Introduction
Nesting success, an important metric in avian demographic studies, is often the focus of conservation efforts for declining species (Sutherland et al. Reference Sutherland, Newton and Green2004). Among birds, survival rates of nests and chicks are influenced by a variety of factors, including seasonal effects (Grant et al. Reference Grant, Shaffer, Madden and Pietz2005), predation (Neuman et al. Reference Neuman, Page, Stenzel, Warriner and Warriner2004), human exploitation (Sok et al. Reference Sok, Claassen, Wright and Ryan2012), disturbance (Yasué and Dearden Reference Yasué and Dearden2006), and environmental factors (Smith et al. Reference Smith, Gilchrist and Smith2007). Recovery of threatened bird species frequently requires mitigation of anthropogenic effects, such as preventing egg harvest (Sok et al. Reference Sok, Claassen, Wright and Ryan2012) or minimising detrimental human activities (Burger et al. Reference Burger, Jeitner, Clark and Niles2004). Conservation becomes more challenging, however, if local human livelihoods are dependent on eggs or chicks for food or trade, or if poverty impedes communities from conserving natural resources (Clements et al. Reference Clements, John, Nielsen, An, Tan and Milner-Gulland2010). In developing countries, where such circumstances are frequently encountered, voluntary conservation action by local communities may be unrealistic (Gjertsen and Niesten Reference Gjertsen and Niesten2010). Thus, incentive-based conservation programmes that involve direct payments to individuals or communities have gained in popularity.
Direct payments for biodiversity conservation, which fall under the broad category of payments for ecosystem services (PES), have frequently been used to protect breeding sea turtles (Ferraro and Gjertsen Reference Ferraro and Gjertsen2009, Gjertsen and Niesten Reference Gjertsen and Niesten2010) and more recently, to protect bird nests, especially in South-East Asia (Sok et al. Reference Sok, Claassen, Wright and Ryan2012, Clements et al. Reference Clements, Rainey, An, Rours, Tan, Thong, Sutherland and Milner-Gulland2013, Wright et al. Reference Wright, Collar, Lake, Net, Rours, Sok, Sum and Dolman2013). Because bird and turtle nesting sites can be monitored relatively easily, they are ideally suited for community-based conservation programmes involving direct payments. Direct payments offer potential advantages over indirect conservation approaches (e.g. legal-policy interventions, education programmes, alternative livelihood development) by being relatively straightforward to implement, cost-effective, and directly focused on a specific conservation outcome such as nesting success (Ferraro Reference Ferraro2001). Furthermore, direct payments provide unambiguous incentives to local communities. In the case of bird nest protection, payments deter community members from harvesting eggs, and instead encourage them to protect nests. Yet, compared to the number of direct payment schemes implemented worldwide, there have been few assessments of the effectiveness of direct payment programmes. Although direct payment nest protection programmes in Cambodia have received some of the most thorough investigation to date (Sok et al. Reference Sok, Claassen, Wright and Ryan2012, Clements et al. Reference Clements, Rainey, An, Rours, Tan, Thong, Sutherland and Milner-Gulland2013, Wright et al. Reference Wright, Collar, Lake, Net, Rours, Sok, Sum and Dolman2013), conflicting results highlight the need to further explore this topic.
The Mekong River and its major tributaries (Sekong and Sesan Rivers) in north-eastern Cambodia and southern Laos contain critical breeding habitat for birds that nest on riverine sand and pebble (shingle) bars. These rivers support regionally important populations of River Tern Sterna aurantia, River Lapwing Vanellus duvaucelii, and Great Thick-knee Esacus recurvirostris (Thewlis et al. Reference Thewlis, Duckworth, Evans and Timmins1998, Timmins and Men Reference Timmins and Men1998, Bezuijen et al. Reference Bezuijen, Timmins and Seng2008). As ground-nesting river channel specialists, this group is particularly sensitive to hydrological changes, predation, and disturbance (Timmins and Men Reference Timmins and Men1998, Claassen Reference Claassen2004). Increased anthropogenic threats from human population and economic growth, and subsequent proliferation of large-scale infrastructure developments such as hydropower, intensification of commercial forestry, agriculture, gold mining, increased hunting and disturbance, climate change, and other land-use activities, have led to population declines regionally (Thewlis et al. Reference Thewlis, Duckworth, Evans and Timmins1998, Claassen Reference Claassen2004, Bezuijen et al. Reference Bezuijen, Timmins and Seng2008) and globally (IUCN 2015). River Tern populations in the Mekong River basin have been especially impacted, having declined by > 75% in the last decade (Timmins Reference Timmins2006, Bezuijen et al. Reference Bezuijen, Timmins and Seng2008). The River Tern will likely be the next species to disappear from the region unless focused conservation action is taken (Goes Reference Goes2013). Furthermore, the Black-bellied Tern Sterna acuticauda has already disappeared from the Mekong basin, likely due to the same factors that threaten the River Tern (Goes et al. Reference Goes, Claassen and Nielsen2010). Despite population declines, riverine sandbar-nesting birds in South-East Asia have been low priorities for conservation until recently. The lack of conservation attention has, in part, been due to large global ranges and larger populations elsewhere in Asia (IUCN 2015). However, loss of regional populations will significantly shrink species’ global ranges and reduce genetic diversity. Thus, it is important to maintain viable regional populations.
To date, few studies have documented the ecology of riverine sandbar-nesting species in Asia. In particular, information on breeding season threats and nesting success are critical to designing effective conservation strategies. Therefore, the objectives of this study were to investigate factors affecting nest survival and likelihood of nest predation or egg harvest, for six sandbar-nesting species (River Tern, River Lapwing, Great Thick-knee, Indian Thick-knee Burhinus indicus, Small Pratincole Glareola lactea and Little Ringed Plover Charadrius dubius). For the River Tern, we also investigated factors affecting chick survival rates. Additionally, we implemented a direct payment programme to increase reproductive success of focal species, especially the River Tern. Specifically, we sought to assess the effectiveness of the direct payment programme to improve reproductive rates. This information has important implications for conservation of sandbar-nesting river birds in South-East Asia, as well as for direct payment for biodiversity conservation programmes.
Methods
Study area and focal species
From 2010 to 2014, we studied six riverine sandbar-nesting bird species along an approximately 60 km stretch of the Mekong River between Stung Treng (13°32′N, 105°58′E) and Kratie (12°29′N, 106°14′E) towns in north-eastern Cambodia (Figure 1). We chose this river stretch because it contains the majority of the populations of key species such as River Tern, River Lapwing, and Great Thick-knee in the Mekong basin (Bezuijen et al. Reference Bezuijen, Timmins and Seng2008). This stretch of river ranges from 1 to 11 km in width and contains approximately 40 permanent islands and hundreds of seasonally emergent sandbars; it has been described in detail by Bezuijen et al. (Reference Bezuijen, Timmins and Seng2008). Nesting habitat consisted of sparsely to moderately vegetated, seasonally-emergent sand and pebble (shingle) bars.
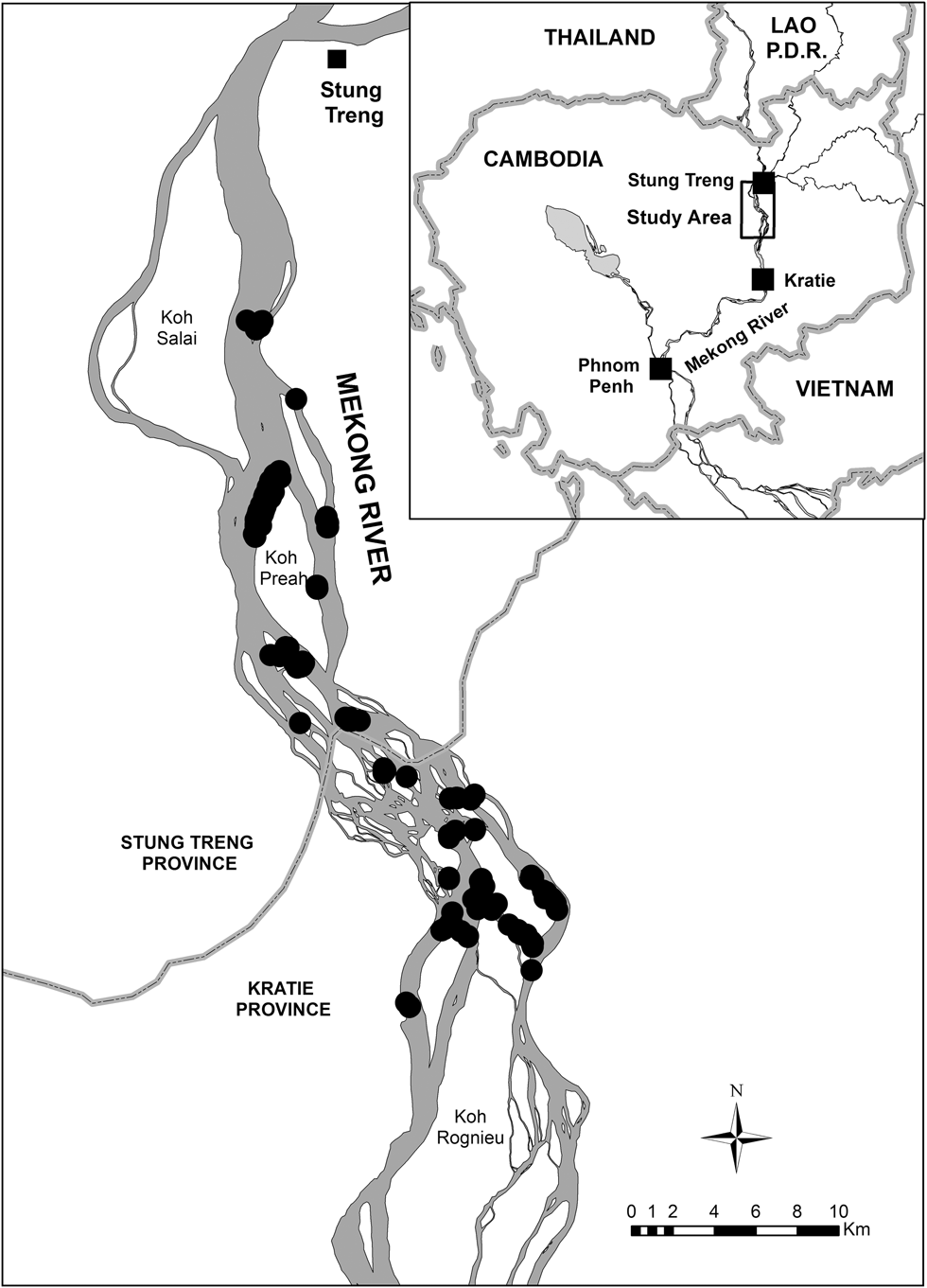
Figure 1. Location of the study area in Cambodia. Black circles represent nest sites that were monitored (2010–2014).
Our study prioritised River Tern, the species of highest regional conservation concern (Goes Reference Goes2013); nest searching efforts, nest protection payments, efforts to recruit community nest protectors, and programme awareness-raising activities were highest for this species. Additional focal species included: River Lapwing, Great Thick-knee, Indian Thick-knee, Small Pratincole, and Little Ringed Plover. The latter two species were included despite their low conservation status (Goes Reference Goes2013, IUCN 2015) because we sought to expand inference to additional species regarding threats and effectiveness of nest protection and to broaden community participation in the programme.
Nest monitoring
From 2010 to 2014, we located and monitored nests during each breeding season (January–May). Nests were located primarily by observing adult behaviour. Nest searching focused on River Tern and River Lapwing. We attempted to locate all River Tern nests within the study area. We located as many River Lapwing nests as time allowed, distributing search efforts spatially to minimise effects of pseudoreplication (Hurlbert Reference Hurlbert1984). Nests of other species were located opportunistically.
Nests were monitored until all eggs hatched or were determined to have failed. Intervals between researcher visits averaged 4.7 ± 3.6 (SD) days. During nest visits, data were collected on nest age, initiation date, fate (success or failure), date of hatch or failure, and cause of failure. Nest age was determined by egg floatation (Westerkov Reference Westerkov1950, Hays and LeCroy Reference Hays and LeCroy1971). Nest initiation date was estimated by back-dating according to nest age, assuming a 1-day laying interval for all species (AHC, pers. obs.). A nest was considered successful if at least one egg hatched. Nest fates were determined by observations of chicks, nest age, parental behaviour, river stage, and signs at the nest such as tracks and eggshell fragments (Mabee Reference Mabee1997).
River Tern chicks were monitored after hatching to assess survival. Chicks left the nest scrape after 1–2 days but remained in the general vicinity until they fledged. Fledging success was based on direct observations of fledged juveniles. Chicks are cryptic and easily overlooked. Therefore, during intermediate site visits, chick survival was assessed from direct observation of chicks or behaviour of breeding adults (carrying fish or exhibiting defensive behaviour). A brood was considered successful if at least one chick fledged.
Nest protection
A direct payment nest protection programme was implemented for focal species; community members were hired to guard nests from potential predators and disturbances. Payment amounts varied among years and species, and were outcome-based, with nest payments varying according to nest fate and cause of failure (Table 1).
Table 1. Community bird nest protection payments (USD) and proportion of nests that were protected on the Mekong River, Cambodia (2010–2014).
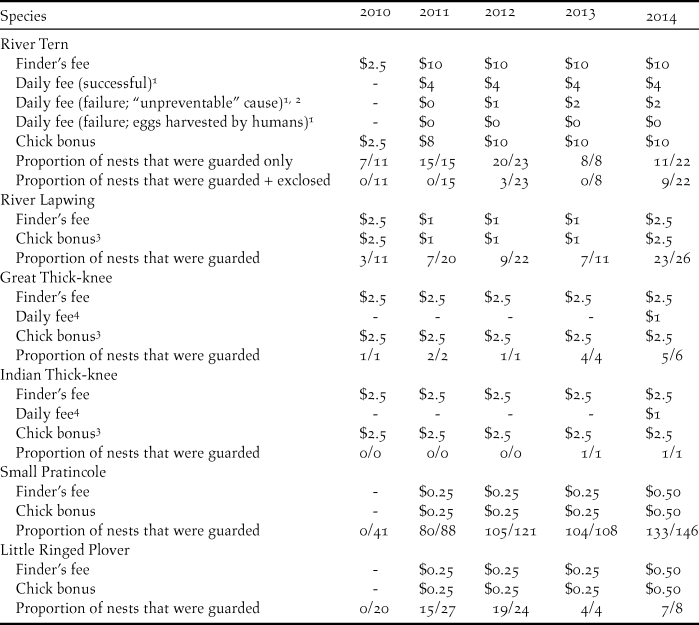
1 Payment per person; two people were hired per nest.
2 All nest failures were considered “unpreventable” except those caused by harvesting by people.
3 Payment amounts were per chick, except in 2010 when payment amounts were per nest if at least one chick hatched.
4 Payment per nest.
In addition to nest guarding, predator exclosures (Smith et al. Reference Smith, Pullin, Stewart and Sutherland2011) were used from 2012 to 2014 to protect a subset (15%) of River Tern nests; nests were selected for exclosure based on logistical feasibility and convenience. Exclosures were erected as soon as possible after locating nests, and remained in place for 2–12 days after chicks hatched (all exclosures were removed prior to fledging). Exclosures consisted of wire-mesh fencing erected around nests and were open on top to allow access by incubating adults. Exclosures were circular, with heights ranging from 0.3 to 0.5 m, and diameters ranging from 3.2 m (10 m length of fencing) in 2012 to 9.5 m (30 m length of fencing) in 2014; diameter was increased each year to improve adult acceptance rates. Small (2 cm) mesh size prevented access by mammalian predators such as rodents. Following installation, exclosures were monitored for up to two hours to ensure acceptance by incubating River Terns. Exclosures were removed if adults did not return to nests within the two-hour monitoring period; we waited ≥ 3 days before re-installing exclosures. If adults returned to nests within two hours, exclosures were left in place; however, if a nest remained untended for 24 hours, we considered the nest abandoned due to exclosure installation.
Statistical analyses
We used an information-theoretic approach (Burnham and Anderson Reference Burnham and Anderson2002) to investigate variation in daily survival rates (DSR) of nests and chicks using the NLMIXED procedure with SAS software (Rotella et al. Reference Rotella, Dinsmore and Shaffer2004; SAS version 9.4, SAS Institute, Cary, NC). We used a logit link function to constrain DSR to be between zero and one. For ease of interpretation, we converted estimates of DSR into nest success (probability of a nest surviving the entire nesting period) or fledging success (probability of a chick surviving until fledging age) by taking the product of predicted DSR for each day of an average nesting or chick period, using appropriate covariate values for each day (Shaffer and Thompson Reference Shaffer and Thompson2007). If nest fate was ambiguous, we calculated DSR on all intervals prior to age of potential hatch (Stanley Reference Stanley2000). We investigated variation in likelihood of nest failure due to egg harvest by humans or predation by animals using Fisher’s exact tests and Chi square (χ2) tests for trends in proportions using R statistical software (version 3.2.2; Bates et al. Reference Bates, Maechler, Bolker and Walker2015, R Development Core Team 2015).
For each response variable (nest survival, likelihood of harvest or predation, and chick survival), we investigated variation according to biologically relevant covariates. We investigated whether nest survival varied according to nest age, initiation date, year, site, or level of nest protection. We considered whether likelihood of egg harvest or nest predation varied according to year or level of nest protection. We examined whether River Tern chick survival varied according to hatching date, chick age, year, site, or level of protection.
We considered day 1 = 1 January and nest age 1 = the day the first egg was laid; we incremented both date and nest age daily. Because we assumed there was variation between years and sites, we included year and site as random effects. We also considered year as a linear trend. Nest protection for River Lapwing, Great Thick-knee, Small Pratincole, and Little Ringed Plover consisted of guarding only; for these species, we treated nest protection as a categorical dummy variable (GUARD; 0 = no protection, and 1 = protection by guarding). For River Terns, a subset of guarded nests and chicks were also protected by exclosures; we therefore considered three different model structures to represent River Tern nest and chick protection: 1) protection as a dummy variable (PROTECT_2; 0 = no protection, and 1 = protection by guarding or exclosure); 2) protection as a discrete variable with 3 levels (PROTECT_3; 0 = no protection, 1 = guarding only, and 2 = guarding plus use of an exclosure), and 3) exclosure as a dummy variable (EXCLOSURE; 0 = no exclosure, and 1 = exclosure).
We used a forward selection approach to model building, starting with the simplest model containing an intercept-only term and sequentially adding variables to each model. First, we considered year and site as a priori random effects and added these effects singly to the intercept-only model. We considered support for a random effect if P < 0.05, or σ2 > 0 and 95% prediction intervals excluded zero (Zuur et al. Reference Zuur, Ieno, Walker, Saveliev and Smith2009). Next, we sequentially added fixed effects such as date, age, protection, and year (linear form). We evaluated models by ranking them according to their Akaike’s Information Criteria values adjusted for small sample sizes (AICc; Burnham and Anderson Reference Burnham and Anderson2002). If a model under consideration included a main effect (linear term) for date, age, or year, we also considered the quadratic term for that covariate. If two main effects were included in a model, we also considered their interaction. We identified the best-supported model in each candidate set based on the lowest AICc value and the largest Akaike weight (w i ), which is the probability of a model being the best in the candidate set (Burnham and Anderson Reference Burnham and Anderson2002). We considered a model to be competitive if it was ≤ 2 AICc units of the best model, as long as it was not merely the best model plus one uninformative parameter (Burnham and Anderson Reference Burnham and Anderson2002, Arnold Reference Arnold2010). Unless otherwise indicated, means are reported ± 1 standard deviation (SD).
Results
From 2010 to 2014 we located 772 nests of six species and monitored them for 7,758 exposure days. Small Pratincole nests dominated the sample (n = 504 nests; 4,326 exposure days), followed by River Lapwing (n = 90 nests; 1,313 exposure days), Little Ringed Plover (n = 83 nests; 951 exposure days), River Tern (n = 79 nests; 947 exposure days), Great Thick-knee (n = 14 nests; 176 exposure days), and Indian Thick-knee (n = 2 nests; 45 exposure days). Community nest protectors guarded 92% of River Tern (77% by guarding only, and 15% by guarding plus an exclosure), 54% of River Lapwing, 93% of Great Thick-knee, 100% of Indian Thick-knee, 84% of Small Pratincole, and 54% of Little Ringed Plover nests (Table 1). The average age of nests at discovery was 5.6 ± 4.7 days for River Terns, 13.9 ± 8.8 days for River Lapwings, 10.5 ± 8.7 days for Great Thick-knees, 8.5 ± 2.1 days for Indian Thick-knees, 11.3 ± 7.2 days for Small Pratincoles, and 8.0 ± 6.0 days for Little Ringed Plovers. For clutches with known initiation and hatching dates, average incubation periods (from day first egg was laid until day first egg hatched) were 23.5 ± 2.6 days for River Terns, 31.0 ± 3.7 days for River Lapwings, 29.0 days for Great Thick-knees, 30.0 ± 2.8 days for Indian Thick-knees, 20.9 ± 3.3 days for Small Pratincoles, and 26.2 ± 3.0 days for Little Ringed Plovers.
Nest survival
Apparent nest success, the proportion of nests that hatched at least one chick, was 47% for River Terns, 56% for River Lapwings, 54% for Great Thick-knees, 100% for Indian Thick-knees, 45% for Small Pratincoles, and 33% for Little Ringed Plovers.
For River Terns, the best-supported model of DSR included an effect of nest protection as a discrete variable with 3 levels (PROTECT_3; Tables 2 and 3). Based on this model, River Tern nest success averaged 0.60 ± 0.12 (SE) for a nest protected by an exclosure plus guarding, 0.29 ± 0.05 (SE) for a nest protected by guarding only, and 0.05 ± 0.05 (SE) for a nest that was not protected (Figure 2).
Table 2. Best-supported models of clutch size, daily nest survival (DSR), and daily chick survival of sandbar-nesting birds on the Mekong River, Cambodia (2010–2014).
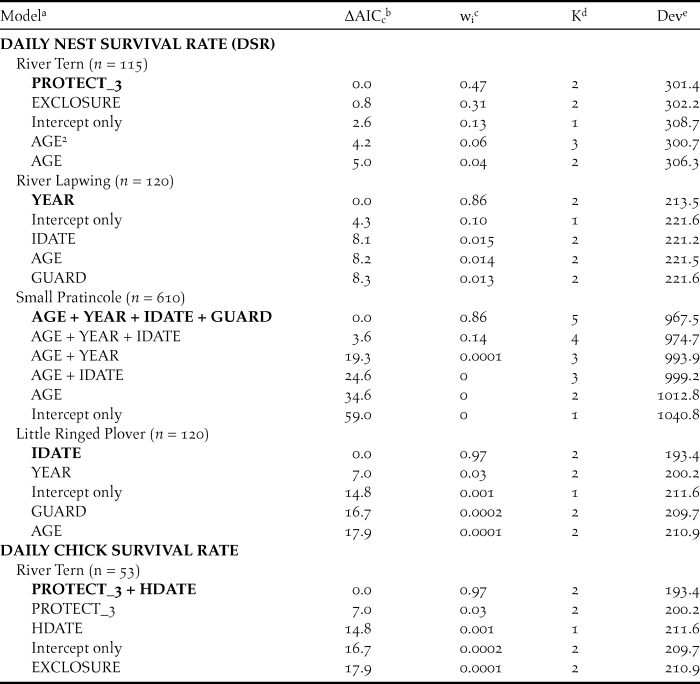
a The top five models, plus intercept-only model if not among the top five models, are presented for each set of analyses. Best-supported models in each candidate set are indicated in bold. Fixed effects included nest age (AGE), nest initiation date (IDATE), hatch date (HDATE), year as a linear trend (YEAR), and nest protection; nest protection was considered as a discrete variable with three levels (PROTECT_3) or categorical dummy variables for nest guarding (GUARD) or use of an exclosure (EXCLOSURE).2 indicates a quadratic effect. Sample sizes (n) indicate number of intervals for DSR and chick survival analyses.
b The difference in AICc value between the model and the best-supported model.
c Akaike weight.
d Number of model parameters.
e Model deviance.
Table 3. Parameter estimates, standard errors, and 95% confidence intervals for best-supported models of daily nest survival (DSR) and daily chick survival of sandbar-nesting birds on the Mekong River, Cambodia (2010–2014).
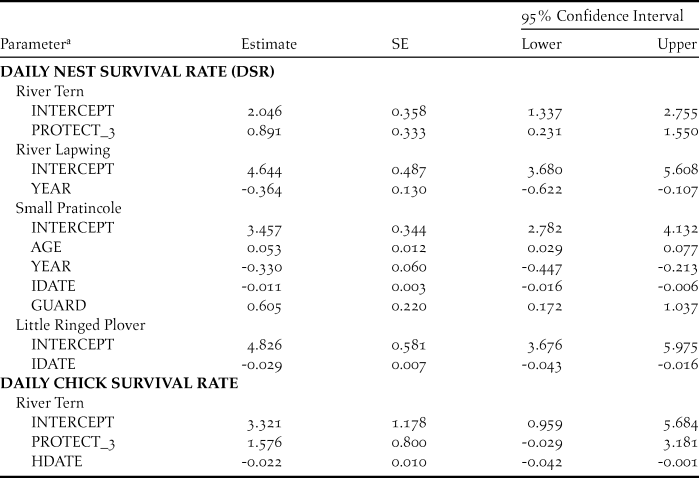
a Model parameters included nest age (AGE), nest initiation date (IDATE), hatch date (HDATE), year as a linear trend (YEAR), and nest protection; nest protection was a discrete variable with three levels (PROTECT_3), or a categorical dummy variable for nest guarding (GUARD).
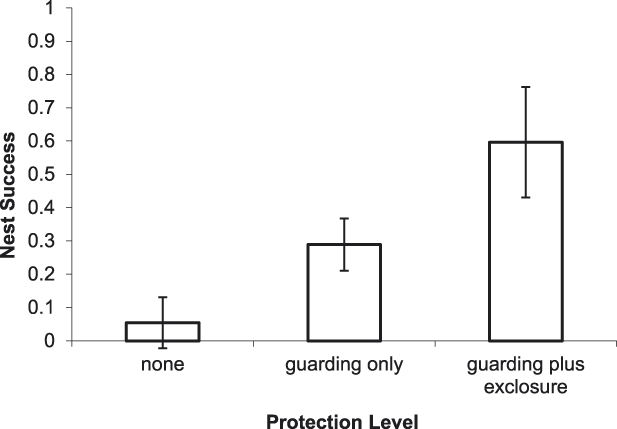
Figure 2. Predicted effect of nest protection on River Tern nest success on the Mekong River, Cambodia (2010–2014). Error bars indicate 85% prediction intervals.
For River Lapwings, the best-supported model of DSR included a linear effect of year (Tables 2 and 3). Based on this model, River Lapwing nest success declined during the study, from 0.65 ± 0.10 (SE) in 2010 to 0.17 ± 0.08 (SE) in 2014.
For Small Pratincoles, the best-supported model of DSR included an effect of nest age, a linear year effect, and effects of nest initiation date and nest protection (Tables 2 and 3). Based on this model, Small Pratincole nest success increased with nest age, from 0.19 ± 0.02 (SE) for a 1-day-old nest to 0.96 ± 0.01 (SE) for a 21-day-old nest that was about to hatch. Also, nest success declined over the study, from 0.51 ± 0.06 (SE) in 2010 to 0.07 ± 0.02 (SE) in 2014 (Figure 3). Additionally, nest success declined from 0.43 ± 0.07 (SE) for early-season nests (6 January) to 0.05 ± 0.02 (SE) for late season nests (8 May). Finally, Small Pratincole nest success averaged 0.21 ± 0.03 (SE) for guarded nests and 0.06 ± 0.03 (SE) for unguarded nests (Figure 3).
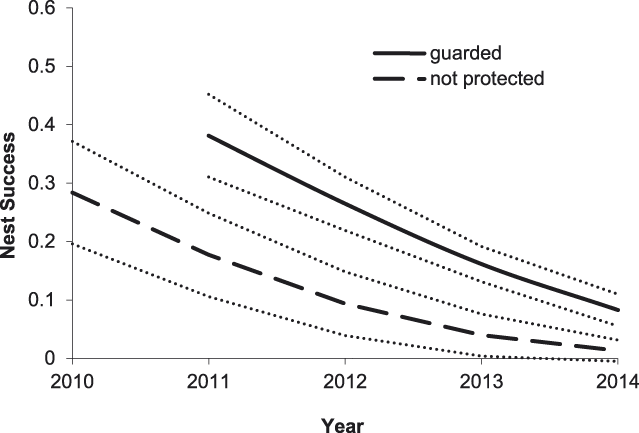
Figure 3. Predicted effects of nest protection and year on Small Pratincole nest success on the Mekong River, Cambodia (2010–2014). Other model covariates were held constant at their mean observed values: nest age = 11.3 days, and nest initiation date = 12 March. Thin dotted lines indicate 85% prediction intervals.
For Little Ringed Plovers, the best-supported model of DSR included an effect of nest initiation date (Tables 2 and 3). Based on this model, Little Ringed Plover nest success declined with later nest initiation, from 0.68 ± 0.11 (SE) for early-season nests (January 21) to 0.01 ± 0.01 (SE) for late-season nests (21 April). We did not model DSR for Great or Indian Thick-knees due to small sample sizes.
Causes of nest failure
River Tern nest failures (n = 43) were caused by predation by animals (47%), egg harvest by humans (26%), abandonment (16% [7% due to exclosure installation; 2% due to the apparent death of the incubating adult by an aggressive Brahminy Kite Haliastur indus; 7% due to other non-investigator related reasons]), flooding (5%), cracked eggs (5%), and trampling by domestic water buffalo (2%). River Lapwing nest failures (n = 39) were caused by animal predation (67%), egg harvest by humans (23%), inundation (5%), trampling by water buffalo (3%), and egg non-viability (3%). All Great Thick-knee nest failures (n = 6) were caused by animal predation (100%). Small Pratincole nest failures (n = 258) were caused by animal predation (69%), egg harvest by humans (15%), abandonment (9% [3% due to heavy rain, 1% due to apparent predation on adults by Peregrine Falcons Falco peregrinus, and 5% due to other non-investigator related reasons]), inundation (4%), trampling by water buffalo (3%), and cracked eggs (1%). Little Ringed Plover nest failures (n = 56) were caused by animal predation (61%), egg harvest by humans (25%), inundation (7%), abandonment (5% [1% due to extreme heat; 4% due to other non-investigator related reasons]), and cracked eggs (2%). Both Indian Thick-knee nests were successful.
Predation by animals was the leading cause of nest failure, accounting for 65% of nest failures for all species pooled. Predator species could be accurately identified from direct observation or clear signs in 19% of nest predation cases, probable predator species was inferred based on some evidence in 36% of cases, and predator species was unknown in 45% of cases. Rats (captured individuals were provisionally identified as Asian house rat Rattus tanezumi) were the primary nest predators of River Terns, Great Thick-knees, and Little Ringed Plovers. Southern Jungle Crow Corvus macrorynchos was the primary nest predator of River Lapwings and Small Pratincoles. Other nest predators included domestic dog, domestic chicken, and Green-backed Heron Butorides striata. Additionally, Great Thick-knees and River Lapwings destroyed four nests of other species. Predation increased over the course of the study for all species pooled (χ2 = 38.94, df = 1, P < 0.001; Figure 4). A significant increase in nest predation occurred during the 2010–2011 period (Fisher’s exact test odds ratio (OR) = 2.12, P = 0.04), but not during 2011–2012 (OR = 0.69, P = 0.32), 2012–2013 (OR = 0.89, P = 0.58), or 2013–2014 (OR = 0.86, P = 0.53). From 2010 to 2014, the proportion of predated clutches increased for River Lapwings (χ2 = 11.32, df = 1, P < 0.001), Small Pratincoles (χ2 = 23.14, df = 1, P < 0.001) and Little Ringed Plovers (χ2 = 10.65, df = 1, P = 0.001), but not River Terns (χ2 = 1.39, df = 1, P = 0.24) or Great Thick-knees (χ2 = 1.81, df = 1, P = 0.18).
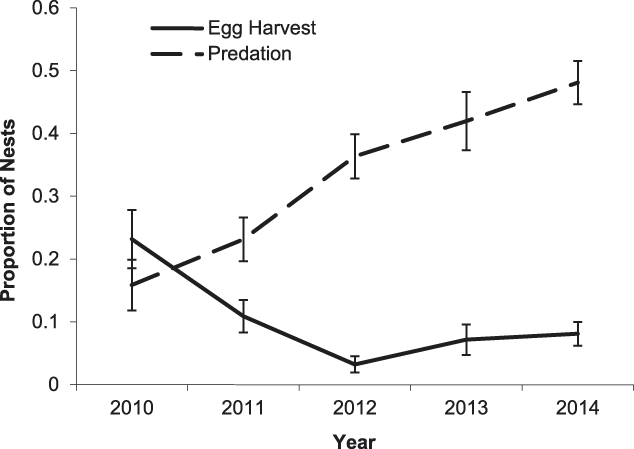
Figure 4. Proportion of nests that failed due to egg harvest by people (solid line) and predation by animals (dashed line) of sandbar-nesting birds on the Mekong River, Cambodia (2010–2014). Nests were pooled for all focal species. Error bars indicate 95% confidence intervals.
Egg harvest by humans was the second leading cause of nest failure, accounting for 18% of nest failures for all species pooled. Clutches were harvested by adults (67%) and children (33%). Egg harvest decreased over the course of the study for all species pooled (χ2 = 9.10, df = 1, P = 0.003; Figure 4). The likelihood of egg harvest decreased significantly during the 2010–2011 (OR = 2.12, P = 0.04) and 2011–2012 periods (OR = 3.38, P = 0.01), but not during 2012–2013 (OR = 0.45, P = 0.16) or 2013–2014 (OR = 0.70, P = 0.54). Over the course of the five-year study (2010–2014), the proportion of harvested clutches decreased for River Terns (χ2 = 6.68, df = 1, P = 0.009) and River Lapwings (χ2 = 8.21, df = 1, P = 0.004), but not Small Pratincoles (χ2 = 1.41, df = 1, P = 0.23) or Little Ringed Plovers (χ2 = 1.15, df = 1, P = 0.28). For River Terns, 50% of unprotected clutches (n = 6), 11% of guarded clutches (n = 64), and 0% of exclosed clutches (n = 7) were harvested by humans. The proportion of harvested River Tern clutches was significantly lower for protected than unprotected nests (χ2 = 6.68, df = 1, P = 0.009). The proportion of clutches that were harvested was also significantly lower for protected nests of Small Pratincoles (OR = 2.98, P = 0.007) and Little Ringed Plovers (OR = 4.39, P = 0.04). Nest protection did not significantly reduce harvest of River Lapwing clutches (OR = 1.22, P = 1).
River tern chick survival
Of 44 River Tern broods that were monitored for the entire chick period, apparent brood success (proportion of broods where at least one chick fledged) was 47%. The average exposure period for chicks was 14.7 ± 8.2 days. For five broods with known fledging dates, the average chick period was 22.0 ± 1.3 days. Of eight broods protected by exclosures, 100% were released prior to fledging; the average length of time broods were kept inside exclosures was 8.0 ± 2.9 days. The best-supported model of River Tern chick survival included protection as a discrete variable with three levels (PROTECT_3) and hatch date (Tables 2 and 3). Based on this model, River Tern chick survival declined during the course of the breeding season, and varied according to level of nest protection, with survival rates being highest for chicks protected by exclosures and lowest for unprotected chicks (Figure 5). River Tern fledging success was 0.82 ± 0.14 (SE) for chicks that were protected by exclosures and nest guarding, 0.40 ± 0.09 (SE) for chicks that were guarded only, and 0.02 ± 0.05 (SE) for unprotected chicks.
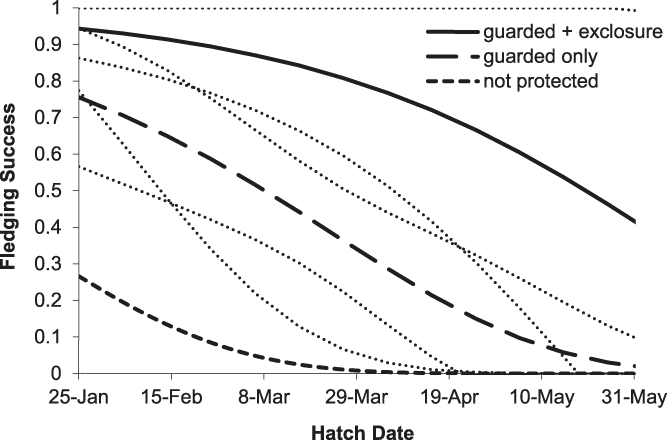
Figure 5. Predicted effects of nest protection and hatching date on River Tern fledging success on the Mekong River, Cambodia (2010–2014). Thin dotted lines indicate 85% prediction intervals.
Discussion
Direct payment programmes have frequently been used to protect threatened birds (Sok et al. Reference Sok, Claassen, Wright and Ryan2012, Clements et al. Reference Clements, Rainey, An, Rours, Tan, Thong, Sutherland and Milner-Gulland2013, Wright et al. Reference Wright, Collar, Lake, Net, Rours, Sok, Sum and Dolman2013) and turtles (Ferraro and Gjertsen Reference Ferraro and Gjertsen2009, Gjertsen and Niesten Reference Gjertsen and Niesten2010). In Cambodia, direct payments for bird nest protection have been effective for some species (Clements et al. Reference Clements, Rainey, An, Rours, Tan, Thong, Sutherland and Milner-Gulland2013), but equivocal for others (Sok et al. Reference Sok, Claassen, Wright and Ryan2012, Wright et al. Reference Wright, Collar, Lake, Net, Rours, Sok, Sum and Dolman2013). Although Sok et al. (Reference Sok, Claassen, Wright and Ryan2012) included the same population of River Terns in their analysis and found little evidence that nest protection was effective, shortcomings of their study were: unguarded control nests were lacking, nest protection was not included as a covariate in the analyses, and nest data were limited to 2011, a year when exclosures were not tested. The current study provides a more comprehensive assessment of the effectiveness of nest protection for River Terns in Cambodia.
Overall, our results indicate that the direct payment nest protection programme was effective in reducing egg harvest and increasing reproductive success rates. The programme was most beneficial for River Terns, the species of highest conservation concern and primary focal species of the programme. In addition to nest guarding, the use of exclosures was an integral component of River Tern nest and chick protection. An exclosed (and guarded) River Tern nest was 2.1 times more likely to be successful than a nest that was guarded only, and 11.0 times more likely to be successful than an unprotected nest (Figure 2). Furthermore, a River Tern brood that was protected by an exclosure was 2.1 times more likely to successfully fledge than a brood that was guarded only, and 52.8 times more likely to fledge than an unprotected brood (Figure 5). Nest protection also improved Small Pratincole nest success; a guarded nest was 3.3 times more likely to succeed than an unguarded nest (Figure 3). Nest protection was especially effective at reducing rates of egg harvest by humans. The decline in egg harvest corresponded to the general pattern of stronger nest protection programme management and implementation in each successive year of the study. During the first year (2010), nest protection was implemented on a small scale, and without a community awareness component. With support from the Worldwide Fund for Nature (WWF – Cambodia programme), the programme was expanded considerably in the second year (2011) and included educational awareness to communities regarding the programme; WWF support continued for the duration of this study and community awareness and participation increased during each successive year. Despite having a stronger programme, a slight increase in egg harvest occurred in 2013 and 2014 (Figure 4); however, the majority of harvested nests during those years were from unprotected sites.
Nest guards were frequently recruited from among the primary resource users (e.g. fishers, farmers) at each breeding location. By enlisting their participation in the nest protection programme, these resource users (as well as their extended families) were therefore effectively removed from the pool of potential egg harvesters. To our knowledge, no nests were harvested by any nest guard or member of their extended families. In fact, nest guards became programme advocates; they spread awareness of the programme and frequently recruited additional nest protectors from among their extended families and communities. All cases of egg harvest occurred when nest protectors were absent from their guard posts; although nest protectors were supposed to guard nests at all times, they occasionally left their posts. However, in one case, a nest guard was able to retrieve a stolen River Tern clutch and return the eggs to the nest, where they later successfully hatched. In another incident, nest guards retrieved two stolen White-shouldered Ibis Pseudibis davisoni chicks and reunited them with their parents (concurrent WWF nest protection programme; Sok et al. Reference Sok, Claassen, Wright and Ryan2012); meanwhile, they assisted the ibis chick thief to locate and protect a River Tern nest.
Despite the effectiveness of the nest protection programme to reduce egg harvest, the programme was less effective against animal predation. Predation, especially by rats and Southern Jungle Crows, increased significantly over the course of the study. In the first year (2010), egg harvest was the primary cause of nest failure, but by the second year (2011), predation surpassed egg harvest as the primary cause of nest failure (Figure 4). The likelihood of nest predation was especially high during the last three years of the study (2012–2014). This pattern of nest predation mirrored observed frequencies of rats and crows in the study area; on average, rat signs and crows were observed once per week during 2010–2011, but sightings increased to several per day during 2012–2014. Nest predation rates and the frequency of predator observations also corresponded to regional weather patterns during the study. 2010 was an especially dry year, but was followed by higher than average rainfall in 2011 and consequent widespread flooding along the Mekong River. Observations of rat signs and crows increased in the following breeding season of 2012, and nest predation rose during that year. The frequency of rat signs and crow sightings, as well as nest predation rates, remained high during subsequent years of the study. Studies of other systems have documented positive effects of precipitation and weather events on rodents and birds (Madsen and Shine Reference Madsen and Shine1999, Jaksic Reference Jaksic2001). We speculate that rat and crow populations increased in the study area due to increased food resources following the 2011 rains and floods. Higher than average rainfall may have led to increased primary (vegetation) or secondary (arthropod) production (Jaksic Reference Jaksic2001), or possibly, detritus that remained after flood waters receded was more abundant than in average rainfall years, benefitting opportunistic scavengers such as rats and crows. In 2016, a drought year, a sharp decline in the rat (but not crow) population occurred such that the frequency of rat signs was similar to pre-2011 levels (AHC, personal observation), providing further anecdotal evidence that precipitation, or a lack thereof, is an important driver of rat population trends.
Nest predation posed a significant threat to sandbar-nesting birds in this study. We recommend that future conservation programmes for these species increase efforts to mitigate threats from nest predation. Exclosures significantly increased nest and chick survival of River Terns (Figures 2 and 5). Their use should be an essential component of future conservation measures for this species. However, using exclosures required considerable effort and care, including timely delivery of exclosure materials to nesting sites. Ensuring that exclosure installation did not lead to nest abandonment proved to be especially challenging. Following five out of 15 installations, exclosures had to be removed because incubating adults did not return to their nests. River Terns abandoned three out of 10 nests, even though adults briefly visited these nests after exclosure installation and we mistakenly assumed acceptance by the adults. To minimise the risk of nest abandonment, conservation staff and nest protectors should receive comprehensive training in proper set-up and monitoring of exclosures. Also, we recommend that larger exclosures be used in the future to minimise abandonments. When adults did accept exclosures and resumed tending their nests, nest and chick survival was 100%; exclosures were completely effective against predation and no mortality of eggs or chicks occurred within exclosures. Although exclosures were designed to deter rodents, they may also have been visual, if not physical, deterrents to avian predators. Unfortunately, all chicks were released from exclosures prior to fledging, and only 64% of chicks released from exclosures survived to fledging age. To increase the chances of successful fledging, we recommend that River Tern chicks be kept inside exclosures until they fledge; importantly, this will require providing chicks with shade and water. In addition to River Terns, exclosures could potentially be used to protect nests of other species; however, exclosure design and implementation should consider the unique behaviour of each species. Additionally, future conservation activities may warrant removal of nest predators, especially seasonal eradication of rats from key River Tern breeding sites. Predator removal measures should be integrated into existing community nest protection activities. For example, community nest protectors could also assist with predator removal activities.
Research activity can potentially cause lower nesting success due to increased detection rates by predators (Carney and Sydeman Reference Carney and Sydeman1999). We therefore took precautions to minimise predation risk, such as postponing nest checks when predators were present and varying approach routes to nests. While exclosed nests were effectively protected from predators, it is possible that predation risk increased for other nearby unfenced nests. Another potential risk was that nest protector encampments may have attracted predators such as rats and crows. However, encounter rates of rat signs and crows were similar for sites with and without human encampments, suggesting that encampments were not the sole factor influencing predator distribution and abundance. Moreover, a sharp decline in the rat population occurred in 2016, despite the continued presence of nest protector encampments. At most sites, human encampments existed before the nest protection programme began; however, prior to the programme, inhabitants of encampments were harvesting nests rather than protecting them. Our results, which show that nest protection activities significantly improved nesting success, suggest that these activities were warranted despite the additional disturbance to nesting birds.
As well as nest protection, reproductive success was influenced by seasonal and age effects. Small Pratincole and Little Ringed Plover nest survival declined with later initiation date (Table 3), and River Tern chick survival declined with later hatching date (Figure 5). Seasonal declines in reproductive success have been documented for a wide range of species, and may result from decreased food resources (Verhulst and Nilsson Reference Verhulst and Nilsson2008), variation in predator dynamics or environmental conditions (Grant et al. Reference Grant, Shaffer, Madden and Pietz2005), decreased physical condition of breeding adults (Arnold et al. Reference Arnold, Hatch and Nisbet2004), or end-of-season time constraints (Verboven and Visser Reference Verboven and Visser1998). For Small Pratincoles, nest survival was also influenced by nest age; older nests had higher survival than younger nests. Studies of other species have documented similar patterns of survival according to nest age (Klett and Johnson Reference Klett and Johnson1982, Grant et al. Reference Grant, Shaffer, Madden and Pietz2005), presumably because the most vulnerable nests failed at a young age. In this study, older nests may have survived longer due to nest site characteristics that made them less vulnerable to harvest, predation, or flooding.
Our results illustrate that direct payments to individuals can be an effective tool for species conservation, at least in terms of biological indicators of success; however, direct payment programmes also have social implications. Direct payments may create or exacerbate negative social dynamics which can undermine programme effectiveness (Sok et al. Reference Sok, Claassen, Wright and Ryan2012, Clements et al. Reference Clements, Rainey, An, Rours, Tan, Thong, Sutherland and Milner-Gulland2013). Furthermore, direct payment initiatives require social responsibility on the part of the payer, and may require taking an adaptive management approach to negotiating with communities (Milne and Niesten Reference Milne and Niesten2009). Although our study did not address social implications per se, we suspect that the nest protection programme’s effectiveness in terms of biological outcomes was influenced by how social aspects were handled. The nest protection programme took an adaptive approach to implementing and disbursing nest payments. Nest payment amounts were assessed annually and adjusted when necessary to ensure that nest protectors were fairly compensated for their time, effort, and travel costs, especially considering the high rates of nest predation and consequent pay reductions. Occasionally, discrepancies regarding nest outcomes arose between nest protector reports and research team observations. In such cases, to minimise potential conflicts, nest protectors were given the benefit of the doubt and payments were made in their favour; thus, the burden of proof was borne by the programme rather than the individual nest protectors. To reduce the potential for nest protectors to abuse the programme by providing false information in anticipation of higher payments, we implemented regular monitoring (of nests and nest protectors), and explained to nest protectors how egg floatation methods allowed us to predict hatching dates. We speculate that the nest protection programme’s emphasis on creating and maintaining positive relationships with participating community members led to higher programme satisfaction and job performance among nest protectors and therefore indirectly contributed to improved reproductive success rates of the target bird species.
Our results clearly demonstrate that nest protection combined with predator exclosures are highly effective at increasing nest success of River Terns and other sandbar-nesting bird species, and are essential for the survival of these species in the Mekong basin. However, the effectiveness of the direct payment nest protection programme was contingent upon having specific and measurable biological indicators, regular monitoring, ability of nest protectors to prevent egg harvest by humans, proper training and use of exclosures to protect nests and chicks from animal predators, and positive relationships between the programme and nest protectors. This study illustrates that multiple biological and social factors should be considered when designing and implementing a direct payment initiative, and can affect programme success. However, these factors are important in any conservation approach. Our study indicates that direct payments can indeed be a useful tool for biodiversity conservation, especially in developing countries where voluntary conservation action by local communities is unrealistic.
Acknowledgements
We would like to thank our partner, the Worldwide Fund for Nature (WWF Cambodia country programme) for their support of River Tern nest protection and conservation; we are especially grateful to G. Congdon, Horm C., Keo B. R., S. Masoomi, Phan C. and G. Ryan. R. Timmins provided guidance during the early stages of this project. Our field team included: T. Kam, Nguon R., Pham, J. Schwilk. and You B. We thank our extensive network of community nest protectors, especially Meak P., Chin L. and their family. This manuscript was improved by comments from two anonymous reviewers.
Funding was provided by the Critical Ecosystem Partnership Fund (CEPF; grant number: 77636-000), Frank McKinney Fellowship in Avian Ethology, Dayton Fund of the Bell Museum of Natural History, and Huempfner Fellowship Fund in the College of Biological Sciences at University of Minnesota. A. H. C. received additional support from the University of Minnesota Graduate School and Conservation Biology Graduate Program, and the National Science Foundation. F. J. C.’s contribution to this work was supported by the USDA National Institute of Food and Agriculture, Hatch project (grant number: 1007020). Research approvals were granted by the Cambodian Fisheries (FiA) and Forestry (FA) Administrations.












