No CrossRef data available.
Article contents
Genetic and non-genetic effects of increased sun and vitamin D exposure: role in the observed healthy changes in cardiometabolic risk factors in Iranian children
Published online by Cambridge University Press: 06 June 2018
Abstract
An abstract is not available for this content. As you have access to this content, full HTML content is provided on this page. A PDF of this content is also available in through the ‘Save PDF’ action button.
- Type
- Invited Commentary
- Information
- Copyright
- © The Authors 2018
References
1. Nikooyeh, B, Abdollahi, Z, Hajifaraji, M et al. (2018) Healthy changes in some cardiometabolic risk factors accompany the summertime higher serum 25-hydroxyvitamin D in Iranian children: National Food and Nutrition Surveillance. Public Health Nutr. Published online: 27 March 2018. doi: 10.1017/S1368980018000630.Google Scholar
2. Ji, L, Gupta, M & Feldman, BJ (2016) Vitamin D regulates fatty acid composition in subcutaneous adipose tissue through Elovl3. Endocrinology 157, 91–97.Google Scholar
3. Cheng, S, So, WY, Zhang, D et al. (2016) Calcitriol reduces hepatic triglyceride accumulation and glucose output through Ca2+/CaMKKβ/AMPK activation under insulin-resistant conditions in type 2 diabetes mellitus. Curr Mol Med 16, 747–758.Google Scholar
4. Zittermann, A, Frisch, S, Berthold, HK et al. (2009) Vitamin D supplementation enhances the beneficial effects of weight loss on cardiovascular disease risk markers. Am J Clin Nutr 89, 1321–1327.Google Scholar
5. Jorde, R & Grimnes, G (2018) Exploring the association between serum 25-hydroxyvitamin D and serum lipids – more than confounding? Eur J Clin Nutr 72, 526–533.Google Scholar
6. Patwardhan, VG, Khadilkar, AV, Chiplonkar, SA et al. (2015) Varying relationship between 25-hydroxy-vitamin D, high density lipoprotein cholesterol, and serum 7-dehydrocholesterol reductase with sunlight exposure. J Clin Lipidol 9, 652–657.Google Scholar
7. Patwardhan, VG, Mughal, ZM, Padidela, R et al. (2017) Randomized control trial assessing impact of increased sunlight exposure versus vitamin D supplementation on lipid profile in Indian vitamin D deficient men. Indian J Endocrinol Metab 21, 393–398.Google Scholar
8. Gaksch, M, Jorde, R, Grimnes, G et al. (2017) Vitamin D and mortality: individual participant data meta-analysis of standardized 25-hydroxyvitamin D in 26916 individuals from a European consortium. PLoS One 12, e0170791.Google Scholar
9. Boucher, BJ (1998) Inadequate vitamin D status: does it contribute to the disorders comprising syndrome ‘X’? Br J Nutr 79, 315–327.Google Scholar
10. Boucher, BJ (2012) Is vitamin D status relevant to metabolic syndrome? Dermatoendocrinology 4, 212–224.Google Scholar
11. Reaven, GM (1988) Banting lecture 1988. Role of insulin resistance in human disease. Diabetes 37, 1595–1607.Google Scholar
13. Alberti, KG, Eckel, RH, Grundy, SM et al. (2009) Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation 120, 1640–1645.Google Scholar
14. Forman, JP, Curhan, GC & Taylor, EN (2008) Plasma 25-hydroxyvitamin D levels and risk of incident hypertension among young women. Hypertension 52, 828–832.Google Scholar
15. Mirhosseini, N, Vatanparast, H & Kimball, SM (2017) The association between serum 25(OH)D status and blood pressure in participants of a community-based program taking vitamin D supplements. Nutrients 9, E1244.Google Scholar
16. Al-Khalidi, B, Kimball, SM, Rotondi, MA et al. (2017) Standardized serum 25-hydroxyvitamin D concentrations are inversely associated with cardiometabolic disease in US adults: a cross-sectional analysis of NHANES, 2001–2010. Nutr J 16, 16.Google Scholar
17. Zhang, R, Li, B, Gao, X et al. (2017) Serum 25-hydroxyvitamin D and the risk of cardiovascular disease: dose–response meta-analysis of prospective studies. Am J Clin Nutr 105, 810–819.Google Scholar
18. Pike, JW & Christakos, S (2017) Biology and mechanisms of action of the vitamin D hormone. Endocrinol Metab Clin North Am 46, 815–843.Google Scholar
19. Trochoutsou, AI, Kloukina, V, Samitas, K et al. (2015) Vitamin-D in the immune system: genomic and non-genomic actions. Mini Rev Med Chem 15, 953–963.Google Scholar
20. Hossein-nezhad, A, Spira, A & Holick, MF (2013) Influence of vitamin D status and vitamin D3 supplementation on genome wide expression of white blood cells: a randomized double-blind clinical trial. PLoS One 8, e58725.Google Scholar
21. Scott, JF, Das, LM, Ahsanuddin, S et al. (2017) Oral vitamin D rapidly attenuates inflammation from sunburn: an interventional study. J Invest Dermatol 137, 2078–2086.Google Scholar
22. Shirts, BH, Howard, MT, Hasstedt, SJ et al. (2012) Vitamin D dependent effects of APOA5 polymorphisms on HDL cholesterol. Atherosclerosis 222, 167–174.Google Scholar
23. Vimaleswaran, KS, Cavadino, A & Hypponen, E (2013) APOA5 genotype influences the association between 25-hydroxyvitamin D and high density lipoprotein cholesterol. Atherosclerosis 228, 188–192.Google Scholar
24. Dopico, XC, Evangelou, M, Ferreira, RC et al. (2015) Widespread seasonal gene expression reveals annual differences in human immunity and physiology. Nat Commun 6, 7000.Google Scholar
25. Grant, WB, Bhattoa, HP & Boucher, BJ (2017) Seasonal variations of US mortality rates: roles of solar ultraviolet-B doses, vitamin D, gene expression, and infections. J Steroid Biochem Mol Biol 173, 5–12.Google Scholar
26. Grant, WB (2011) An estimate of the global reduction in mortality rates through doubling vitamin D levels. Eur J Clin Nutr 65, 1016–1026.Google Scholar
27. Garland, CF, Kim, JJ, Mohr, SB et al. (2014) Meta-analysis of all-cause mortality according to serum 25-hydroxyvitamin D. Am J Public Health 104, e43–e50.Google Scholar
28. Veloudi, P, Jones, G & Sharman, JE (2017) Effectiveness of vitamin D supplementation for cardiovascular health outcomes. Pulse (Basel) 4, 193–207.Google Scholar
29. Lappe, J, Watson, P, Travers-Gustafson, D et al. (2017) Effect of vitamin D and calcium supplementation on cancer incidence in older women: a randomized clinical trial. JAMA 317, 1234–1243.Google Scholar
30. Grant, WB & Boucher, BJ (2017) Randomized controlled trials of vitamin D and cancer incidence: a modeling study. PLoS One 12, e0176448.Google Scholar
31. Grant, WB, Boucher, BJ, Bhattoa, HP et al. (2018) Why vitamin D clinical trials should be based on 25-hydroxyvitamin D concentrations. J Steroid Biochem Mol Biol 177, 266–269.Google Scholar
32. Raed, A, Bhagatwala, J, Zhu, H et al. (2017) Dose responses of vitamin D3 supplementation on arterial stiffness in overweight African Americans with vitamin D deficiency: a placebo controlled randomized trial. PLoS One 12, e0188424.Google Scholar
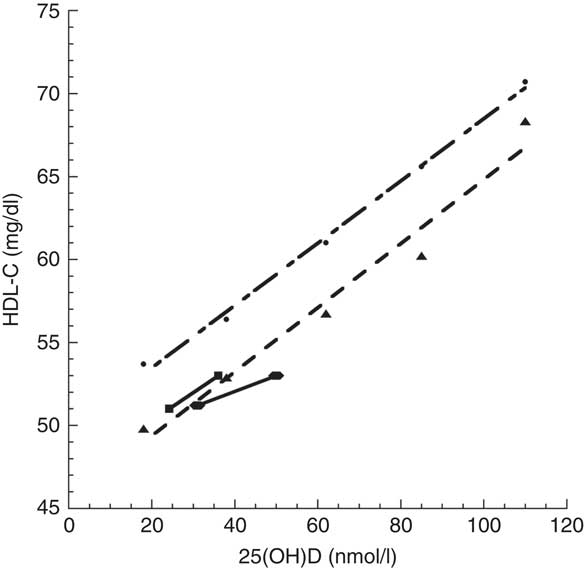
Fig. 1 Relationship of HDL-cholesterol (HDL-C) concentration with 25-hydroxyvitamin D (25(OH)D) concentration: comparison between Jorde and Grimnes’ study(5) (, Norway, standard diet; , Norway, low fish oils) and Nikooyeh et al.’s study(1) (, Iran, boys; , Iran, girls)





The recent Public Health Nutrition paper by Nikooyeh and colleagues( Reference Nikooyeh, Abdollahi and Hajifaraji 1 ) reports on seasonal latitudinal variations in serum 25-hydroxyvitamin D (25(OH)D) concentration, serum lipid status, height-for-age Z-score and BMI for 530 apparently healthy children aged 5–18 years in six regions of Iran. Serum 25(OH)D concentrations increased from 31 (sd 16) to 50 (sd 30) nmol/l for boys and from 27 (sd 18) to 43 (sd 29) nmol/l for girls. The primary reason for this difference was that boys spent more time in the sun and were less likely to use sunscreen than girls. More importantly, HDL-cholesterol (HDL-C) concentration, a frequently used biomarker of cardiovascular health, increased significantly in summer compared with winter for both boys and girls in Iran. In addition, there were inverse correlations between seasonal changes in serum 25(OH)D concentration and changes in BMI, TAG and total cholesterol, and a direct correlation with height-for-age Z-score; all healthy cardiometabolic risk factor changes.
The changes in weight and height with season may reflect increased exercise and growth rates in summer compared with winter; however, other observed healthy risk factor changes are likely due to the measured increase in serum 25(OH)D concentration. In this commentary, we describe how the known functions of vitamin D impact these changes in cardiovascular risk markers and this builds confidence in the prospect that the beneficial observed risk factor changes are driven by increases in vitamin D status.
The reduction in TAG in summer may reflect the changes in weight, but increases in vitamin D status could contribute directly to this effect since calcitriol, the active hormonal metabolite of vitamin D, reduces NEFA synthesis( Reference Ji, Gupta and Feldman 2 ) and can inhibit hepatic TAG synthesis( Reference Cheng, So and Zhang 3 ). One randomized controlled trial has shown that vitamin D enhanced the beneficial effects of weight loss on serum TAG( Reference Zittermann, Frisch and Berthold 4 ).
In an earlier cross-sectional Norwegian study involving 17 083 individuals older than 40 years, HDL-C concentration was also significantly correlated with 25(OH)D concentration( Reference Jorde and Grimnes 5 ), where mean concentration ranged from ~18 to 110 nmol/l. Graphical comparison of these studies shows that the Norwegian 25(OH)D concentration–HDL-C concentration relationship matches the Iranian relationship reasonably well, although the HDL-C concentration for Iranian boys in summer( Reference Nikooyeh, Abdollahi and Hajifaraji 1 ) was below the linear fit to Jorde and Grimnes’ data( Reference Jorde and Grimnes 5 ) for low fish oil intake by ~2 mg/dl (Fig. 1). This finding may be due to a non-linear relationship between UVB exposure and HDL-C concentration as reported in two studies in Pune, India (18·5°N), since low UVB exposure increased HDL-C concentration but high UVB exposure reduced HDL-C concentration( Reference Patwardhan, Khadilkar and Chiplonkar 6 , Reference Patwardhan, Mughal and Padidela 7 ).
Fig. 1 Relationship of HDL-cholesterol (HDL-C) concentration with 25-hydroxyvitamin D (25(OH)D) concentration: comparison between Jorde and Grimnes’ study( Reference Jorde and Grimnes 5 ) ( , Norway, standard diet;
, Norway, standard diet;  , Norway, low fish oils) and Nikooyeh et al.’s study(
Reference Nikooyeh, Abdollahi and Hajifaraji
1
) (
, Norway, low fish oils) and Nikooyeh et al.’s study(
Reference Nikooyeh, Abdollahi and Hajifaraji
1
) ( , Iran, boys;
, Iran, boys;  , Iran, girls)
, Iran, girls)
The implications of these findings warrant consideration. First, is HDL-C an important risk factor for CHD? A recent review found that HDL-C concentration >51 mg/dl was not a significant risk-reduction factor after adjustment for other recognized risk factors( Reference Gaksch, Jorde and Grimnes 8 ), although HDL-C concentration <51 mg/dl was a significant risk factor, after adjustment for other recognized risk factors.
Vitamin D deficiency has been shown to be linked to the metabolic syndrome( Reference Boucher 9 , Reference Boucher 10 ) or syndrome ‘X’( Reference Reaven 11 , Reference Reaven 12 ). The hallmarks of the metabolic syndrome include three or more of large waist size, raised TAG, low HDL-C concentration, hypertension, hyperglycaemia, increased insulin resistance and non-alcoholic fatty liver disease( Reference Alberti, Eckel and Grundy 13 ). Raising serum 25(OH)D concentration can raise HDL-C concentration, reduce the risk of hypertension( Reference Forman, Curhan and Taylor 14 , Reference Mirhosseini, Vatanparast and Kimball 15 ) and reduce fasting glucose( Reference Al-Khalidi, Kimball and Rotondi 16 ). Serum 25(OH)D concentration has been found to be inversely correlated with CVD in a meta-analysis of observational studies, finding a pooled risk ratio of 0·90 (95 % CI 0·86, 0·94) for each 25 nmol/l increment in 25(OH)D concentration( Reference Zhang, Li and Gao 17 ).
The first mechanism to consider in explaining the effects of increased vitamin D status on HDL-C concentration is vitamin D’s role in changing gene expression, which can be induced by higher serum 25(OH)D concentrations. It is well established that virtually all biological activities of the active metabolite of vitamin D are mediated by the vitamin D receptor, a nuclear receptor protein that functions to control expression of genes and gene networks in specific cell types( Reference Pike and Christakos 18 ). However, other effects considered to be non-genomic induction are driven by the active form of vitamin D through an increased intracellular Ca2+ concentration( Reference Trochoutsou, Kloukina and Samitas 19 ).
A second genetic explanation stems from vitamin D supplementation studies that have shown up- and down-regulation of many genes. In one study, three individuals were given 10 µg (400 IU) of cholecalciferol (vitamin D3) daily and five were given 50 µg (2000 IU) of cholecalciferol daily for two months, and white blood cells were sampled to monitor changes. The cholecalciferol supplementation that improved serum 25(OH)D concentrations was associated with at least a 1·5-fold alteration in the expression of 291 genes in these white blood cells. There also was a significant difference in the expression of sixty-six genes at baseline between individuals with vitamin D deficiency (serum 25(OH)D<50 nmol/l) and those without (serum 25(OH)D>50 nmol/l). After cholecalciferol supplementation, gene expression of these sixty-six genes was similar for both groups( Reference Hossein-nezhad, Spira and Holick 20 ). In another study, twenty-five adults were randomized to receive placebo or a single oral dose of cholecalciferol of 1250 µg (50 000 IU), 250 µg (100 000 IU) or 5000 µg (200 000 IU)( Reference Scott, Das and Ahsanuddin 21 ). Adults receiving 5000 µg were much more likely to have expression of many genes flipped from up- to down- or from down- to up-regulated. The results of gene expression flipping were grouped into two clusters for skin barrier repair genes. Those with increased expression of the skin barrier repair genes had a mean 25(OH)D concentration of ~106 nmol/l, while those with no increase in skin barrier repair gene expression had a mean 25(OH)D concentration of ~75 nmol/l.
A third possible mechanism is described in two papers that identified 25(OH)D-dependent effects of APOA5 polymorphisms on circulating HDL-C( Reference Shirts, Howard and Hasstedt 22 , Reference Vimaleswaran, Cavadino and Hypponen 23 ). Shirts et al.( Reference Shirts, Howard and Hasstedt 22 ) described a 25(OH)D receptor binding site-modifying APOA5-promoter polymorphism associated with lower HDL-C in 25(OH)D-deficient individuals( Reference Shirts, Howard and Hasstedt 22 ). The other identified a borderline interaction between the SNP rs12272004 (near the APOA5) and serum 25(OH)D on HDL-C (P for interaction=0·05)( Reference Vimaleswaran, Cavadino and Hypponen 23 ).
A fourth line of evidence supporting vitamin D’s role in the observed changes in cardiometabolic risk factors concerns the widespread seasonal variations in gene expression that have been shown, including genes recognized as biomarkers of increased risks of CVD( Reference Dopico, Evangelou and Ferreira 24 ). While Dopico et al.’s study( Reference Dopico, Evangelou and Ferreira 24 ) did not identify changes in 25(OH)D concentration as the driver, based on the preceding discussion, it seems likely that it is a contributor.
Studies of the seasonality of disease and mortality rates find that rates are often higher in winter than in summer. For example, death rates in the USA are 25 % higher in winter than in summer, with CVD being a major contributor to that seasonal variation( Reference Grant, Bhattoa and Boucher 25 ), suggesting that keeping serum 25(OH)D concentration >90 nmol/l year-round may reduce death rates significantly. This estimate was supported by calculations based on increasing 25(OH)D concentration from 54 to 110 nmol/l globally( Reference Grant 26 ), as well as a meta-analysis of mortality rates found in relationship to serum 25(OH)D concentrations in thirty-two independent studies( Reference Garland, Kim and Mohr 27 ). Thus, the study by Nikooyeh et al.( Reference Nikooyeh, Abdollahi and Hajifaraji 1 ) provides further support for beneficial effects of seasonal changes in available UVB dose, and in 25(OH)D concentration, that may have a role in reducing mortality rates.
Clinical trials conducted by 2017 had not shown that vitamin D supplementation reduces the risks of CVD( Reference Veloudi, Jones and Sharman 28 ) but this finding fails to question the appropriateness of the design of the clinical trials. Most vitamin D clinical trials have been based on the assumptions implicit in trials of pharmaceutical drugs, namely that the only source of the agent is that provided in the trial and that there is a linear dose–response relationship; however, neither assumption is satisfied for vitamin D. The primary determinant of health outcomes in clinical trials with high doses of vitamin D is the achieved 25(OH)D concentration, not the vitamin D dose used. A recent clinical trial supplementing with high-dose cholecalciferol supports this concept that clinical trial efficacy should not be based on the success of the dose used to alter disease biomarkers, but on the improved change in 25(OH)D concentration associated with improved disease risk( Reference Lappe, Watson and Travers-Gustafson 29 , Reference Grant and Boucher 30 ). A recent paper outlined how to base clinical trials of vitamin D on measurements of 25(OH)D concentrations( Reference Grant, Boucher and Bhattoa 31 ). However, results of two clinical trials reported after those papers had been published have shown reductions in biological risk factors for CVD. In one, hypertensive patients who increased cholecalciferol doses from ~50 to ~150 µg/d (~2000 to ~6000 IU/d), with increases in their 25(OH)D concentrations from ~82 to 110 nmol/l, did show reductions in systolic blood pressure by ~16 mmHg and in diastolic blood pressure by ~12 mmHg( Reference Mirhosseini, Vatanparast and Kimball 15 ), while blood pressure changes in normotensive subjects were not significant. In the second trial, seventy overweight African Americans (aged 13–45 years) with serum 25(OH)D concentrations <50 nmol/l were given monthly cholecalciferol doses equivalent to 15, 50 or 100 µg/d (600, 2000 or 4000 IU/d) v. placebo( Reference Raed, Bhagatwala and Zhu 32 ). The beneficial change in carotid–femoral pulse wave velocity was significantly greater for 100 µg/d (−10·4 %; 25(OH)D=33 nmol/l at baseline, 89 nmol/l at 8 weeks, 87 nmol/l at 16 weeks) than for 50 µg/d (−2·0 %; 25(OH)D=40 nmol/l at baseline, 76 nmol/l at 8 weeks, 90 nmol/l at 16 weeks); as was the change in carotid–radial pulse wave velocity at −8·0 % after 100 µg/d v. +1·2 % after 15 µg/d (25(OH)D=35 nmol/l at baseline, 53 nmol/l at 8 weeks, 57 nmol/l at 16 weeks). While these two clinical trials did not assess HDL-C concentrations, they did show that vitamin D supplementation at high enough doses can reduce biological risk markers for CVD in those at increased risk; furthermore, these two trials also demonstrate that it is not always necessary to enrol people with deficiency in order to be able to find significant beneficial effects of cholecalciferol supplementation if the cholecalciferol dose is high enough. In the vitamin D3 plus calcium trial for cancer, mean serum 25(OH)D concentration increased from 83 to 109 nmol/l in the treatment arm but decreased from 82 to 79 nmol/l in the control arm( Reference Lappe, Watson and Travers-Gustafson 29 ).
We are currently in the ‘golden age’ of vitamin D research with many exciting findings being reported each year on the non-skeletal effects of vitamin D. Given the difficulty of conducting vitamin D clinical trials, observational studies such as the one reported by Nikooyeh et al.( Reference Nikooyeh, Abdollahi and Hajifaraji 1 ) play very important roles in identifying and quantifying effects and paving the way for additional studies, as well as serving to raise awareness of the importance of sun exposure and cholecalciferol for human health.
Acknowledgements
Financial support: This research received no specific grant from any funding agency in the public, commercial or not-for-profit sectors. Conflict of interest: W.B.G. receives general research funding from Bio-Tech Pharmacal, Inc. (Fayetteville, AR, USA). Authorship: W.B.G. wrote the first draft of this commentary, which was revised by W.B.G. and B.J.B. Ethics of human subject participation: Not applicable.