans bore convoluted dental plates on the lower jaw, a larger posterior and smaller anterior, that occluded with a single pair of more elongated plates mounted to the undersurface of the neurocranium. Anterior to the upper plates were at least two series of helodontid teeth (Stahl, Reference Stahl and Schultze1999). The larger posterior teeth were broad but narrow antero-posteriorly. This dental plate appears to have been an anterior plate from the right side of the lower jaw.
Although mainly reported from the lower Carboniferous of Ireland, Belgium, and England (Stahl, Reference Stahl and Schultze1999), an isolated lower jaw of this species has also been described from the lower Carboniferous (Serpukhovian) Bear Gulch Limestone of Montana (Lund and Grogan, Reference Lund and Grogan1997).
Genus Deltodus Morris and Roberts, Reference Morris and Roberts1862
(ex Agassiz Ms., Reference Agassiz1859)
Deltodus cf. D. angularis Newberry and Worthen, Reference Newberry and Worthen1866
Occurrence
Surprise Canyon Formation; Travertine Canyon, lower member; (USNM PAL 412170, 603798); Stairway Canyon, upper member; (USNM PAL 412168); latest Mississippian (Serpukhovian).
Description
Three specimens are attributed to this species. USNM PAL 412168 (Fig. 14.3) is an incomplete, small, anterior mandibular dental plate with a convex occlusal surface; oval in outline, 10 mm long and 5.8 mm wide. USNM PAL 603798 (Fig. 14.4, 14.5) is a small, posterior, mandibular tooth, roughly triangular with part of the anterior point missing, and is12.4 mm long and 8.9 mm wide. A broad, convex ridge is present along the mesial edge of the dental plate and this broadens to form much of the lingual edge. Distally a trough starts near the anterior edge and broadens and shallows towards the lingual edge. USNM PAL 412170 (Fig. 14.6) is a large, thickened dental fragment that probably formed part of an upper jaw dental plate.
Material
Anterior mandibular dental plate: USNM PAL 412168; posterior mandibular dental plate: USNM PAL 603798; upper jaw dental plate fragment: USNM PAL 412170.
Remarks
Stahl and Hansen (Reference Stahl and Hansen2000) described an associated dentition of Deltodus angularis from the upper Carboniferous of Ohio. They showed that the lower jaw had two small anterior plates on either side of the symphysis, and that behind these on each side was a larger triangular posterior plate with a trough and ridge that occluded with the upper jaw dental plate. In the upper jaw was a single large plate on each side with a labial ridge that occluded with the trough in the posterior mandibular plate.
This species has been reported from several U.S. localities, including Illinois (Newberry and Worthen, Reference Newberry and Worthen1866), Ohio (Stahl and Hansen, Reference Stahl and Hansen2000), West Virginia (Lund et al., Reference Lund, Garton, Weishampel and Englund1979), Colorado (Lockley, Reference Lockley1984), and Arizona (Elliott et al., Reference Elliott, Irmis, Hansen and Olson2004).
Deltodus cf. D. cingulatus Newberry and Worthen, Reference Newberry and Worthen1866
Occurrence
Surprise Canyon Formation, Granite Park Section 2, lower member (USNM PAL 412148), and Rampart Cave Section, lower member (USNM PAL 412146); latest Mississippian (Serpukhovian).
Description
Both specimens appear to be tooth plates from the lower jaw on the right side. The larger and more complete specimen (USNM PAL 412146; Fig. 14.7) is an elongated (38.9 mm long) crushing tooth, curved, with a prominent convex ridge along the radial axis that is broadest at the lingual margin. The ridge narrows as it arches towards the labial margin and ends in an acute angle. Tooth is broken along the distal margin and is missing the furrow that broadens into a wing in complete specimens. Convex occlusal surface is marked by a series of ridges and furrows that cross it parallel to lingual margin of the plate. The second specimen (USNM PAL 412148; Fig. 14.8) is smaller than the first (26.0 mm long), but shows a similar morphology. The distal furrow and wing are broken away and the occlusal surface is marked by a series of ridges and grooves.
Material
Two right mandibular tooth plates: USNM PAL 412146, USNM PAL 412148.
Remarks
The holotype of D. cingulatus is a single tooth collected by Orestes St. John from the Mississipian Chester Limestone of Illinois, and described by Newberry and Worthen (Reference Newberry and Worthen1866, p. 99, pl. 9, fig. 6). Although many of the speciemens described in Newberry and Worthen (Reference Newberry and Worthen1866) found their way to the Smithsonian Insitution Paleobiology collections, the current whereabouts of the holotype of D. cingulatus is unknown. Croneis (Reference Croneis1927) erected a pleisotype to D. cingulatus (MCZ 5322) from the Chesterian (Serpukhovian) Fayetteville Formation.
Deltodus sp.
Occurrence
Surprise Canyon Formation, middle member, West Stairway Canyon; latest Mississippian (Serpukhovian); lower Watahomigi Formation, Three Springs Canyon, Lower Pennsylvanian (Bashkirian).
Description
Large tooth plate (USNM PAL 4121773; Fig. 14.9) in aboral view showing a slightly concave surface; 25.5 mm long and 16.0 mm wide with a roughly pentagonal outline. Based on the shape and on the thickened (labial) edge thinning lingually, this is probably an upper jaw tooth plate. Because it is only preserved in aboral aspect, it is not possible to identify it further. A series of smaller tooth plate fragments (USNM PAL 412174) were also collected from the Surprise Canyon Formation. A second upper tooth plate (USNM PAL 412145; Fig. 14.10) was collected from the Watahomigi Formation. This specimen measures ~30 mm long and 25 mm wide.
Material
Tooth plate: USNM PAL 412173; three tooth fragments: USNM PAL 412174; upper tooth plate: USNM PAL 412145.
Remarks
McKee (Reference McKee1982) also listed the isolated teeth of “Deltodus” occurring in the Watahomigi Formation in the Grand Canyon from Guano Cave, Parashant Canyon and Separation Canyon. Unfortunately these specimens cannot be located, therefore the identifications of these records cannot substantiated.
Subclass Elasmobranchii Bonaparte, Reference Bonaparte1838
Order, Family indeterminate
Genus Amelacanthus Maisey, Reference Maisey1982b
Amelacanthus sp.

Figure 15. Chondrichthyan dermal spines from the Surprise Canyon Formation. (1) Amelacanthus sp. USNM PAL 412150 spine fragment in matrix; (2) Acondylacanthus sp., USNM PAL 412149 large spine fragment in matrix. Scale bars = 1 cm.
Occurrence
Surprise Canyon Formation, lower member, Quartermaster Canyon, section 2. Latest Mississippian (Serpukhovian).
Description
Fin spine fragment 24.8 mm long and 5.8 mm wide, with four broad rounded ridges extending the length of the fragment and separated by intercostal grooves. One ridge forms the anterior margin of the spine and is the broadest (1.2 mm wide), while the most posterior spine is the narrowest (0.8 mm wide). The ridges are smoothly rounded with a shiny enameled layer over the surface and with coarsely and irregularly crenulated edges.
Material
Fin spine fragment: USNM PAL 412150.
Remarks
Although this is a small fragment, the characteristic smooth ribs with a shiny enameloid surface layer are characteristic of Amelacanthus. Maisey (Reference Maisey1982b) described this genus and included four species from the British lower Carboniferous. This genus was subsequently identified from the Pennsylvanian of Nebraska (Maisey, Reference Maisey1983), where it was associated with spines of Acondylacanthus, Bythiacanthus, and ‘Physonemus.’ It was also recognized in the Naco Formation of central Arizona (Elliott et al., Reference Elliott, Irmis, Hansen and Olson2004), where it was also associated with fin spines of Acondylacanthus. Separation into species is based on the number and size of the ribs and the pattern of bifurcation, but the fragment described here is too small to show those features.
Genus Acondylacanthus St. John and Worthen, Reference St. John and Worthen1875
Acondylacanthus sp.
Occurrence
Surprise Canyon Formation, lower member, Granite Park, section 2. Latest Mississippian (Serpukhovian).
Description
Poorly preserved fin spine fragment, broken at both ends and with most of the bone removed by weathering. Total length 118 mm, width 19.5 mm; specimen has a slight longitudinal curve. One short section (32 mm long) shows an external impression with longitudinal ridges 1.0–1.5 mm wide. Ridges are broadly rounded, not bifurcating, and do not have denticulate margins. The convex (presumed anterior) margin is broadly rounded and composed of several ridges.
Material
Fin spine fragment: USNM PAL 412149.
Remarks
This genus is recognized by its smooth, parallel, rounded ribbing. Separation into species rests on cross-sectional shape, which is not preserved in this specimen. Acondylacanthus is known from Ohio (Hansen, Reference Hansen1986, Reference Hansen, Feldmann and Hackathorn1996), Iowa (St. John and Worthen, Reference St. John and Worthen1875), Colorado (Itano et al., Reference Itano, Hauk and Lockley2003), and Arizona (Elliott et al., Reference Elliott, Irmis, Hansen and Olson2004).
Discussion of ecology and environmental preferences of the chondrichthyans of the Surprise Canyon and Watahomigi formations and their global relationships
Assemblage structure of the Surprise Canyon Formation chondrichthyans
The sharks of the Surprise Canyon Formation were diverse and distributed through many of the paleovalleys during the west to east marine transgression and periods of fluvial deposition. It should be stated that much of the material presented here was the byproduct of conodont studies (Martin, Reference Martin1992; Martin and Barrick, Reference Martin, Barrick, Billingsley and Beus1999) or fortuitous recovery during the 1980s field surveys measuring the geologic sections of the Surprise Canyon Formation (Billingsley et al., Reference Billingsley, Beus, Grover, Billingsley and Beus1999). This method of collecting the vertebrate material may have skewed the natural composition of the Surprise Canyon shark assemblage because looking for vertebrate remains was not the priority of the research. However, due to the meticulous stratigraphic and biogeographic data that were recorded for the Surprise Canyon Formation (Billingsley and Beus, Reference Billingsley, Beus, Billingsley and Beus1999b, appendices 1–3; Billingsley et al., Reference Billingsley, Beus, Grover, Billingsley and Beus1999; Martin and Barrick, Reference Martin, Barrick, Billingsley and Beus1999), the following observations can be made for the Surprise Canyon shark assemblage:
Presence of holocephalan chondrichthyans in fluvial/estuarine systems of the lower member of the Surprise Canyon Formation.—Holocephalan dental plates are the most common of the macro-chondrichthyan fossil material collected from the Surprise Canyon Formation, with the majority of them collected from the fluvial lower member siltstone, sandstone, and conglomerate beds. These dental plates show little to no wear that would indicate transportation or re-working (Irmis and Elliott, Reference Irmis and Elliott2006). This suggests that these holocephalans were possibly inhabiting estuarine and fluvial environments during the beginning of Surprise Canyon deposition. Living holocephalans primarily occur in deeper marine habitats, although there are a few records of holocephalans occurring in esturaries (Bigelow and Schroeder, Reference Bigelow, Schroeder and Tee-Van1953). Fossil evidence of holocephalans occurring in nonmarine habitats is rare. A holocephalan within an estuarine system is known from a complete specimen from the upper Pennsylvanian Tijearas member of the Atrasado Formation of central New Mexico (Hodnett and Lucas, Reference Hodnett, Lucas, Lucas and Sullivan2015). The new specimens from the lower member of the Surprise Canyon Formation, as well as other materials elsewhere, suggest that perhaps upper Paleozoic holocephalans had a wider range of salinity tolerance and environmental preferences than their modern descendents.
West to east distinction in the falcatid chondrichthyans in the middle member.—Three falcatid symmoriform chondrichthyians are presently known from the middle member of the Surprise Canyon Formation. Of these three, Denaea williamsi and “falcatid indet 1” are known from a substantial number of teeth from the stratigraphic samples made by Martin (Reference Martin1992). What is clear is that there was a west to east distinction for the distribution of these two forms. Falcatid indet 1 is presently only known from the Bat Tower type section at section 15 for the Surprise Canyon Formation, which at the time of deposition was closer to the development of the deeper open-water portion of the Surprise Canyon embayment. Teeth of D. williamsi are known from the Bat Tower type section, but only from two teeth collected from section 13, a slightly shallower depositional setting. The majority of the specimens of D. williamsi were collected from the eastern Blue Mountain Canyon sections, which represent shallower near shore environments.
Lund et al. (Reference Lund, Greenfest-Allen and Grogan2012, Reference Lund, Greenfest-Allen and Grogan2015) demonstrated that the fishes of the Bear Gulch limestone had distinct distribution patterns within the embayment due to regional environmental conditions and the corresponding ecomorphological changes. Of interest here is that the stethacanthoid taxa of the Bear Gulch fish assemblage as a whole had a fairly even distribution throughout the embayment, but Lund et al. (Reference Lund, Greenfest-Allen and Grogan2012, Reference Lund, Greenfest-Allen and Grogan2015) noted that Falcatus was more common in the marginal, bay mouth/basin and reef habitat zones, while Damocles and “other paleoselachii” were more common in the upper bay habitat zone.
Euselachian diversity
The diversity of euselachian grade taxa within the Surprise Canyon Formation assemblage is relatively high. At present, six distinct tooth taxa are known from the Surprise Canyon Formation, primarily from the near shore deposits of the lower/middle member transition at Burnt Spings Canyon, the shallow Blue Mountain Canyon section, and the deeper Bat Tower 2 section of the middle members. Of these six taxa, Microklomax carrieae n. gen. n. sp., Cooleyella platera n. sp., and Amaradontus santuccii n. gen. n. sp.were found relatively commonly within the above sections while cf. Mesomodus sp., Novaculodus billingsleyi n. gen. n. sp., and Cooleyella fordi are only known from one or two teeth in total. The record of euselachian grade taxa is rather poor at Bear Gulch, with only a few undescribed forms being mentioned in reviews of the fauna (Lund et al., Reference Lund, Greenfest-Allen and Grogan2012, Reference Lund, Greenfest-Allen and Grogan2015). This shortage of data on the euselachian diversity at Bear Gulch should be short lived because new taxa are currently being studied (R. Lund and E. Grogan, personal communication, 2015–2016). There are no other late Serpukhovian records of euselachians in North America at present. Eurasian records of Serpukhovian/Bashkirian euselachians are known primarily from a few isolated teeth. The anachronistid neoselachian Ginteria fungiforma Duffin and Ivanov, Reference Duffin and Ivanov2008 was recorded from the Steshev Formation (early Serpukhovian) of the Moscow District, Russia (Duffin and Ivanov, Reference Duffin and Ivanov2008); the basal Euselachian Gissarodus flabellatus Ivanov, Reference Ivanov, Lucas, DiMichelle, Barrick, Schneider and Spielmann2013 was recorded from the Khodzhir-Bulak Formation (late Serpukhovian-early Bashkirian) in the Gissar Mountains of south-eastern Uzbekistan (Ivanov, Reference Ivanov, Lucas, DiMichelle, Barrick, Schneider and Spielmann2013); and Protacrodus sp. was recorded from the Bashkirian borehole studies of the North Urals (Ivanov, Reference Ivanov1999).
The temporal extension of Clairina
The enigmatic taxon Clairina was previously only known from the Upper Devonian (Famennian) of Morocco, Poland, and Germany, as the single species, C. marocensis Derycke, Reference Derycke1992 (Ginter et al., Reference Ginter, Hampe, Duffin and Schultze2010). However, the new occurrence of Clairina in the Surprise Canyon Formation extends this taxon by ca. 28 Myr to the latest Mississippian. Although the two specimens are broken, the unworn condition of both specimens and the fact that they are separated by two stratigraphic intervals at the Bat Tower 2 type section rules out the possibility that these specimens are reworked from older sediments. The karst formation of the paleo-valleys of the Surprise Canyon Formation occured in the underlying Redwall Limestone, a Lower Mississippian formation, below which is the Middle Devonian Temple Butte Formation.
The Late Mississippian/Early Pennsylvanian marine shark assemblages and their global relationship
The small, however surprisingly diverse, sample of chondrichthyan taxa from the Surprise Canyon and Watahomigi formations of the Grand Canyon adds to the relatively few records of sharks from the Mississippian to Pennsylvanian transition during the middle Carboniferous. Below is a relative comparison with the currently known chondrichthyan assemblages for the latest Mississipian and Early Pennsylvanian:
Latest Mississippian (Serpukhovian)
In North America there are at least four marine fish assemblages from the Serpukhovian. Stahl and Cicimurri (Reference Stahl and Cicimurri2005) described a chondrichthyan assemblage from the Monteagle Limestone in northern Alabama, which consisted of isolated large teeth of at least fifteen taxa. The Surprise Canyon assemblage and the Monteagle Assemblage share the occurrence of the taxa Cladodus, Cochliodus, and Deltodus, but differ at the species level. At Monteagle, at least two species of Cladodus have been identified, “C.” newmani? Tuomey, Reference Tuomey and Mallett1858 and “C.” magnificus Tuomey, Reference Tuomey and Mallett1858 (now synonymized with Saivodus striatus, vide Duffin and Ginter, Reference Duffin and Ginter2006); two species of Cochliodus, C. leidyi and C. cf C. vanhorni; and Deltodus sp. (Stahl and Cieimurri, Reference Stahl and Cicimurri2005). The Fayetteville Formation of Arkansas has presently produced a small but significant collection of nearly articulated chondrichthyan specimens, many preserving a degree of three-dimensionality. Taxa described thus far include Carcharopsis wortheni Newberry and Worthen, Reference Newberry and Worthen1866 (Lund and Mapes, Reference Lund and Mapes1984; Bronson et al., Reference Bronson, Mapes and Maisey2018), “Cobelodus” sp. (Maisey, Reference Maisey2007), and Ozarcus mapesae (Pradel et al., Reference Pradel, Maisey, Tafforeau, Mapes and Mallatt2014). Of the taxa from the Fayetteville assemblage, only Ozarcus mapesae may have ties with the Surprise Canyon assemblage in the form of the indeterminate falcatid teeth. Although both the teeth of Ozarcus and the indeterminate taxa from the Surprise Canyon Formation are small, the teeth of Ozarcus have only been identified and described from three-dimensional segmentation data (Pradel, et al., Reference Pradel, Maisey, Tafforeau, Mapes and Mallatt2014). This method presently does not show the fine details commonly used to describe chondrichthyan teeth, and drawing a relationship among the different taxa is at best broadly speculative and inconclusive.
The Manning Canyon Shale assemblage of central Utah has also preserved a small but well-preserved vertebrate fauna of either marine or brackish influence (Mickle, Reference Mickle2011). At present, isolated shark teeth (Miller, Reference Miller1981), an acanthodian (Schultze, Reference Schultze1990), a microsaur (Caroll et al., Reference Carrol, Bybee and Tidwell1991), and three actinopterygians (Mickle, Reference Mickle2011) have been described. Of the chondrichthyans, Miller (Reference Miller1981) described two isolated cladodont teeth, which he tentatively referred to Cladodus sp. based on their five-cusped structure, but as two separate taxa. The smaller of the two specimens (BYU 4370) has a well-developed basolabial depression, similar in structure to Glikmanius (Ginter et al., Reference Ginter, Ivanov and Lebedev2005). It is difficult to determine which species of Cladodus the larger of the two specimens (BYU 4369) (Miller, Reference Miller1981) represents. However, the narrow proportions of the median and intermediate cusps in this specimen does eliminate C. marginatus from consideration. The Manning Canyon Shale assemblage is the closest geographic location to the Surprise Canyon assemblage because both locations were in close proximity to the paleoequator (Fig. 16). However, comparison between these two chondrichthyan assemblages is inconclusive at this time because the Manning Canyon Shale assemblage has only a small chondrichthyan component.
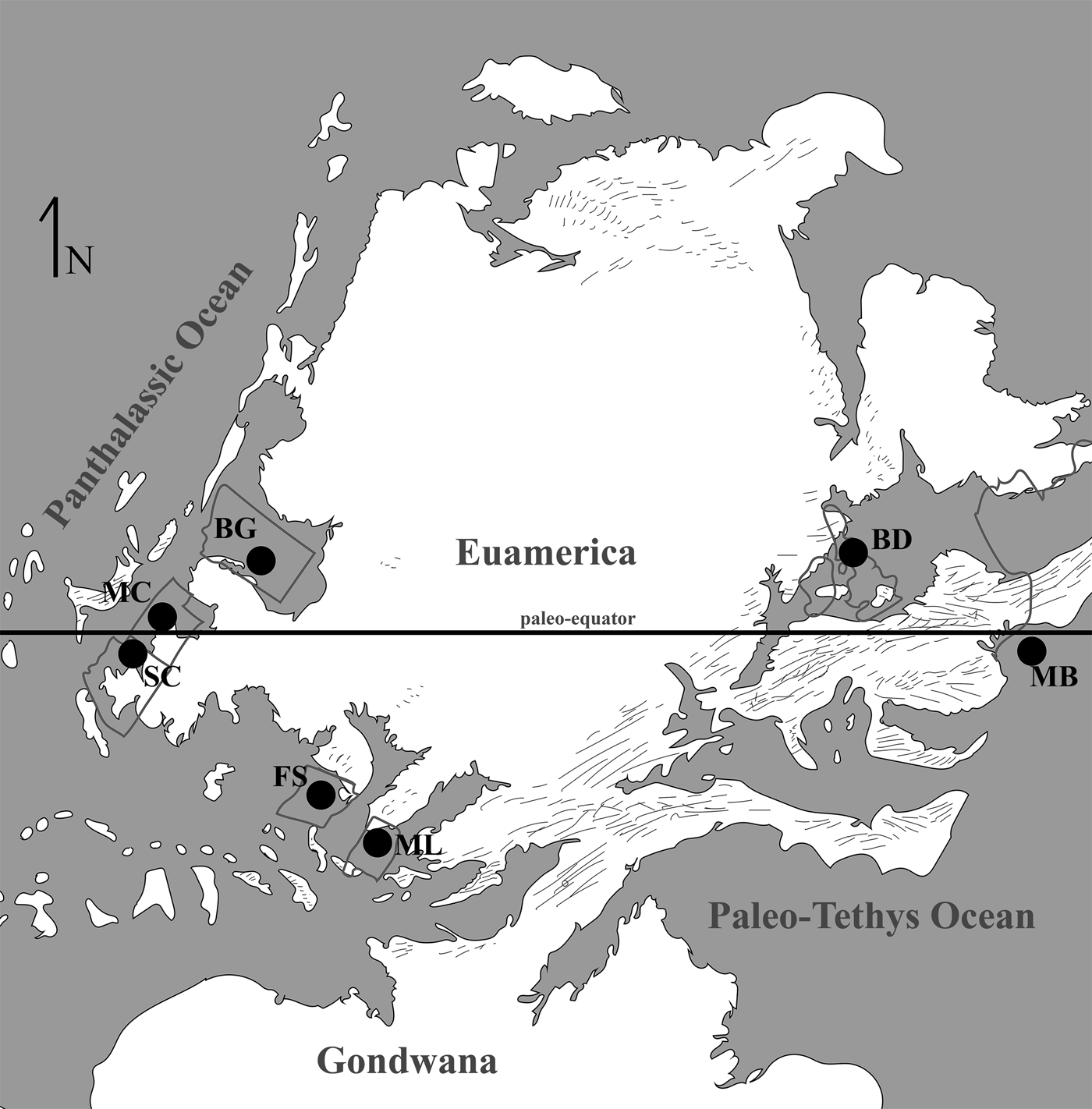
Figure 16. Paleogeography of the Latest Mississippian (Serpukhovian) of Euamerica and the primarily known marine fish assemblages. BD, Bearsden assemblage, Scotland; BG, Bear Gulch Limestone assemblage, Montana; FS, Fayetteville Shale assemblage, Arkansas; MB, Moscow Basin (Serpukhovian type section) assemblage, Russia; MC, Manning Canyon Shale assemblage, central Utah; ML, Monteagle Limestone, Alabama; SC, Surprise Canyon Formation assemblage, Grand Canyon, Arizona.
The Bear Gulch Limestone in Montana is perhaps the best-known locality not only in North America, but for the global record of fish for the Late Mississippian. Approximately seventy-five nominal genera have been identified (Lund et al., Reference Lund, Greenfest-Allen and Grogan2015), and of these seventy-five taxa, thirty-six are chondrichthyans (Lund et al., Reference Lund, Greenfest-Allen and Grogan2015). At the family level, the Surprise Canyon assemblage shares with the Bear Gulch assemblage at least seven familes (Thrinacodontidae, Stethacanthidae, Falcatidae, Ctenacanthidae, Gregoriidae, Debeeriidae, and Cochliodontidae). Both assemblages share at the order level the presence of the Petalodontiformes, Eugenodontiformes, Orodontiformes, and Helodontiformes, which, with the exception of the Petalodontiformes, are yet to be described in detail from Bear Gulch (Lund et al., Reference Lund, Grogan and Fath2014, Reference Maisey2015). However, the Surprise Canyon assemblage does differ significantly from the Bear Gulch assemblage in the presence of the xenacanthimorph Bransonella, the elasmobranch Clairina, members of the Protacrodontidae, and members of the Anachronistidae. There is an also a latitudinal difference between the two locations. The Bear Gulch assemblage was located near the 12° North latitude, which influenced an arid seasonal deposition (Lund et al., Reference Lund, Greenfest-Allen and Grogan2012), whereas the Surprise Canyon assemblage was within a tropical equatorial zone with greater fluvial and estuarine depositional influences (Fig. 16).
The Eurasian marine record of Serpukhovian chondrichthyans is known primarily from tooth-taxon assemblages. The type section for the Serpukhovian is located in the Moscow Basin (Fig. 16), and contains chondrichthyan assemblages typical of the East European Platform (Duffin and Ivanov, Reference Duffin and Ivanov2008). Chondrichthyans known from the East European Platform include the anchronistid euselachians Ginteria fungiforma (Duffin and Ivanov, Reference Duffin and Ivanov2008), cf. Thrinacodus gracia (Ginter and Turner, Reference Ginter and Turner2010), and the xenacanthomorph Bransonella lingulata (Ivanov and Ginter, Reference Ivanov and Ginter1996; Hampe and Ivanov, Reference Hampe and Ivanov2007). Ivanov (Reference Ivanov1999) reported indeterminate ‘cladodont'grade teeth of symmoriforms and ctenacanths from the South Island of the Novaya Zemlya Archipelago and the Russian Arctic. The Bearsden and East Kilbride marine/non-marine chondrichthyan assemblages of the Manse Burn Formation of Northern Scotland are at present the only Eurasian Serpukhovian localities that preserve articulated or disarticulated chondrichthyan endoskeletal material. Taxa such as the symmoriids Denaea sp., “Symmorium” sp., Gutturensis neilsoni Sequeira and Coates, Reference Sequira and Coates2000, and Akmonistion zangerli, the euselachian Tristychius arcuatus Agassiz, Reference Agassiz1843, and the holocephalan Deltoptychius sp. have been recorded from both localities (Dick et al., Reference Dick, Coates and Rolfe1986; Stahl, Reference Stahl and Schultze1999; Sequeira and Coates, Reference Sequira and Coates2000; Coates and Sequeira, Reference Coates and Sequeira2001). It can be argued that there is little published data to support a relationship between the Eurasian Serpukhovian chondrichthyans and the Surprise Canyon assemblage with the exception of the possible cf. Thrinacodus gracia (Ginter and Turner, Reference Ginter and Turner2010). Bransonella is represented on the East European Platform by B. lingulata, while B. nebraskensis is known from the Surprise Canyon assemblage.
Earliest Pennsylvanian (Bashkirian)
In Europe, the chondrichthyan record is composed of tooth-taxa assemblages. Ivanov (Reference Ivanov1999) reported on the teeth and dermal denticles of Protacrodus, symmoriids, ctenacanths, and other neoselachians from the North Urals, an isolated tooth plate of Lagarodus from the River Shar'yu of the Chernyshev Ridge, and stethacanthid and Denaea-like teeth, and neoselachian dermal denticles from the South Island of the Novaya Zemlya Archipelago. Ivanov (Reference Ivanov, Lucas, DiMichelle, Barrick, Schneider and Spielmann2013) also reported on a Late Serpukhovian–Early Bashkirian marine chondrichthyan assemblage from the Gissar Mountains of Uzbekistan. The taxa described here include the symmoriids Denaea cf. D. williamsi and Stethacanthulus decorus Ivanov, Reference Ivanov1999, the euselachian Gissarodus flabellatus, and indeterminate dermal denticles (Ivanov, Reference Ivanov, Lucas, DiMichelle, Barrick, Schneider and Spielmann2013). In North America, the best known Bashkirian fish assemblage is from the Joggins Formation of Nova Scotia. The chondrichthyans and other fishes identified here have been reported as representing a euryhaline assemblage (Carpenter et al., Reference Carpenter, Falcon-Lang, Benton and Grey2015). Chondrichthyans described from Joggins include Ctenacanthus sp., Orthacanthus cf. denticulatus Davis, Reference Davis1880, an indeterminate xenacanth, and the chondrichthyan Ageleodus pectinatus Agassiz, Reference Agassiz1843 (Carpenter et al., Reference Carpenter, Falcon-Lang, Benton and Grey2015). Additionally, a chondrichthyan assemblage from the the late Bashkirian Black Prince Limestone of the Swisshelm Mountains of southern Arizona includes xenacanths, cladodont-grade teeth, helodontids, and petalodonts (Johnson and Thayer, Reference Johnson and Thayer2009). Of the xenacanths from the Black Prince Limestone, Bransonella was recorded from numerous specimens which Johnson and Thayer (Reference Johnson and Thayer2009) tentatively identified as B. ?nebraskensis, B. ?lingulata, B. sp. A, and B. sp. B, respectively. The B. ?lingulata specimen from the Black Prince Limestone, if valid, would be the earliest record of B. lingulata in North America. Presently, this record suggests the distribution of B. lingulata to North America occurred after the Serpukhovian. A Demoinesian record of B. lingulata is now recognized from the Buckhorn Asphalt Quarry in southern Oklahoma (Ivanov et al., Reference Ivanov, Seuss and Nützel2017). The Watahomigi Formation of the Grand Canyon is at present the only known fully marine locality yielding chondrichthyans and includes Hokomata parva n. gen. n. sp. and Deltodus sp.
Conclusions
The chondrichthyan assemblage from the Latest Mississippian (Serpukhovian) Surprise Canyon Formation of the western Grand Canyon is remarkably diverse with a total of thirty-one taxa, identified from teeth and dermal elements. This assemblage best compares with the Bear Gulch Limestone assemblage from central Montana. The Surprise Canyon assemblage provides a range extension of the paraselachians Srianta and Heteropetalus, which previously were only known from the Bear Gulch Limestone assemblage. However, the Surprise Canyon assemblage differs from the Bear Gulch assemblage in its greater number of euselachian taxa and the presence of the xenacanthimorph Bransonella nebraskensis and the elasmobranch Clairina sp. The protacrodonts from the Surprise Canyon assemblage represent an ecological split from the generalized Protacrodus tooth model (Ginter et al., Reference Ginter, Hampe, Duffin and Schultze2010), with the more durophagus dentition of Microklomax carrieae n. gen. n. sp. and the more hypocarnivorous dentition of Novaculodus billingsleyi n. gen. n. sp. The anachronistid euselachians also show a greater diversity with the additions of Cooleyella platera n. sp. and Amaradontus santuccii n. gen. n. sp.
Following an overview of the Serpukhovian Euamerica marine chondrichthyan assemblages (Fig. 16), we tentatively suggest there may have been an eastern and western distinction between the Eurasian assemblages. All Eurasian and North American assemblages share taxa at the order, family, and in some cases, genus levels, but neither faunas show similarity at the species level. This suggests that after the closing of the Rheic Ocean during the early Carboniferous (Nance et al., Reference Nance, Gutierrez-Alonso, Keppie, Linnemann, Murphy, Quesada, Strachan and Woodcock2012), many of these taxonomic groups were already in place. However, the closure of the Rheic Ocean provided the catalyst for these groups to further evolve in isolation from one another, resulting in the differences we see at the species level. The one exception to this tentative speciation model is the presence of Thrinacodus gracia from the Moscow Region of Russia. Ginter and Turner (Reference Ginter and Turner2010) recognized two partial teeth, which they referred to cf. Thrinacodus gracia from a larger collection made by A. Ivanov in the Kalionovskie Vyelki Quarry near Moscow, Russia. The presence of these teeth in Eurasia suggests a connection between the western North American chondrichthyan assemblages while Eurasia was still present, or that the origin and distribution of T. gracia occurred earlier, before the closure of the Rheic Ocean.
The poor record of marine chondrichthyans of the Early Pennsylvanian Bashkirian is only slightly supplemented by the new record from the Watahomigi Formation in the western Grand Canyon. The presence of the new diplodoselachian xenacanth, Hokomata parva n. gen. n. sp., within the interval of the Watahomigi Formation indicates that some xenacanth taxa were capable of living in marine conditions. Most of the marine records of Bashkirian chondrichthyans are from Eurasia (Ivanov, Reference Ivanov1999, Reference Ivanov, Lucas, DiMichelle, Barrick, Schneider and Spielmann2013), while in North America the chondrichthyans are primarily euryhaline (Johnson and Thayer, Reference Johnson and Thayer2009; Carpenter et al., Reference Carpenter, Falcon-Lang, Benton and Grey2015). The records of “Deltodus” by McKee (Reference McKee1982) from the Guano Cave, Parashant Canyon, and Separation Canyon stongly suggest additional chondrichthyan fossils could be found in the Watahomigi Formation of the western Grand Canyon and could expand the data for the Bashkirian chondrichthyan assemblages.
In conclusion, the specimens described here from the Surprise Canyon and Watahomigi formations of the western Grand Canyon most likely represent a small fraction of the fauna present during those depositional events. Unfortunately, these specimens do not fully illuminate the transition of chondrichthyan assemblages across the Mississippian/Pennsylvanian boundary. Further work, particularly with the Watahomigi Formation, could expand our knowledge of this transition.
Acknowledgments
We thank C. Brugger-Schorr for bringing the conodont residue material to our notice, which in turn inspired this project. Special thanks to J. Wittke for SEM imagery and A. Millhouse in the Paleobiology Department at the Smithsonian for her assistance in the collections and for specimen loans. We greatly appreciate assistance from D. and J. Gillette for helping us with collection information at the Museum of Northern Arizona. Conversations with and suggestions from A. Ivanov greatly improved this project. JPH particularly would like to thank his academic advisors, E. Grogan and R. Lund, for sharing their knowledge and data on chondrichthyans and the Bear Gulch Limestone during his tutelage at SJU. We thank our reviewers, S. Turner, A. Ivanov, and C. Underwood, for providing useful comments that helped to improve this manuscript.




















