Dysfunctional energy metabolism underlies the development of obesity and obesity-related complications such as hepatic steatosis, diabetes and heart disease(Reference Murray, Lygate and Cole1). It is a well-known fact that carbohydrate is the most prevalent source of energy for monogastric mammals and humans. Carbohydrates not only provide the substrate for the Krebs cycle, but also act as a regulator for lipid metabolism(Reference Ghusain-Choueiri and Rath2, Reference Reddy and Hashimoto3). Upon consumption of excess carbohydrate, digestion yields simple sugars that are converted to pyruvate (glycolysis), which is either oxidised to provide energy or channelled into pathways for synthesis of fatty acids (lipogenesis) when energy is available(Reference Reddy and Hashimoto3). The coordinated regulation of these metabolic processes allows the efficient utilisation of dietary carbohydrates, and key enzymes involved in carbohydrate metabolism are tightly regulated by hormones and dietary nutrients(Reference Uyeda and Repa4).
Previous studies have indicated that in monogastric mammals the metabolic responses induced by different dietary carbohydrates are widely variable(Reference Yudkin5, Reference MacDonald and Braithwaite6). In humans, isoenergetic replacement of dietary starch with sucrose, in the short term, resulted in elevated plasma TAG and cholesterol concentrations(Reference MacDonald and Braithwaite6, Reference Reiser, Hallfrisch and Michaelis7) and impaired glucose tolerance(Reference Reiser, Handler and Gardner8). Similar adverse effects of high sucrose consumption have been reported for non-human primates and a number of laboratory animals(Reference Ghusain-Choueiri and Rath2, Reference Camp, Southern and Bidner9). Starch, acting as the main energy source of the daily diet, is the most prevalent carbohydrate consumed by monogastric mammals. However, the metabolic responses induced by different dietary starches may vary widely depending upon their sources and polymer structures(Reference Stevneb, Sahlström and Svihus10, Reference Fugui, Zhenzhen and Ju11). For instance, starches that are high in amylopectin are easily digested, which may lead to a rapid increase in blood glucose and insulin concentration, whereas starches with more amylose may result in moderate glycaemic and insulinaemic responses(Reference Deng, Wu and Bing12–Reference Bird, Brown and Topping14). Although these metabolic responses were previously studied in both humans and other monogastric mammals, still less is known about the mechanisms behind these responses. More importantly, few studies have investigated the relationship between hepatic lipid metabolism and starch composition. Therefore, the aim of the present study was to evaluate the metabolic responses of weaned pigs fed with different dietary starches, and mechanisms behind these responses were investigated on a molecular basis.
Materials and methods
Starches
Purified cassava starch (CS, 80 % amylopectin and 20 % amylose) and maize starch (MS, 70 % amylopectin and 30 % amylose) were purchased from Chengdu food market (Chengdu, Sichuan, China).
Animals and diets
The experimental protocols used in the present study were approved by Sichuan Agricultural University Institutional Animal Care and Use Committee. Sixteen weaned pigs (Duroc × Landrace × Yorkshire) with an average initial body weight of 7·37 (sem 0·25) kg were selected and randomly allotted to two dietary treatments with equal numbers of males and females in each group. The experimental diet was formulated on the basis of nutrient requirements established by the National Research Council (1998) for 5–10 kg pigs(15). Either CS or MS was used as the sole dietary energy source. There were no discrepancies for other nutrient components. Dietary amino acids were supplied by dehulled soyabean meal, extruded soyabean and fishmeal, and vitamin and minerals were supplied by vitamin and mineral supplements (Table 1). Synthetic dl-methionine was added to the diets to meet minimal methionine–cystine requirements.
Table 1 Ingredient and chemical composition of experimental diets (as fed-basis)

CS, cassava starch; MS, maize starch.
* Supplied (per kg diet): Fe as FeSO4·7H2O, 100 mg; Mn as MnSO4·7H2O, 40 mg; Zn as ZnO, 80 mg; Cu as CuSO4·5H2O, 10 mg; Se as NaSeO3, 0·3 mg and I as KI, 0·3 mg.
† Supplied (per kg diet): 5·7 mg vitamin A, 36·7 mg vitamin E, 0·01 mg vitamin D, 1·1 mg vitamin K (menadione dimethylpyrimidinoe bisulfate), 5 mg vitamin B1, 15 mg riboflavin, 25 mg niacin, 30 mg d-pantothenic acid and 0·05 mg vitamin B12.
Animal housing and tissue sampling
The pigs were housed individually in metabolism cages (0·7 × 1·5 m) with woven wire flooring in an environmentally controlled room (22–24°C) and were given ad libitum access to water through a water nipple. They were hand-fed four times/d (08.00, 12.00, 16.00 and 20.00 hours) in bowl feeders to make sure fresh feed was available, and were allowed a 7 d adjustment to the experimental diets. The diet adjustment period was followed by a 21 d experimental period. Weights and feed consumption of the pigs were determined daily throughout the trial. The blood samples were collected by venepuncture at 07.00 hours on day 14. At the end of the trial, pigs were euthanised with an intravenous injection of pentobarbital sodium (50 mg/kg body weight) and the liver samples were collected, weighted and stored at − 80°C.
Biochemical analysis
The liver and serum lipids were extracted and purified(Reference Folch, Lees and Sloane Stanley16). TAG and cholesterol levels were measured using the method described by Hercberg et al. (Reference Hercberg, Galan and Preziosi17). Insulin, growth hormone (GH) and glucagon levels were measured using electrochemiluminescence immunoassays (Roche Diagnostics, Meylan, France). All the assay kits were purchased from Tosoh Corporation (Kyoto, Japan). The activities of liver glucose-6-phosphate dehydrogenase, fatty acid synthase, acyl-CoA oxidase and 3-hydroxy-3-methylglutaryl-CoA reductase were assayed according to methods described by Ide et al. (Reference Ide, Watanabe and Sugano18).
RNA extraction
Total RNA was isolated from liver using TRIzol (Invitrogen, Carlsbad, CA, USA) and further purified by RNeasy Mini Kit (Qiagen, Valencia, CA, USA). All the procedures were carried out as per the manufacturer's protocol. The concentration of RNA was determined using spectrophotometry based on absorbance at 260 nm and integrity was monitored using an Agilent 2100 Bioanalyzer (Agilent Technologies, Santa Clara, CA, USA).
Real-time RT-PCR
Real-time PCR primers were designed (Takara, Dalian, Liaoning, China) to assay six genes related to lipid metabolism (Table 2). β-Actin was used as the reference gene. Briefly, 500 ng RNA was reverse transcribed using high-capacity cDNA Reverse Transcription Kit (PN 4368814; Invitrogen) for each pig. Real-time RT-PCR for six target genes and the housekeeping gene were performed using Applied Biosystems (Foster City, CA, USA) Power SYBR Green PCR Master Mix in a Bio-Rad iCycler with minor modifications (Bio-Rad, Hercules, CA, USA). Fluorescein was added at a final concentration of 10 nm as the reference dye. Cycling conditions were as follows: 95°C for 5 min, forty-five cycles of 95°C for 30 s, appropriate annealing temperature (Table 2) for 30 s, 72°C for 30 s, followed by 72°C for 5 min, 95°C for 1 min, 55°C for 1 min, followed by a melt curve analysis of eighty cycles of 10 s at 55°C with a 0·5°C increase every cycle.
Table 2 Primer sequences of genes selected for analysis by real-time RT-PCR

Temp, temperature.
Statistical analysis
Gene expression data from replicate samples were averaged and analysed using the Pfaffl(Reference Pfaffl19) method to measure the difference between the cassava and maize cycle threshold values. Growth performance, metabolic enzymes and serum data were analysed by SPSS 13.0 (SPSS, Inc., Chicago, IL, USA). Determination of statistical significance was carried out by independent-sample t test. Data were expressed as means with their standard errors. Differences with P < 0·05 were considered to be significant.
Results
Growth performance
The growth parameters are reported in Table 3. There were no significant differences (P>0·05) in either average daily body weight gain or feed intake between the CS and MS groups during the 21 d experimental period; weight gains were 378·9 (sem 21·3) and 386·7 (sem 28·2) g/d in the CS and MS groups respectively. The daily feed intakes of the CS and MS groups were 492·7 (sem 29·1) and 509·4 (sem 35·6) g/d respectively.
Table 3 Effects of different dietary starches on growth performance, metabolic hormones and hepatic lipid concentrations in weaned pigs
(Mean values with their standard errors)
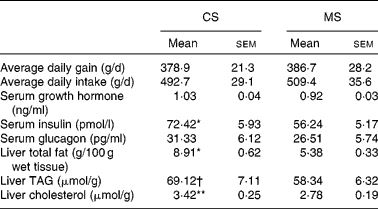
CS, cassava starch; MS, maize starch.
Mean value was significantly different from that of the MS group: * P < 0·05, ** P < 0·01.
† Mean value tended to be significantly different from that of the MS group (P < 0·1).
Serum metabolites and hormones
No significant difference in serum glucose concentration was observed between the two groups (Fig. 1). However, ingestion of CS acutely increased the serum cholesterol concentration (2·04 (sem 0·13) v. 1·54 (sem 0·10) mmol/l, P < 0·05). In addition, the serum TAG concentration increased by 14·9 % in the CS group (Fig. 1). There were no significant differences in serum GH and glucagon concentrations between the two groups (P>0·05). In contrast, ingestion of CS significantly elevated the serum insulin concentration (Table 3).

Fig. 1 Effect of different dietary starches on serum glucose and lipid concentrations. Mean values were significantly different: *P < 0·05, † P < 0·10. ![]() , Cassava starch (CS); □, maize starch (MS).
, Cassava starch (CS); □, maize starch (MS).
Lipid content and metabolic enzymes in liver
Ingestion of CS significantly elevated the liver total fat and cholesterol concentration (P < 0·05). In addition, the TAG concentration increased by 18·5 % in this group (Table 3). The activities of several critical enzymes involved in lipid metabolism were measured (Fig. 2). No significant difference was observed for the activity of glucose-6-phosphate dehydrogenase between the two groups (P>0·05). However, ingestion of CS significantly elevated the fatty acid synthase and 3-hydroxy-3-methyl-glutaryl-CoA reductase activities in the liver (P < 0·05). The activity of acyl-CoA oxidase was lower in the CS group than that in the MS group (P < 0·05).

Fig. 2 Effect of different dietary starches on the activities of lipid metabolic enzymes. Mean values were significantly different: *P < 0·05, † P < 0·10. ![]() , cassava starch (CS); □, maize starch (MS). G6PD, glucose-6-phosphate dehydrogenase; FAS, fatty acid synthase; ACOX1, acyl-CoA oxidase 1; HMGR, HMG-CoA reductase.
, cassava starch (CS); □, maize starch (MS). G6PD, glucose-6-phosphate dehydrogenase; FAS, fatty acid synthase; ACOX1, acyl-CoA oxidase 1; HMGR, HMG-CoA reductase.
Hepatic gene expression
Quantitative real-time RT-PCR assays were designed for six genes expressed in liver. The genes were selected based on their involvement in lipid metabolism or their importance as components of the metabolic process. The present results indicated that ingestion of CS significantly elevated the transcription of lipogenic genes such as fatty acid synthase and 3-hydroxy-3-methylglutaryl-coenzyme A reductase (Fig. 3). However, the expression of lipolytic genes such as acyl-CoA oxidase 1 (acox1) and pparα decreased 1·18- and 1·24-fold respectively (P < 0·05). No significant differences were observed for diacylglycerol acyltransferase and carnitine palmitoyltransferase 1A transcription between the two groups (P>0·05).

Fig. 3 Effect of different dietary starches on hepatic gene expression. The relative expression was calculated as the ratio of target gene to internal reference gene. Mean values were significantly different: *P < 0·05; **P < 0·01. ![]() , Cassava starch; □, maize starch; fasn, fatty acid synthase; hmgr, 3-hydroxy-3-methylglutaryl-coenzyme A reductase; acox1, acyl-CoA oxidase 1; cpt1a, carnitine palmitoyltransferase 1A; dgat, diacylglycerol acyltransferase.
, Cassava starch; □, maize starch; fasn, fatty acid synthase; hmgr, 3-hydroxy-3-methylglutaryl-coenzyme A reductase; acox1, acyl-CoA oxidase 1; cpt1a, carnitine palmitoyltransferase 1A; dgat, diacylglycerol acyltransferase.
Discussion
Starch is the most important energy source for monogastric mammals. However, the digestibilities of starches from different sources are widely variable(Reference Stevneb, Sahlström and Svihus10, Reference Fugui, Zhenzhen and Ju11, Reference Englyst, Veenstra and Hudson13). Starches with a high amount of amylose are difficult to hydrolyse, whereas fully gelatinised amylopectin is easily digested, which can serve as a source of rapidly digestible starch and cause a stronger glycaemic and insulinaemic responses(Reference Stevneb, Sahlström and Svihus10–Reference Englyst, Veenstra and Hudson13). In the present study, the growth performance of weaned pigs was not affected by different dietary starches. However, ingestion of CS significantly elevated the serum cholesterol (32·4 %) and insulin (28·8 %) concentrations (P < 0·05). Previous studies have indicated that ingestion of carbohydrate results in elevated blood glucose, which rapidly triggers insulin release from β-cells of the endocrine pancreas(Reference Fugui, Zhenzhen and Ju11, Reference Wolever20). Unexpectedly, no significant difference was observed for serum glucose concentration between the two groups. This should be attributed, in part, to the elevated serum insulin concentration in the CS group (Table 3) and the time point for blood collection (blood samples were collected before the first meal in the morning). According to a previous report, the average retention time of starch digestion in small intestine is about 4 h, postprandial circulated glucose as well as other metabolites may change periodically(Reference Stevneb, Sahlström and Svihus10). The present results, however, agree well with previous findings showing that ingestion of a quickly digested carbohydrate significantly elevated the plasma TAG and insulin concentrations in rats(Reference Scribner, Pawlak and Ludwig21). Furthermore, a stronger insulinaemic response was previously observed both in humans(Reference Behall, Scholfield and Canary22) and other monogastric animals(Reference Krezowski, Nuttall and Gannon23–Reference Liu, Zhang and Bin25) after ingestion of starches high in amylopectin. We also measured the concentrations of serum GH and glucagon. GH is a protein-based polypeptide hormone capable of stimulating growth and cell reproduction and regeneration in humans and other animals, whereas both insulin and glucagon are important hormones involved in carbohydrate metabolism. In the present study, no significant differences were observed for serum GH and glucagon concentration between the two groups.
The liver is the central player in whole-body energy homoeostasis. We have found that ingestion of CS deposited more lipids in the liver (Table 3). Similar results were observed in a rat model, which showed that a diet high in rapidly absorbed carbohydrate causes hepatic steatosis(Reference Scribner, Pawlak and Ludwig21). In the present study, the liver total fat concentration exceeded 8 % in the CS group, which indicated a moderate fatty liver. The elevated liver fat concentration in the CS group might result from the elevated insulin concentration since it has long been looked as one of the most important hormones to activate the transcription of lipogenic enzymes(Reference Uyeda and Repa4, Reference Horton, Goldstin and Brown26). This hypothesis was also verified by the measurements of the enzyme activities produced in liver tissues (Fig. 2). Fatty acid synthase plays a key role in fatty acid synthesis, whereas 3-hydroxy-3-methylglutaryl-CoA reductase is the rate-controlling enzyme of the mevalonate pathway that produces cholesterol and other isoprenoids(Reference Smith, Witkowski and Joshi27, Reference Meigs, Roseman and Simoni28). The present results indicated that the activities of both enzymes were elevated in the CS group (P < 0·05). However, ingestion of CS significantly decreased the activity of acyl-CoA oxidase – a key enzyme involved in the fatty acid β-oxidation pathway(Reference Kawaquchi, Tsubotani and Seyama29, Reference Osumi, Hashimoto and Ui30).
To explore the mechanisms behind these metabolic responses, we analysed the transcription levels of six important genes involved in lipid metabolism. Ingestion of CS significantly activated the transcription of lipogenic genes – fatty acid synthase and 3-hydroxy-3-methylglutaryl-coenzyme A reductase (Fig. 3). The acox1-encoded protein is the first enzyme of the fatty acid β-oxidation pathway, and defects in this gene result in accumulation of very long-chain fatty acids in the body(Reference Kim, Sohn and Ahn31). We found that the transcription of acox1 was down-regulated in the CS group (Fig. 3). The real-time PCR results agree well with the enzyme activities produced in the liver (Fig. 2). The PPARα-encoded protein is an important transcriptional factor involved in the regulation of energy metabolism(Reference Michalik, Auwerx and Berger32). In the present study, the transcription of PPARα was down-regulated in the CS group (Fig. 3). PPARα belongs to the PPAR subfamily of nuclear receptor and facilitates energy combustion by activating the transcription of catabolic genes (i.e. FABP3, CYP4A1 and ADIPO; Fatty acid binding protein 3, cytochrome P450 and adiponectin, respectively) involved in lipid catabolism(Reference Evans, Barish and Wang33). The role of PPAR in hepatic steatosis has been fully investigated(Reference IP, Farrell and Robertson34). The present results indicated that the ingestion of starches with more amylopectin may increase the incidence of hepatic steatosis by down-regulation of the PPARα signalling pathway. Therefore, PPARα can be a valuable target for nutritional intervention during the process of steatosis, and starches with less amylopectin may help prevent or treat obesity and fatty liver in humans.
In summary, the present results suggested that the metabolic responses of weaned pigs fed with different dietary starches may vary widely depending on their composition, and ingestion of starches that are high in amylopectin not only induces a stronger insulinaemic response, but also leads to an up-regulation of lipogenesis and steroidogenesis in the liver.
Acknowledgements
J. H. and B. Y. participated in the experimental design, carried out the molecular and biochemical experiments, participated in data interpretation and helped draft the manuscript. B. Y. conceived the study. D. C. and K. Z. directly supervised the project, participated in its experimental design and data interpretation. J. H. was also responsible for writing the manuscript. The authors state that there are no conflicts of interest in this field. This work was supported by Youth Fund Project of Sichuan Ministry of Education (grant no. 00924200) and Program for Changjiang Scholars and Innovative Research Team in University (grant no. IRTO555-5), China Ministry of Education.










