The combination of pegylated (PEG) interferon-alpha (IFN-α) plus ribavirin is established standard treatment of hepatitis C virus (HCV) infection. Antiviral therapy including IFN-α, however, is associated with significant psychiatric side-effects such as depressive symptoms, fatigue and insomnia. Reference Dieperink, Willenbring and Ho1–Reference Schaefer, Capuron, Friebe, Diez-Quevedo, Robaeys and Neri3 The reported prevalence of INF-associated major depressive episodes is 20–40%, and up to 70% of patients may develop mild to moderate depressive episodes with a significant impact on their quality of life. This is likely to negatively affect adherence to treatment and may represent a major cause of treatment discontinuation. Reference Schaefer, Capuron, Friebe, Diez-Quevedo, Robaeys and Neri3,Reference Schaefer, Hinzpeter, Mohmand, Janssen, Pich and Schwaiger4 Recently, pre-emptive treatment with antidepressants, notably selective serotonin reuptake inhibitors (SSRIs), demonstrated a reduction of the incidence of mild to severe major depressive episodes in HCV-infected patients during antiviral treatment. Reference Sarkar and Schafer5 The largest trial in patients without prior psychiatric disorders to date was conducted with escitalopram. When compared with controls without pre-emptive treatment, a lower incidence and severity of depression, defined by the presence of DSM-IV major depressive episodes criteria and Montgomery–Åsberg Depression Rating Scale (MADRS) scores, was observed during HCV treatment. Reference Schaefer, Sarkar, Knop, Effenberger, Friebe and Heinze6 Although antidepressant pre-treatment showed a high efficacy in reducing overall depression rates and the use of SSRIs was safe without negative effects on antiviral response, it also needs to be considered that a significant proportion of patients do not develop clinically relevant depressive episodes. Thus, a pharmacological prophylactic treatment might put them at risk of harm without apparent benefits. Concomitant treatment with antidepressants may cause additional side-effects including sexual dysfunction, insomnia, nausea and visual and cardiac symptoms. Reference Gumnick and Nemeroff7 Hence, pre-emptive antidepressant treatment should be limited to patients who are at increased risk for depression, and reliable identification of high-risk patients for INF-associated depressive syndromes is warranted. Screening criteria to identify those patients who might have the greatest benefits from pre-emptive antidepressant therapy are warranted for limiting potential disadvantages and maximising potential benefits of psycho-pharmacological pre-treatment in routine care. The aim of the present post hoc analysis was to identify neuropsychiatric risk factors that predict the development of depression during antiviral treatment with IFN-α.
Method
Settings and participants
This article reports on a total of 91 people with chronic HCV infection who participated in a prospective controlled trial investigating the prevention of depressive symptoms with escitalopram during antiviral treatment with PEG-IFN-α and ribavirin (for detailed information see Schaefer et al Reference Schaefer, Sarkar, Knop, Effenberger, Friebe and Heinze6 ) and who were randomised to placebo treatment.
All patients were treatment-naive to HCV treatment, older than 18 years and had a chronic HCV infection with serum HCV-RNA levels of 1000 IU/mL or higher. Psychiatric exclusion criteria were a lifetime diagnosis of an affective disorder, drug misuse in the past 12 months, treatment with antidepressants during the past 3 years or a history of any other Axis I disorder according to DSM-IV criteria. 8 Medical exclusion criteria were pre-treatment with IFN or immunotherapy, other chronic infections or a severe somatic comorbid condition. The study protocol was approved by the ethics committee of the Charité – University Medicine Berlin and confirmed by local ethics committees. The study was conducted in accordance with the principles of the Declaration of Helsinki and local laws and regulations. All participants provided written informed consent. The trial is registered at ClinicalTrials.gov: NCT00136318.
Treatment
Eligible patients participated in a multicentre, double-blind, prospective, randomised and placebo-controlled phase-III study consisting of three different study periods as described elsewhere. Reference Schaefer, Sarkar, Knop, Effenberger, Friebe and Heinze6 The original study compared pre-emptive treatment with escitalopram v. placebo in patients undergoing antiviral therapy with PEG-IFN-α2a plus ribavirin; here we report on the 91 patients who had been randomised to placebo. During a pre-treatment period of 14 weeks before antiviral therapy was started, patients were monitored for spontaneously developing symptoms of depression. Next, all patients received antiviral therapy with PEG-IFN-α2a plus ribavirin (Roche, Grenzach-Wyhlen, Germany). Patients with HCV genotype 1 or 4 were treated for 48 weeks with PEG-IFN-α2a at a dose of 180 μg per week, and ribavirin at a dose of 1000 mg per day (body weight <75 kg) or 1200 mg per day (body weight ≥75 kg). Patients with HCV genotype 2 or 3 received PEG-IFN-α2a (180 μg/week) and ribavirin (800 mg daily) for 24 weeks. Doses of ribavirin and PEG-IFN-α2a could be adjusted in response to side-effects. Benzodiazepines were not allowed during the trial.
Psychiatric assessments and end-points
Psychiatric assessments were performed 14, 8, and 2 weeks before antiviral therapy was started as well as after 2, 4, 12 and 24 and, for patients with genotypes 1 and 4, 48 weeks of antiviral therapy and, finally, 24 weeks after antiviral treatment (follow-up assessment). The Mini-International Neuropsychiatric Interview was used at baseline as a screening tool to detect pre-existing psychiatric disorders. Reference Sheehan, Lecrubier, Sheehan, Amorim, Janavs and Weiller9 The Structured Clinical Interview for DSM-IV Disorders was used to verify the incidence of major depressive disorder before and during treatment. Reference First, Spitzer, Gibbon and Williams10 In addition we used the MADRS Reference Montgomery and Asberg11 and the 21-item Beck Depression Inventory (BDI) Reference Beck, Ward, Mendelson, Mock and Erbaugh12 to define and identify the incidence of all clinically relevant (mild to moderate) depressive symptoms. The MADRS scale consists of 10 items assessing symptoms associated with depression (apparent and reported sadness, inner tension, reduced sleep, reduced appetite, loss of concentration, lassitude, inability to feel, pessimistic thoughts and suicidal thoughts), each item scored from 0 to 6 according to severity. We applied three different definitions of depressive mood disorder: (a) those who fulfilled criteria of a current major depressive episode according to DSM-IV (independent of severity), and two groups defined on the basis of MADRS scores independent of fulfilling full DSM-IV major depressive episodes criteria; (b) MADRS scores ≥13 were used as cut-off for clinically relevant depression; (c) MADRS ≥25 for severe depression. Reference Schaefer, Sarkar, Knop, Effenberger, Friebe and Heinze6,Reference Montgomery and Asberg11,Reference Zimmerman, Posternak and Chelminski13 In addition, a self-rating instrument, the BDI was administered to screen for obvious differences in clinician-rated MADRS scores and patient’s account and to identify single items associated with the later development of depression under treatment. The Trail Making Test A/B (TMT A/B) and the Regensburger verbal fluency test were applied to evaluate cognitive function at baseline. Reference Reitan and Wolfson14,Reference Aschenbrenner, Tucha and Lange15 In addition, language production was assessed using two different word fluency tasks of the Regensburger verbal fluency test, measuring semantic and phonematic word fluency. The Clinical Assessment Geriatric Scale (also called Sandoz Clinical Assessment-Geriatric (SCAG)) was applied to measure mental-mnestic disturbances (confusion, mental alertness, impairment of recent memory, disorientation) with higher values indicating greater impairment. Reference Shader, Harmatz and Salzman16
Statistical analysis
Descriptive analysis was applied for the frequencies of primary outcome parameters (major depressive episodes, MADRS score ≥13, ≥25, sustained virologic response and adverse events). Chi-squared tests were used for categorical data and t-test for continuous data to compare the rate of patients with and without depressive symptomatology (according to MADRS score ≥13, ≥25 and DSM-IV criteria), baseline characteristics (age, gender, body mass index (BMI), genotype 1 and 4 v. 2 and 3, baseline MADRS score, BDI score, TMT A/B, word fluency, SCAG, study site (four major sites; all other small sites were combined into one category)) and MADRS score at week 2 and 4 after the beginning of IFN therapy. Multiple logistic regression analysis was used to calculate odds ratios of the incidence of IFN-associated depressive symptomatology (MADRS ≥13, major depressive episodes, MADRS ≥25) adjusted for gender, age, BMI, genotype (1 and 4 v. 2 and 3), baseline MADRS >3 and baseline BDI >4. We further evaluated single-item scores of the MADRS scale to screen for potential risk factors for developing depressive episodes during antiviral therapy. To evaluate the relationship between specific subclinical depressive symptoms at baseline and the development of an IFN-associated depressive syndrome (MADRS scores ≥13, major depressive episodes, MADRS scores ≥25), items measured on the MADRS scale were used to perform different regression trees. Independent variables were the 10 single items of the MADRS scale (apparent and reported sadness, inner tension, inability to feel, loss of concentration, pessimistic thoughts, reduced sleep, reduced appetite, lassitude and suicidal thoughts). On the basis of decision trees, we defined cut-off points for each symptom of depression of the MADRS scale at baseline to discriminate between patients with or without IFN-associated depression. There is a need for cut-off points for subclinical depressive symptoms to construct rules for making predictions about individual cases and assist in identifying homogeneous groups with high or low risk for the development of IFN-associated depression. To evaluate the influence of subclinical cognitive dysfunction at baseline, multiple logistic regression analysis was used to calculate the odds ratios of the incidence of IFN-associated depression (MADRS ≥13, major depressive episodes, MADRS ≥25) adjusted for TMT A/B, semantic and phonematic word fluency and the SCAG score.
For the primary end-point (MADRS score ≥13), we dealt with missing MADRS assessments by multiple imputation. We generated a mean of 10 imputed samples by a linear model using all MADRS assessments, age and BMI. For each patient and sample, the maximum MADRS score was subsequently computed and dichotomised at a MADRS score of 13. For all imputed samples, a logistic model tested differences between treatment groups with the sole factor group. Combined point estimates and the covariance matrix of the model parameters were subsequently calculated by the Rubin rule. Finally, the combined P-value, difference of depression rates with 95% confidence intervals were calculated by the delta method. The multiple imputation samples were generated by using SPSS version 19.01 for Windows. Patients without HCV RNA measurements at follow-up were considered non-responders to antiviral treatment. All tests and confidence intervals were two-sided, and the level of significance was α = 0.05. A statistical analysis was performed by using SPSS Statistics, version 19.0 for Windows.
Results
Development of depressive symptoms during antiviral treatment
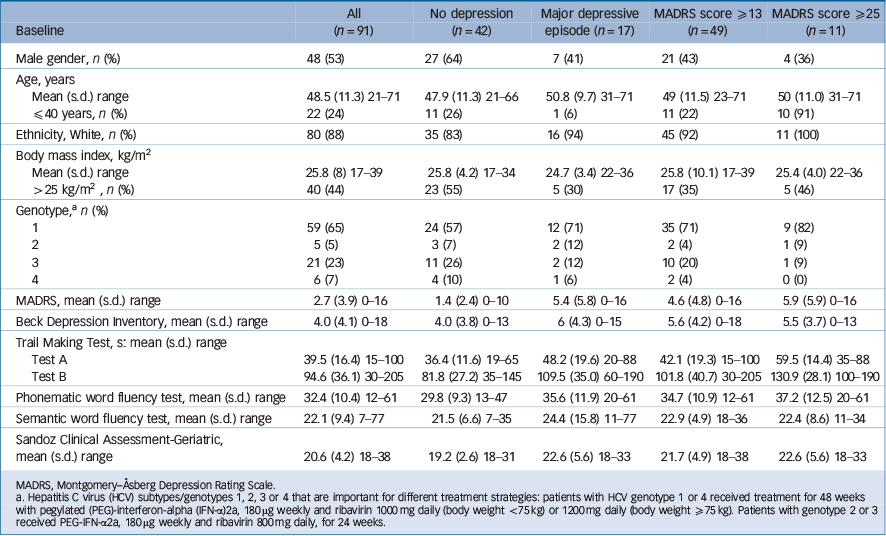
None of the 91 patients exhibited any clinically relevant depressive syndromes (defined as a MADRS score ≥13) before the initiation of INF treatment. A total of 88% (80/91) of the patients finished antiviral therapy according to the protocol. The overall incidence of adverse events was 87%. Dose reduction of PEG-IFN-α2a was necessary for 19% (n = 17) of patients and ribavirin dose was decreased in 29% (n = 26) of patients. Of the 91 patients, 54% (n = 49, 95% CI 48–69%) developed MADRS scores ≥13 during antiviral therapy, 19% developed a major depressive episode according to DSM-IV criteria (n = 17, 95% CI 12–28%) and 12% had severe depressive syndromes (MADRS score ≥25) (n = 11, 95% CI 7–21%) (Table 1).
Baseline factors and INF-associated depression
Baseline factors were compared between patients with any kind of depressive syndrome during antiviral therapy with PEG-IFN-α2a and ribavirin (MADRS score ≥13), severe depressive syndrome (MADRS score ≥25), major depressive episode (defined by DSM-IV criteria) and patients without depression. No differences between patients with depression and those without were found for age, genotype and BMI. However, more women (n = 28, 65%) than males (n = 21, 44%) developed depressive syndromes (MADRS ≥13, χ2 = 4.166, d.f. = 1, P 0.041).
Patients with clinically relevant depression during IFN treatment had significant higher MADRS (t = –3.858, d.f. = 89, P<0.001) and BDI scores (t = –2.012, d.f. = 88, P = 0.05) at base-line and exhibited significantly higher MADRS scores after 2 (t = –4.155, d.f. = 89, P<0.001) and 4 weeks (t = –4.861, d.f. = 88, P<0.001) of antiviral treatment. Patients who developed a major depressive episode during antiviral therapy demonstrated higher MADRS scores at baseline (t = –2.437, d.f. = 88, P = 0.017), and after 2 (t = –4.245, d.f. = 89, P<0.001) and 4 weeks (t = –3.071, d.f. = 89, P = 0.013) of antiviral treatment. In contrast to this, no significant differences were found between individuals
Table 1 Comparison of pre-treatment demographic and clinical characteristics between patients without and with depressive syndromes during antiviral treatment
| Baseline | All (n = 91) |
No depression
(n = 42) |
Major depressive episode (n = 17) |
MADRS score ≥13
(n = 49) |
MADRS score ≥25
(n = 11) |
|---|---|---|---|---|---|
| Male gender, n (%) | 48 (53) | 27 (64) | 7 (41) | 21 (43) | 4 (36) |
| Age, years | |||||
| Mean (s.d.) range | 48.5 (11.3) 21-71 | 47.9 (11.3) 21-66 | 50.8 (9.7) 31-71 | 49 (11.5) 23-71 | 50 (11.0) 31-71 |
| ≤40 years, n (%) | 22 (24) | 11 (26) | 1 (6) | 11 (22) | 10 (91) |
| Ethnicity, White, n (%) | 80 (88) | 35 (83) | 16 (94) | 45 (92) | 11 (100) |
| Body mass index, kg/m2 | |||||
| Mean (s.d.) range | 25.8 (8) 17-39 | 25.8 (4.2) 17-34 | 24.7 (3.4) 22-36 | 25.8 (10.1) 17-39 | 25.4 (4.0) 22-36 |
| >25 kg/m2, n (%) | 40 (44) | 23 (55) | 5 (30) | 17 (35) | 5 (46) |
| Genotype,Footnote a n (%) | |||||
| 1 | 59 (65) | 24 (57) | 12 (71) | 35 (71) | 9 (82) |
| 2 | 5 (5) | 3 (7) | 2 (12) | 2 (4) | 1 (9) |
| 3 | 21 (23) | 11 (26) | 2 (12) | 10 (20) | 1 (9) |
| 4 | 6 (7) | 4 (10) | 1 (6) | 2 (4) | 0 (0) |
| MADRS, mean (s.d.) range | 2.7 (3.9) 0-16 | 1.4 (2.4) 0-10 | 5.4 (5.8) 0-16 | 4.6 (4.8) 0-16 | 5.9 (5.9) 0-16 |
| Beck Depression Inventory, mean (s.d.) range | 4.0 (4.1) 0-18 | 4.0 (3.8) 0-13 | 6 (4.3) 0-15 | 5.6 (4.2) 0-18 | 5.5 (3.7) 0-13 |
| Trail Making Test, s: mean (s.d.) range | |||||
| Test A | 39.5 (16.4) 15-100 | 36.4 (11.6) 19-65 | 48.2 (19.6) 20-88 | 42.1 (19.3) 15-100 | 59.5 (14.4) 35-88 |
| Test B | 94.6 (36.1) 30-205 | 81.8 (27.2) 35-145 | 109.5 (35.0) 60-190 | 101.8 (40.7) 30-205 | 130.9 (28.1) 100-190 |
| Phonematic word fluency test, mean (s.d.) range | 32.4 (10.4) 12-61 | 29.8 (9.3) 13-47 | 35.6 (11.9) 20-61 | 34.7 (10.9) 12-61 | 37.2 (12.5) 20-61 |
| Semantic word fluency test, mean (s.d.) range | 22.1 (9.4) 7-77 | 21.5 (6.6) 7-35 | 24.4 (15.8) 11-77 | 22.9 (4.9) 18-36 | 22.4 (8.6) 11-34 |
| Sandoz Clinical Assessment-Geriatric, mean (s.d.) range | 20.6 (4.2) 18-38 | 19.2 (2.6) 18-31 | 22.6 (5.6) 18-33 | 21.7 (4.9) 18-38 | 22.6 (5.6) 18-33 |
MADRS, Montgomery-Åsberg Depression Rating Scale.
a. Hepatitis C virus (HCV) subtypes/genotypes 1, 2, 3 or 4 that are important for different treatment strategies: patients with HCV genotype 1 or 4 received treatment for 48 weeks with pegylated (PEG)-interferon-alpha (IFN-α)2a, 180 μg weekly and ribavirin 1000 mg daily (body weight <75 kg) or 1200 mg daily (body weight ≥75 kg). Patients with genotype 2 or 3 received PEG-IFN-α2a, 180 μg weekly and ribavirin 800 mg daily, for 24 weeks.
with depression and those without for the BDI score (P = 0.19). Patients who developed severe depressive syndromes (MADRS score ≥25) also showed significantly higher MADRS scores at baseline (t = –2.440, d.f. = 89, P = 0.017), as well as after 2 (t = 4.552, d.f. = 89, P<0.001) and 4 weeks (t = 2.976, d.f. = 80, P = 0.004) of antiviral treatment. No influence on the development of major depressive episodes or a severe depressive syndrome was found for gender, age, genotype and BMI.
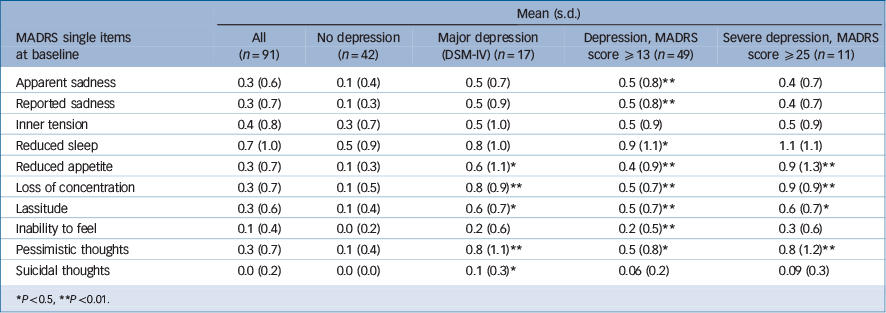
Table 2 shows the single-item analysis of the MADRS scale before antiviral treatment was initiated. Patients who developed any kind of an IFN-associated depressive syndrome (MADRS score ≥13) had significantly higher scores in the following single MADRS items at baseline: apparent sadness (P = 0.009), reported sadness (P = 0.002), inability to feel (P = 0.015), loss of concentration (P = 0.001), pessimistic thoughts (P = 0.005), reduced sleep (P = 0.050), reduced appetite (P = 0.009) and lassitude (P = 0.004). Patients with major depressive episodes had higher scores on the following five MADRS items at baseline: reduced appetite (P = 0.016), loss of concentration (P<0.001), lassitude (P = 0.016), pessimistic thoughts (P = 0.001) and suicidal thoughts (P = 0.022). The development of severe depression (MADRS score ≥25) during antiviral therapy was associated with higher scores for reduced appetite (P = 0.001), loss of concentration (P = 0.001), lassitude (P = 0.045) and pessimistic thoughts (t = 2.636, d.f. = 90, P = 0.010).
We also examined the possible role of cognitive function for the development of depression with the following neuropsychological tests: TMT A, TMT B and the Regensburger word fluency test. Pre-treatment results were compared between patients with and without IFN-associated depressive syndromes. Patients with any clinically relevant depressive syndrome during therapy (MADRS ≥13) showed lower scores on TMT B (t = 2.580, d.f. = 83, P = 0.012) and semantic word fluency (t = 2.219, d.f. = 85,
Table 2 Pre-treatment depression symptoms on the Montgomery-Åsberg Depression Rating Scale (MADRS) items
| Mean (s.d.) | |||||
|---|---|---|---|---|---|
| MADRS single items at baseline |
All (n = 91) |
No depression
(n = 42) |
Major depression (DSM-IV) (n = 17) |
Depression, MADRS score ≥13 (n = 49) |
Severe depression, MADRS
score ≥25 (n = 11) |
| Apparent sadness | 0.3 (0.6) | 0.1 (0.4) | 0.5 (0.7) | 0.5 (0.8)Footnote ** | 0.4 (0.7) |
| Reported sadness | 0.3 (0.7) | 0.1 (0.3) | 0.5 (0.9) | 0.5 (0.8)Footnote ** | 0.4 (0.7) |
| Inner tension | 0.4 (0.8) | 0.3 (0.7) | 0.5 (1.0) | 0.5 (0.9) | 0.5 (0.9) |
| Reduced sleep | 0.7 (1.0) | 0.5 (0.9) | 0.8 (1.0) | 0.9 (1.1)Footnote * | 1.1 (1.1) |
| Reduced appetite | 0.3 (0.7) | 0.1 (0.3) | 0.6 (1.1)Footnote * | 0.4 (0.9)Footnote ** | 0.9 (1.3)Footnote ** |
| Loss of concentration | 0.3 (0.7) | 0.1 (0.5) | 0.8 (0.9)Footnote ** | 0.5 (0.7)Footnote ** | 0.9 (0.9)Footnote ** |
| Lassitude | 0.3 (0.6) | 0.1 (0.4) | 0.6 (0.7)Footnote * | 0.5 (0.7)Footnote ** | 0.6 (0.7)Footnote * |
| Inability to feel | 0.1 (0.4) | 0.0 (0.2) | 0.2 (0.6) | 0.2 (0.5)Footnote ** | 0.3 (0.6) |
| Pessimistic thoughts | 0.3 (0.7) | 0.1 (0.4) | 0.8 (1.1)Footnote ** | 0.5 (0.8)Footnote * | 0.8 (1.2)Footnote ** |
| Suicidal thoughts | 0.0 (0.2) | 0.0 (0.0) | 0.1 (0.3)Footnote * | 0.06 (0.2) | 0.09 (0.3) |
* P<0.5
** P<0.01.
P = 0.029) before IFN treatment was started. Patients with a major depressive episode during IFN therapy demonstrated significantly lower scores on TMT A (t = 2.104, d.f. = 83, P = 0.038), and semantic word fluency (t = 2.265, d.f. = 85, P = 0.026) compared with patients without depression at baseline. Finally, patients with a severe depressive syndrome (MADRS score ≥25) had significantly lower scores in the TMT A (t = 3.927, d.f. = 87, P<0.001) and TMT B at baseline (t = 3.105, d.f. = 83, P = 0.003).
With respect to the SCAG, patients with IFN-associated depressive syndrome and major depressive episodes had significant higher scores before INF therapy was started (MADRS ≥13: t = 2.901, d.f. = 88, P = 0.005; major depressive episodes: t = 2.256, d.f. = 88, P = 0.027).
Prediction of INF-associated depression
A multivariable regression analysis was performed to identify those risk factors that maximally discriminated between patients who developed any kind of a clinically relevant depressive syndrome (MADRS scores ≥13) during IFN treatment and those who remained without clinically relevant depressive mood
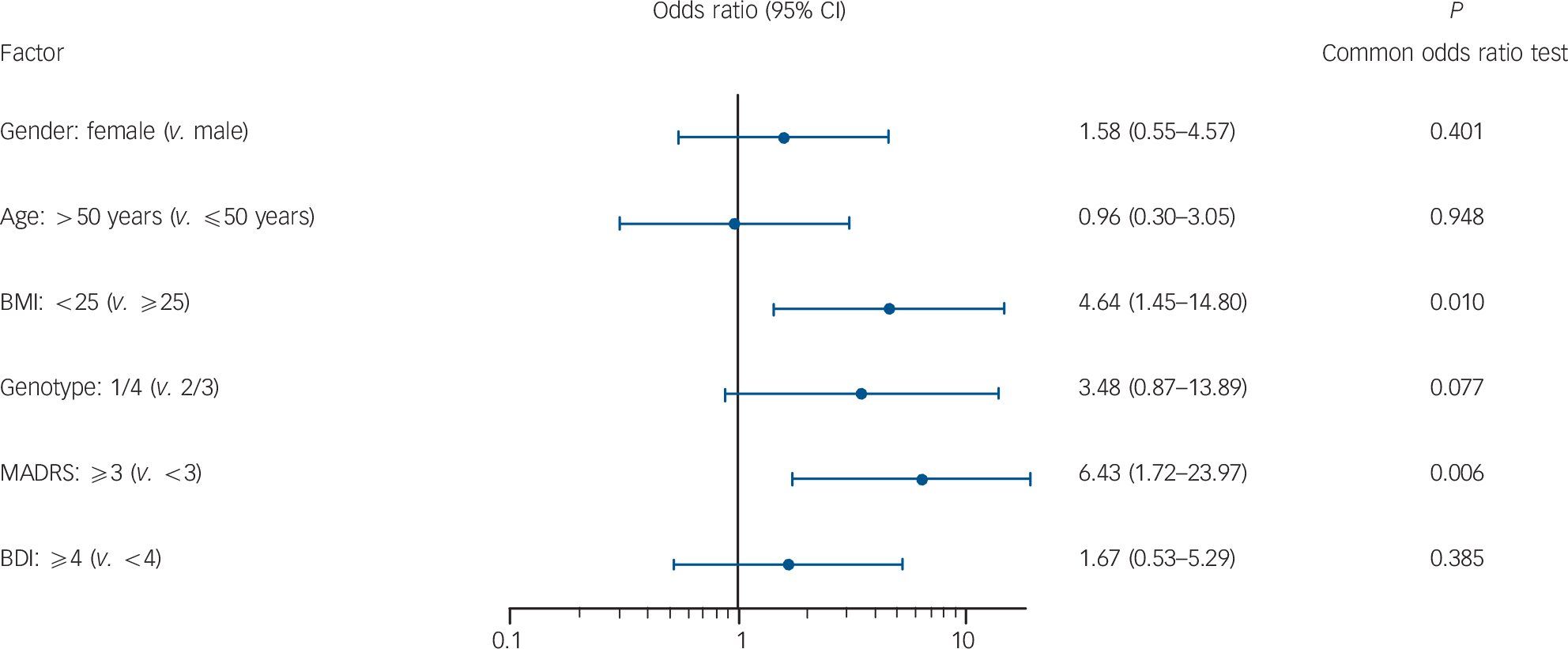
Fig. 1 Odds ratios for developing any clinically relevant depressive syndrome (Montgomery-Åsberg Depression Rating Scale (MADRS) scores ≥13) during interferon treatment.
BMI, body mass index; BDI, Beck Depression Inventory.
changes (Fig. 1). A baseline MADRS score of 3 or higher was associated with a 6.4-fold increased higher risk for the development of an IFN-associated clinically relevant depressive syndrome (95% CI 1.7–24.0, P = 0.006). A BMI less than 25 at baseline resulted in a significantly higher risk for the development of a clinically relevant depressive syndrome (OR = 4.63, 95% CI 1.5–14.8, P = 0.010). In contrast, logistic regression analysis revealed that neither major depressive episodes according to DSM-IV criteria nor severe depressive syndromes (MADRS score ≥25) were associated with any baseline factors (data not shown).
A multiple logistic regression analysis was used again to calculate odds ratios of the incidence of INF-associated depressive syndromes (MADRS ≥13, major depressive episodes, MADRS ≥25) adjusted for TMT A, TMT B, Regensburger verbal fluency test semantic and phonematic word fluency and the SCAG score. Reduced performance on the TMT A (OR = 1.1, 95% CI 1.0–1.1, P = 0.027) and TMT B (OR = 1.0, 95% CI 1.0–1.1, P = 0.033) predicted the development of severe depression (MADRS ≥25) during antiviral treatment. Higher scores on the SCAG (OR = 1.23, 95% CI 1.0–1.4, P = 0.014) was found to be the only relevant risk factor for the development of an INF-associated depressive syndrome (MADRS ≥13) during antiviral therapy.
Decision tree for the prediction of INF-associated depression
As a MADRS score of 3 or higher was determined as the only significant predictor for an INF-associated depressive syndrome, we analysed the individual items of the MADRS. Items were included in the decision tree regression analyses in order to define cut-off points for each symptom of depression on the MADRS scale at baseline to discriminate between patients with or without an IFN-induced depressive syndrome (Fig. 2). Patients were classified according to their values on the MADRS items as independent variables, and according to the emergence of a depressive syndrome (MADRS ≥13). The final decision tree analysis distinguished between patients with clinical depression and those without with a sensitivity of 73.6%. For developing INF-induced depression (MADRS ≥13), statistically significant predictors at baseline evaluation were reported sadness, loss of concentration, pessimistic thoughts and reduced sleep.
In total, 89% (n = 16) of patients with a baseline score for ‘reported sadness’ greater than 0 developed a clinically relevant

Fig. 2 Decision tree analyses using single symptom items on the Montgomery-Åsberg Depression Rating Scale (MADRS) to predict interferon-alpha (INF-α)-induced depression during antiviral treatment.
Four MADRS items were found by decision tree analysis to predict IFN-α associated depression: reported sadness, loss of concentration, pessimistic thoughts and reduced sleep, with the highest predictive value for the item reported sadness. The final decision tree analysis distinguished between patients with clinical depression from those without, with a sensitivity of 73.6% (correct predicted depression rate 73.5%, correct predicted non-depression rate 73.8%). The overall IFN depression rate was 53.8% (n = 49) compared with 46.2% (n = 42) for those without interferon-induced depression. IFN-α depression, INF-α-induced depression defined by MADRS ≥13.
depressive syndrome during IFN-therapy. Further analyses revealed that 75% (n = 9) of patients without reported sadness, but with loss in concentration (score >0) developed an INF-associated depressive syndrome (MADRS ≥13). The prediction rate for absence of depression with INF-treatment (specificity) for this decision tree was 73.8%.
Discussion
Main findings
Clinically relevant depressive symptoms emerged in up to 70% of patients with chronic hepatitis C who received antiviral therapy including IFN-α. Reference Schaefer, Hinzpeter, Mohmand, Janssen, Pich and Schwaiger4,Reference Schafer, Wittchen, Seufert and Kraus17 Two recently published meta-analyses demonstrated that pre-emptive antidepressant treatment with SSRIs significantly reduces the incidence of IFN-associated depression. Reference Sarkar and Schafer5,Reference Jiang, Deng, Zhang, Chen, Chen and Ruan18 However, strategies are demanded that help to discriminate between patients at risk of INF-induced depression and those who are not. Our results show significant differences at baseline between patients with and without IFN-associated depressive syndromes. Pre-existing subclinical depressive mood (MADRS ≥3) resulted in a 6.4-fold increased risk of clinically relevant IFN-associated depressive syndromes in patients without a history of psychiatric disorders. By using decision tree analysis, four single items of the MADRS scale – reported sadness, loss of concentration, pessimistic thoughts and reduced sleep – stood out in predicting the development of a clinically relevant depressive syndrome (MADRS ≥13) with a sensitivity of 73.8%. A total of 89% (n = 16) of patients with reported sadness at baseline (MADRS single item, score >0) developed a clinically relevant depressive syndrome during IFN-therapy.
Our results are in line with previous studies demonstrating that patients with higher MADRS scores at baseline have a higher risk for the development of depressive symptoms during cytokine therapy. Reference Schaefer, Capuron, Friebe, Diez-Quevedo, Robaeys and Neri3,Reference Capuron and Ravaud19–Reference Miyaoka, Otsubo, Kamijima, Ishii, Onuki and Mitamura21 Capuron and colleagues identified similar mood (sadness, pessimistic thoughts) and behavioural symptoms (sleep disturbance) as significant predictors of depressive symptoms at the end of the first month of cytokine treatment. Reference Capuron, Ravaud, Miller and Dantzer20 These findings support the notion that even subclinical deterioration of the affective state of patients before the initiation of INF therapy may indicate an increased individual vulnerability for the development of clinically significant IFN-associated depressive symptoms. Interestingly, the number or severity of these symptoms seem not to be decisive factors. Rather, our results indicate an ‘all-or-nothing’ effect: the risk of developing clinically relevant depressive symptoms during IFN treatment was increased whenever patients reported any kind of subclinical depressive symptoms, especially sadness and in addition sleep disturbance, pessimistic thoughts and lack of concentration. Thus, screening of patients for any degree of these pre-existing symptoms is advisable.
The predictive value of subclinical depressive symptoms before the beginning of interferon therapy shows similarities with the concept of subsyndromal symptomatic depression. This has been defined as minimal depressive symptoms below the diagnostic threshold for minor, dysthymic or major depressive disorders. Reference Judd, Rapaport, Paulus and Brown22–Reference Judd, Akiskal and Paulus24 Subsyndromal symptomatic depression is frequently observed in patients with unipolar major depressive disorder and is considered as an integral component of the symptomatic course of illness. Reference Judd, Paulus, Wells and Rapaport23,Reference Judd, Akiskal, Maser, Zeller, Endicott and Coryell25 In addition, the presence of subsyndromal symptomatic depression shows significant associations with a higher prevalence of future and past major depressive episodes. Reference Judd, Akiskal and Paulus24,Reference Broadhead, Blazer, George and Tse26–Reference Sherbourne, Wells, Hays, Rogers, Burnam and Judd28 A similar relationship seems to exist for INF-associated depression and subclinical depressive symptoms before the beginning of antiviral therapy. However, it should be emphasised that despite major depression being a serious and highly prevalent side-effect of INF therapy, most affected patients will respond to acute treatment with SSRIs, Reference Kraus, Schafer, Schoettker, Keicher, Weissbruch and Hofbrauer29 or depression might be prevented by pre-emptive antidepressant treatment. Reference Sarkar and Schafer5,Reference Schaefer, Sarkar, Knop, Effenberger, Friebe and Heinze6 Thus, even with the increased risk for depression, it is not indicated to exclude patients with minimal depressive symptomatology from antiviral treatment with IFN-α. Reference Schaefer, Capuron, Friebe, Diez-Quevedo, Robaeys and Neri3
Another important and novel finding is that severe depression can be predicted by neuropsychological tests measuring cognitive flexibility (TMT A and B). It was evident that inferior cognitive performance predicts especially severe depression before the start of INF treatment. This increased vulnerability to severe depression was even found in patients without a history of any affective disorders. With respect to subjective cognitive problems as measured by the SCAG, patients with IFN-associated depressive syndromes (MADRS ≥13) had significantly higher scores before INF treatment was started.
Cognitive impairment and HCV infection
Cognitive difficulties are associated with impaired work productivity. Reference Jaeger, Berns, Uzelac and Davis-Conway30 Of note, treatment-naive patients with chronic hepatitis C infection have an already increased risk of mild cognitive impairment. Reference Schaefer, Capuron, Friebe, Diez-Quevedo, Robaeys and Neri3,Reference Tillmann, Wiese, Braun, Wiegand, Tenckhoff and Mössner31 Chronic infection with HCV is suspected to cause lasting changes to the immune system and to neurotransmission, leading to mild cognitive impairment. Reference Schaefer, Hinzpeter, Mohmand, Janssen, Pich and Schwaiger4,Reference Forton, Hamilton, Allsop, Grover, Wesnes and O'Sullivan32 Different hypotheses have been proposed to explain the occurrence of cognitive impairment in HCV infection, such as a direct action of the virus on the central nervous system (CNS), a delayed action of the virus on the CNS mediated by viral replication in neurons and cognitive deterioration as a side-effect of the inflammatory process. Reference Senzolo, Schiff, D'Aloiso, Crivellin, Cholongitas and Burra33 In contrast, a recently published study demonstrated no evidence of an association between HCV infection and cognitive impairment. Reference Abrantes, Torres and de Mello34 The authors argue that a lack of rigorous selection criteria in previous studies may have resulted in an overestimation of the prevalence of cognitive impairment associated with HCV by including patients with a history of alcohol and/or illegal drug misuse, patients using psychotropic drugs or patients with depression. Reference Abrantes, Torres and de Mello34 However, our trial, which excluded patients with psychiatric disorders, now confirms the presence of at least mild cognitive impairment in a subgroup of treatment-naive patients with chronic hepatitis C independent from depressive symptoms.
On the other hand, and independent from HCV infection, two recent meta-analyses support the presence of cognitive impairment in patients with major depressive disorder. Reference McDermott and Ebmeir35,Reference Snyder36 These studies demonstrate that executive function mediated by the prefrontral cortex and measured with the TMT B is affected by depression, which may have an impact on cognitive performance. The finding that a reduced performance on TMT A and TMT B predicts the development of severe depression (MADRS ≥25) lends further support to a depression–executive dysfunction syndrome. Reference Alexopoulos37 Executive dysfunction is also one of the clinical expressions of abnormalities in the frontostriatal-limbic circuitry that might predispose to late-life depression.
BMI
In our trial a BMI ≥25 at baseline resulted in a significantly reduced risk of developing clinically relevant depression with INF treatment. Obesity is one of the core characteristics that has been associated with lower sustained viral response rates in patients with chronic hepatitis Reference Negro and Clément38 and might be associated with a higher relapse rate. Reference Rivero-Juarez, Mira, Camacho, Neukam, Perez-Camacho and Caruz39 Therapy with IFN-α in these patients might have less clinical antiviral effects, resulting in lower rates of neuropsychiatric side-effects.
Limitations
Although the present study generally supports the utility of the TMT as a screening tool for HCV patients before the beginning of IFN therapy, further investigations are clearly required. For example, additional cognitive tests should be conducted in future studies to evaluate whether other cognitive functions also predict severe IFN-associated depression (for example, attention or memory). A more extensive evaluation would provide a stronger and more rigorous basis for assessing the potential impact of cognitive functions at the beginning of INF therapy in relation to INF-associated depression. Although a reasonable number of patients was included, numbers did not allow for a more precise identification of risk factors for developing DSM-IV major depressive episodes or severe depression (MADRS ≥25). Finally, multiple testing might limit the results.
Implications
In summary, our data demonstrate that pre-existing subclinical depressive mood significantly increases the risk of clinically relevant IFN-associated depressive syndromes in patients without a history of psychiatric disorders. Mild cognitive disturbances are an independent risk factor for the development of severe depression. Scoring on the MADRS single items reported sadness, loss of concentration, pessimistic thoughts and reduced sleep prior to treatment, or a MADRS total score >3 at baseline may identify patients at risk of treatment-emergent depression. Pre-emptive treatment with antidepressants is recommended in these high-risk patients.
Acknowledgements
We thank Professor Heinz Grunze for a critical review of the paper and helpful advice.









eLetters
No eLetters have been published for this article.