Vitamin D insufficiency/deficiency has been considered a public health problem worldwide because of its implications in the bone tissue, as well as in maintaining the normal homeostasis of Ca and P which is already well known( Reference Forrest, Stuhldreher and Kimberly 1 – Reference Binkley, Ramamurthy and Krueger 3 ). In tropical countries this insufficiency/deficiency has not been cited as a major concern, since cutaneous synthesis from sun exposure is the major source of vitamin D in most individuals. However, some studies have reported a significant insufficiency/deficiency prevalence even in countries with adequate sun exposure, as in the case of Brazil( Reference Holick 4 , Reference Peters, dos Santos and Fisberg 5 ). Peters et al. ( Reference Peters, dos Santos and Fisberg 5 ) reported that 60 % of adolescents from the state of São Paulo had vitamin D insufficiency, despite having collected their data during the summer.
An adequate vitamin D level is essential in all stages of life, and there is consensus that the best status indicator of this vitamin is the serum concentration of calcidiol (25(OH)D)( Reference Binkley, Ramamurthy and Krueger 3 ). Low levels of calcidiol directly affect Ca absorption and bone mineralization. However, recent studies have indicated that deficiency of this vitamin may confer a risk factor for the development of endocrine and metabolic diseases( Reference Borges, Martini and Rogero 6 , Reference Foss 7 ). Studies suggest that vitamin D may be involved in various processes, such as cell differentiation/proliferation and hormone secretion, as well as in the immune system and in chronic diseases such as obesity, glucose intolerance, increased blood pressure and lipid abnormalities( Reference Foss 7 – Reference Kelly, Brooks and Dougherty 9 ).
Despite the scientific interest in the topic, most studies investigating the consequences of hypovitaminosis D in relation to metabolic disorders have focused their attention on adults and the elderly. However, it is necessary to consider that adolescence is the strategic period for promoting healthy habits and preventing the development of overweight/obesity, which damages the current physical and mental health of the individual( 10 ), besides being considered a predictor of overweight in adulthood, atherosclerotic disease, hypertension and metabolic disorders( Reference Lloyd, Langley-Evans and McMullen 11 ).
In Brazil, while the accelerating increase of overweight/obesity in adolescence has been reported in national studies( 12 ), there are few studies aimed at investigating the vitamin D status of healthy adolescents. Considering the impact on the metabolic profile of health, the present study aimed to evaluate the frequency of deficiency/insufficiency of vitamin D in adolescents and its relationship to overweight and metabolic disorders.
Methods
The study was cross-sectional, conducted with eutrophic and overweight adolescents aged 15 to 17 years from high schools in the city of Juiz de Fora.
Study design
The city of Juiz de Fora, located in the region of Minas Gerais in south-east Brazil, had a population of 497 778 inhabitants of whom 487 822 (98 %) lived in the urban area in 2010( 13 ).
The sample size was calculated using the following premises: population size of adolescents aged 15–17 years in Juiz de Fora of 18 814( 14 ), prevalence of vitamin D insufficiency of 62 %( Reference Peters, dos Santos and Fisberg 5 ), error tolerance of 8 %, 95 % confidence interval, significance level of 5 % and estimated loss of 10 %, resulting in a total of 165 adolescents. Participating schools were selected based on calculating the number of public and private institutions needed to represent the region (fifteen schools). All adolescents in the age group of the study, enrolled in selected schools, were invited to participate in nutritional status screening to evaluate their nutritional status. By random selection and based on the study inclusion criteria (non-use of drugs interfering with glucose and lipid metabolism, non-use of supplemental Ca and/or vitamin D, not pregnant/lactating and having already experienced menarche for girls( Reference Colli 15 ), presence of axillary and facial hair for boys( Reference Colli, Coates and Guimarães 16 )), the authors contacted eighty-three pairs of adolescents (one eutrophic and one overweight). Six adolescents declined participation because they were already in nutritional monitoring with other professionals. Thus, a sample of 160 adolescents were eligible to participate in the study, with seventy-seven being eutrophic (BMI < 85th percentile for age and sex) and eighty-three overweight (BMI ≥ 85th percentile). The groups were similar in age, gender and type of school (public or private).
Anthropometry and body composition
Weight was taken on a digital electronic scale (Tanita® model BC 553, Arlington Heights, IL, USA), with a maximum capacity of 150 kg and 100 g graduations, and height using a portable stadiometer (Alturexata®, Belo Horizonte, MG, Brazil) with a height range of 2 m, in 0·1 cm graduations. Weight and height were measured according to the techniques advocated by Jellife( Reference Jelliffe 17 ). Nutritional status was assessed by BMI according the WHO recommendations( Reference de Onis, Onyango and Borghi 18 ). Excessive weight was defined as BMI above 1 sd.
Waist circumference (WC) was measured with a non-elastic flexible tape (TBW®, São Paulo, SP, Brazil) with the adolescent in a standing position, at the end of expiration( Reference Jelliffe 17 ). Abdominal adiposity was estimated using cut-off points corresponding to the 90th centiles( 19 ).
Percentage of body fat (%BF) was estimated from the resistance and reactance values provided by a horizontal tetrapolar bioimpedance analyser (Biodynamics® model 310, Seattle, WA, USA). For this measure, adolescents followed the protocol( 20 ). The values provided by the bioimpedance analyser were used in the equation proposed by Houtkooper et al. ( Reference Houtkooper, Lohman and Going 21 ). %BF was analysed according to the classification proposed by Lohman( Reference Lohman 22 ).
Biochemical and clinical assessment
Blood samples were collected at the schools by technically qualified professionals and analysed in the Clinical Analysis Laboratory at the University Hospital, Federal University of Juiz de Fora. The samples were collected by venepuncture in the antecubital region, from individuals who had fasted for 12 h, to measure plasma glucose, insulin, total cholesterol (TC), HDL-cholesterol (HDL-C), LDL-cholesterol (LDL-C), TAG, 25(OH)D and parathyroid hormone (PTH).
Blood lipids were analysed according to the Brazilian Cardiology Society( 23 ). Impaired glucose tolerance was analysed according to the American Diabetes Association( 24 ). Fasting plasma insulin ≥15 μU/ml was considered hyperinsulinaemia( 23 ). Insulin resistance was assessed using the homeostasis model assessment (HOMA-IR), with HOMA-IR ≥ 3·16 being considered the presence of insulin resistance( 23 ).
Serum 25(OH)D levels were considered the most useful marker of total vitamin D exposure from both endogenous synthesis and dietary intake. Serum 25(OH)D levels were measured by a RIA kit. The cut-off points used to classify vitamin D deficiency and insufficiency were ≤25 nmol/l (≤10 ng/ml) and between 25 and 75 nmol/l (10–30 ng/ml), respectively( Reference Bischoff-Ferrari, Giovannucci and Willett 25 ). The intact PTH level was measured by a chemiluminescence immunoassay, with values between 15 and 65 pg/ml being considered normal( Reference Peters, dos Santos and Fisberg 5 ).
Sitting blood pressure was measured using an automatic unit with the right arm at the same level as the heart, using an appropriate cuff size. This equipment (Omron® HEM 907, Vernon Hills, IL, USA) was validated against mercury sphygmomanometers according to the international validation protocol( Reference El Assaad, Topouchian and Darné 26 ). Blood pressure was measured three times at 5 min intervals and was considered the average of the second and third measurements. The classification individually took into account gender, age and height based on parameters of the Brazilian Hypertension Society( 27 ).
Dietary assessment
Dietary intake was assessed by a 3 d food record, obtained over three non-consecutive days, one on the weekend( Reference Willett and Stampfer 28 ). The food record method for evaluation of nutrient intakes was used because of its high specificity in describing foods and food preparation methods( 27 ). The adolescents were informed about self-reporting the food records, with the food consumed being recorded in household measures, as well as the brand of the manufactured product. When an adolescent returned his/her records, a dietitian checked the record contents using photo albums and clarified the information with the adolescent. Standardization of recipes and portion sizes was carried out in order to analyse the results( Reference Philippi 29 , Reference Pinheiro, Lacerda and Benzecry 30 ).
Nutrient intakes were calculated using Diet-Pro software version 4·0. The foods or preparations that did not appear in the software's database were inserted according to data from tables of food composition( Reference Philippi 29 , Reference Pinheiro, Lacerda and Benzecry 30 ) and food labels. The assessment of adequate vitamin D intake was carried out following the recommendations from the Dietary Reference Intakes (DRI)( 31 ). To control the effect of energy consumption on the micronutrients evaluated, we used the nutrient residual method proposed by Willett and Stampfer( Reference Willett and Stampfer 28 ).
Physical activity and sun exposure
Physical activity was assessed using the questionnaire developed and validated for adolescents by Florindo et al. ( Reference Florindo, RomeroII and Peres 32 ), which allows the calculation of a weekly physical activity score. To classify the level of physical activity, we used the final score as a dichotomous variable, using the cut-off point of ≥300 min/week as moderate or vigorous physical activity and <300 min/week as light or sedentary physical activity( Reference Pate, Freedson and Sallis 33 ).
Sun exposure was evaluated by asking whether the adolescents used sunscreen daily (yes or no), and if they performed outdoor physical activities (yes or no) and their frequency (min/week).
Data were collected by trained staff at the schools participating in the project. A room was properly assembled and equipped for this purpose.
Statistical analysis
The database construction and statistical analyses were done using the statistical software package SPSS for Windows version 17·0.
The Kolmogorov–Smirnov normality test was used to determine if the distribution of values was normal in all variables. According to the distribution of variables in the normal curve, Student's t test, the Mann–Whitney test, the Kruskal–Wallis test and ANOVA were used to compare two or three groups. When a statistical difference was detected among three groups, Bonferroni's test and Tukey's test were then performed to assess specific differences. Pearson or Spearman correlation tests were also employed.
Results
A total of 160 adolescents were evaluated in the present study. Their mean age was 16 years, 55·6 % were male, 48·1 % were eutrophic and 51·9 % were overweight.
The characteristics of the population, overall and by nutritional status, are presented in Table 1. Adolescents with excess weight had significantly higher mean values of WC, %BF, TC, LDL-C, TAG, insulin, HOMA-IR, systolic and diastolic blood pressure (P < 0·05). Lower mean serum levels of 25(OH)D and HDL-C were found in the excess weight group (P < 0·05; Table 1). Higher prevalence of hypercholesterolaemia, hypertriacylglycerolaemia, hyperinsulinaemia, reduced HDL-C, systolic and diastolic hypertension was found in the excess weight group (P < 0·05).
Table 1 Distribution of anthropometry, body composition, biochemical profile and clinical characteristics according to nutritional status among adolescents (n 160) aged 15 to 17 years, Juiz de Fora city, Brazil

IQR, interquartile range; WC, waist circumference; %BF, percentage of body fat; FFM, fat-free mass; TC, total cholesterol; HDL-C, HDL-cholesterol; LDL-C, LDL-cholesterol; HOMA-IR, homeostasis model assessment of insulin resistance; 25(OH)D, calcidiol; PTHi, intact parathyroid hormone; SBP, systolic blood pressure; DBP, diastolic blood pressure.
*Significant result.
†These variables were parametric (mean and standard deviation) and compared between groups using Student's t test; otherwise, variables were non-parametric (median and IQR) and compared between groups using the Mann–Whitney test.
The mean serum 25(OH)D level was 24·0 nmol/l. Vitamin D deficiency was observed in 1·25 % (n 2) of the adolescents, both from the excess weight group. Insufficiency was observed in 70·6 % of the adolescents (72·2 and 68·8 % from the normal and excess weight groups, respectively).
No statistical difference in vitamin D intake, demographic and behavioural variables was observed according to serum 25(OH)D level. Serum 25(OH)D levels were statistically lower in adolescents with excess body fat, abdominal obesity, hypercholesterolaemia, insulin resistance, hyperinsulinaemia and hypertension (P < 0·05; Table 2). Furthermore, the levels of serum 25(OH)D had a significant inverse correlation with BMI, WC, %BF, TC, LDL-C, insulin, HOMA-IR and PTH (P < 0·05).
Table 2 Serum 25(OH)D levels according to demographic, behavioural and intake variables among adolescents aged 15 to 17 years, Juiz de Fora city, Brazil

25(OH)D, calcidiol; IQR, interquartile range; %BF, percentage of body fat; WC, waist circumference; TC, total cholesterol; HDL-C, HDL-cholesterol; LDL-C, LDL-cholesterol; PTHi, intact parathyroid hormone; HOMA-IR, homeostasis model assessment of insulin resistance; BP, blood pressure.
a,bMedian values within a column with unlike superscript letters were significantly different (P < 0·05).
*Significant result.
†These values were compared between groups using the Kruskal–Wallis test; otherwise, the Mann–Whitney test was used.
Daily sunscreen use and the practice of physical activities in sunlight were reported by 16·2 and 31·5 % of the adolescents, respectively. The mean duration of physical activities in sunlight was 80 min/week. There was no difference in serum 25(OH)D concentration between adolescents who did/did not use sunscreen daily, nor between adolescents who did/did not practise physical activity in sunlight. These behaviours did not differ between adolescents with or without excess weight.
The macronutrient distribution was adequate according to the Acceptable Macronutrient Distribution Ranges( 34 ) in eutrophic and overweight adolescents. No differences in energy, protein, fat, Ca, saturated fat and cholesterol intakes were observed between groups. Significantly more adolescents with excess weight had low vitamin D and carbohydrate intakes compared with the eutrophic group (P = 0·009 and P = 0·015, respectively).
The mean vitamin D intake (2·18 μg/d) was lower than the Estimated Average Requirement (EAR) in both groups. The main sources of vitamin D reported by teenagers were milk, eggs and butter. This intake did not differ between adolescents with and without excess weight, and daily milk consumption was reported by only 57 % of the adolescents. Only 7·2 % of the excess weight group and 15·6 % of the eutrophic group reached the EAR for vitamin D (10 μg/d). Lower BMI and WC were observed in the third (highest) tertile of vitamin D intake for all adolescents (P < 0·05; Table 3). There was no statistical correlation between vitamin D intake and serum 25(OH)D level (r = 0·151; P = 0·56).
Table 3 Distribution of anthropometry, body composition, biochemical profile and clinical characteristics according to tertile of dietary vitamin D intake among adolescents aged 15 to 17 years, Juiz de Fora city, Brazil
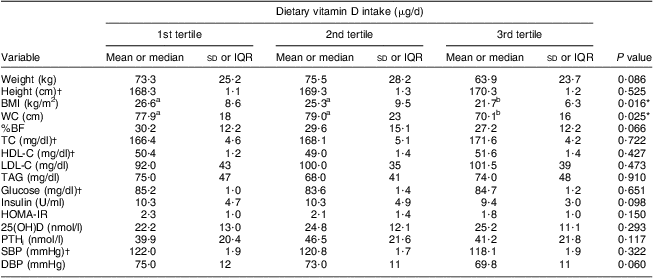
IQR, interquartile range; WC, waist circumference; %BF, percentage of body fat; TC, total cholesterol; HDL-C, HDL-cholesterol; LDL-C, LDL-cholesterol; HOMA-IR, homeostasis model assessment of insulin resistance; 25(OH)D, calcidiol; PTHi, intact parathyroid hormone; SBP, systolic blood pressure; DBP, diastolic blood pressure.
a,bMean values within a row with unlike superscript letters were significantly different (P < 0·05).
*Significant result.
†These variables were parametric (mean and standard deviation) and assessed with ANOVA using the Tukey test for post hoc comparisons; otherwise, variables were non-parametric (median and IQR) and assessed with the Kruskal–Wallis test using the Bonferroni test for post hoc comparisons.
Discussion
The present study found that a significant proportion (∼70 %) of Brazilian adolescents had vitamin D insufficiency even in a tropical country. These results are lower than those observed by Delvin et al. ( Reference Delvin, Lambert and Levy 35 ) who, in an evaluation of Canadian children and adolescents, and using the same proposed classification of 25(OH)D levels, found a 93 % rate of suboptimal levels of this vitamin. In Brazil, until the present day, there are few studies on the prevalence of hypovitaminosis D in adolescence. Peters et al. ( Reference Peters, dos Santos and Fisberg 5 ) studied healthy adolescents in São Paulo and observed a 62·1 % rate of insufficiency using the same proposed classification of 25(OH)D levels. These results indicate that although hypovitaminosis D is lower in Brazil due to the tropical climate, a significant prevalence of insufficiency of this vitamin is seen when compared with milder climates.
In the present study, the serum 25(OH)D levels were statistically lower in adolescents with excess weight, abdominal obesity, hypercholesterolaemia, insulin resistance, hyperinsulinaemia and hypertension (P < 0·05). The lower serum 25(OH)D levels in overweight individuals has been reported previously in the scientific literature( Reference Harel, Flanagan and Forcier 8 , Reference Kelly, Brooks and Dougherty 9 , Reference Delvin, Lambert and Levy 35 ). It is believed that low serum vitamin D levels in the obese are not just a consequence of reduced sun exposure. Some evidence suggests that this deficiency may be linked to vitamin D storage in adipocytes, decreasing its bioavailability and activating the hypothalamus to develop a cascade of reactions that results in increased feelings of hunger and decreased energy expenditure( Reference Snijder, Van Dam and Visser 36 ). This situation can also generate increased levels of PTH and a consequent decrease in insulin sensitivity( Reference Kelly, Brooks and Dougherty 9 ).
The inadequacy of the lipid profile observed in the present study has been described by the WHO as a problem in this age group in a number of countries( 10 ). It is noteworthy that the result showing the association of BMI with clinical and biochemical variables indicates the need for constant intervention with adolescents, thus reinforcing the importance of specific health care for this age group( Reference Celermajer and Ayer 37 ).
Several authors have discussed an extra-skeletal function of vitamin D and found that individuals with high levels of TC, LDL-C, insulin and blood pressure had serum 25(OH)D concentrations statistically lower than those with normal levels of these variables( Reference Borges, Martini and Rogero 6 , Reference Foss 7 , Reference Delvin, Lambert and Levy 35 ). Kelly et al. ( Reference Kelly, Brooks and Dougherty 9 ), studying the association of serum 25(OH)D levels and glucose profiles in obese and non-obese children, found that low vitamin D levels were associated with increased plasma insulin and insulin resistance after adjustment for BMI and puberty. Some authors have observed that lower serum 25(OH)D levels are associated with blood pressure( Reference Peters, dos Santos and Fisberg 5 , Reference Snijder, Van Dam and Visser 36 ). However, caution is needed before assuming any direct association. It is essential to consider the well-known influence between high BMI and excess weight, biochemical profile and blood pressure( Reference Lloyd, Langley-Evans and McMullen 11 , Reference Hill, Cotter and Mitchell 38 ).
The Institute of Medicine reviewed the DRI for vitamin D and concluded that a causal role of vitamin D in skeletal health provided the necessary basis for the EAR and RDA. However, for non-skeletal outcomes including CVD, diabetes and autoimmune disorders, the evidence was inconsistent and inconclusive as to causality, and insufficient for DRI development( Reference Pinheiro, Lacerda and Benzecry 30 , Reference Ross, Manson and Abrams 39 ). The mean vitamin D intake was lower than the EAR values in both groups in the present study; mean dietary vitamin D intake was inadequate for adolescents either with or without excess weight, and was significantly lower in adolescents with excess weight. Lower BMI and WC were observed in the third tertile of vitamin D intake for all adolescents (P < 0·05).
The low vitamin D intake among adolescents has also been documented in other countries( Reference Peters, dos Santos and Fisberg 5 , Reference Au, Economos and Goodman 40 ). The dietary sources of vitamin D are limited, and food sources such as salmon, tuna and cod-liver oil are not usually consumed by Brazilian teenagers. The exception is milk, a food source consumed on a regular basis, where daily consumption was reported by 57 % of adolescents. Furthermore, the unhealthy eating habits common at this stage of life, such as the habit of skipping breakfast, high consumption of soft drinks and processed products rather than foods that are sources of vitamins and minerals, and replacing dinner with snacks, can explain the reduction of these nutrients in the diet( Reference Lloyd, Langley-Evans and McMullen 11 ). There was no association between vitamin D intake and serum 25(OH)D levels. The limitations of the food composition tables that comprise the databases in the dietary assessment software may have contributed to this result.
The other potential dietary source of vitamin D intake is supplements. However, none of the adolescents in our study were taking any vitamin D supplements. The use of supplements and foods fortified with vitamin D is not common in Brazil. In the present study, the fact that a substantial proportion of Brazilian adolescents had vitamin D insufficiency indicated that dietary sources of vitamin D are not adequate to maintain the recommended levels of vitamin D status. Similar results have been documented in a study conducted in São Paulo, Brazil( Reference Peters, dos Santos and Fisberg 5 ).
In addition to dietary vitamin D intake and supplements, the other source of vitamin D is exposure of the skin to sunlight. However, there was no difference in serum 25(OH)D concentration between adolescents who do/do not use sunscreen daily, as well as between adolescents who do/do not practise physical activity in sunlight. These behaviours did not differ between adolescents with or without excess weight.
Some limitations of the present study should be considered. It is noteworthy that, although using a random sample, the study is not representative of the whole population and so extrapolation of the results must be made with caution. However, the authors believe that the results are important because vitamin D insufficiency is a global problem, even in sunny environments like Brazil, and the study is one of the few performed in developing countries that evaluated vitamin D status and its association with metabolic disorders in adolescents. It is unclear whether the association between low serum vitamin D and metabolic disorders is causal, much less the direction of causality. Low serum vitamin D is contributing to the increased prevalence of metabolic disorders or metabolic disorders increase the risk of low serum vitamin D, or both. Another limitation is that the present study did not evaluate daily activities in sunlight like walking and driving a car, only physical activities. More detailed information about sun exposure is necessary to investigate the variation of serum concentration of 25(OH)D. It is important to recognize the limitations of dietary assessment when studying the relationship between food intake and adiposity, such as under-reporting. However this information bias did not affect the results in relation to vitamin D intake, which was lower in adolescents with excess weight, as seen in the literature.
Some strengths of our study should also be considered. First, the inclusion of adolescents with normal and excess weight allows for control of the effect of BMI on clinical and biochemical parameters, and understanding the direct association of vitamin D on these parameters. Second, the selection of postpubertal adolescents to control biological markers characteristic of late sexual maturation( 14 , Reference Colli 15 ), such as menarche and the presence of axillary and facial hair, allowed us to reduce the influence of the sexual maturation process on body composition and metabolic changes.
Conclusion
Our results support the negative association of serum 25(OH)D levels with non-skeletal outcomes in Brazilian adolescents, such as obesity, abdominal obesity, hypercholesterolaemia, insulin resistance, hyperinsulinaemia and hypertension. Furthermore, lower BMI and WC were observed in the third tertile of vitamin D intake for all adolescents.
Although hypovitaminosis D is lower in Brazil due to the tropical climate, a significant frequency of insufficiency of this vitamin is seen when compared with milder climates. The high frequency of vitamin D insufficiency is primarily nutritional and reflects a low vitamin D intake. Vitamin D fortification of foods and/or the use of vitamin D supplements need to be considered to raise the vitamin D intake in the adolescent population, even in a sunny country like Brazil.
The present study is one of few performed in developing countries that evaluated vitamin D status and its association with overweight and metabolic disorders in adolescents. These results are useful to health practitioners and should raise concerns in assessment and management of patients/clients. Randomized clinical trials are of utmost importance to test the effects of vitamin D on non-skeletal outcomes, as well as possible mediating effects of sun exposure, adiposity and genetic factors on this relationship.
Acknowledgements
Sources of funding: This work was supported by the Fundação de Amparo a Pesquisa do Estado de Minas Gerais (FAPEMIG) under grant number 01571-09. Conflicts of interest: The authors declare no conflict of interest. Ethical information: The study was conducted according to the guidelines laid down in the Declaration of Helsinki and all procedures involving human subjects/patients were submitted to and approved by the Ethics Committee of the Universidade Federal de Juiz de Fora. Study participants and their guardians were informed about the study objectives. Participation was voluntary and was allowed only after signing of an informed consent by parents or guardians. Authors’ contributions: All of the authors have participated sufficiently in the conception and design of this work and the analysis of the data, as well as the writing of the manuscript, to take public responsibility for it. Acknowledgements: The authors thank FAPEMIG for financing the project, the Nutrition Department of the Federal University of Juiz de Fora for support and the adolescents for their participation in the study.







