Adequate maternal thyroid function during pregnancy is crucial for optimal fetal development, especially during the first 20 weeks when the fetal thyroid does not produce its own thyroid hormones (TH)(Reference Korevaar, Medici and Visser1). Proper thyroid function is highly relevant because the development of the fetal central nervous system is dependent on TH(Reference Korevaar, Medici and Visser1). Nutritional (trace) elements such as Se, Zn and Cu are involved in a diversity of mechanisms including: (i) the regulation of cellular function, (ii) growth and maintenance, and (iii) neuromodulation(Reference Maret2). A deficiency of trace elements may result in alterations in immune function, cognition and growth and development(Reference Saeed, Nadeem and Ahmed3,Reference Wessels, Maywald and Rink4) .
For adult females, normal plasma Se concentrations vary according to location. In Europe, they are around 1·1 µmol/l, whereas in the USA, they are more often 1·6 µmol/l(Reference Rayman5). Except perhaps for regions with high plasma Se concentration, plasma Se may fall in pregnancy owing to plasma volume expansion(Reference Rayman5). Zn is important for many physiological functions such as growth, immune function and reproduction(Reference Andreini, Banci and Bertini6). As part of the adaptive immune system, T cells are particularly sensitive to deficiency of Zn that is needed for their maturation and the maintenance of a balance between different T-cell subsets(Reference Wessels, Maywald and Rink4). Cu has a crucial role in mitochondrial respiration, erythropoiesis, myelin formation, hormone synthesis, antioxidant protection and immune function(Reference Bost, Houdart and Oberli7). Cu is important for the functioning of other dietary elements including Fe. While Se and Cu have a role only in 2–3 dozen proteins, Zn is a constituent of about every tenth protein, that is, over 3000 human proteins are believed to be Zn proteins(Reference Lowe8). For the trace elements Zn and Cu, the plasma reference ranges for adult females are 10–18 and 10–24 µmol/l, respectively(Reference Andreini, Banci and Bertini6,Reference Lowe8) . In a study from China, reference ranges for Cu and Zn were established in different groups of women at a number of gestational ages and were compared with those of 552 non-pregnant matched controls(Reference Zhang, Yuan and Liu9). It was unclear whether the figures referred to plasma or serum concentrations and supplement intake was not recorded. The reference range for Cu was significantly lower for non-pregnant women (aged 21–38 years), at 7·9–24·9 µmol/l, than for any assessment during gestation. The non-pregnant reference range of Zn was 9·7–16·9 µmol/l which only slightly differed from that in pregnancy(Reference Zhang, Yuan and Liu9). In a recent Norwegian study of 2982 pregnant women, the median plasma Se/Zn/Cu concentrations assessed at 18 weeks’ gestation were 1·29, 7·35 and 24·2 µmol/l, respectively(Reference Caspersen, Thomsen and Haug10). However, as the authors reported themselves, this was a highly selected sub-group of the original cohort of 112 789 pregnant women accrued over a period of 10 years (1999–2008) making a bias likely(Reference Caspersen, Thomsen and Haug10). A major limitation in determining standard reference ranges of trace elements during pregnancy is the widespread use of supplement intake during that life stage. In several Western countries, up to 70 % of women take pregnancy supplements (folic acid, vitamin D, specific trace elements)(Reference Mousa, Naqash and Lim11). Thus, a large sample size is needed in order to find subgroups of women not taking supplements containing these trace elements. These sub-groups should preferentially share similar characteristics with the main group, enabling researchers to adapt the reference ranges to the general pregnant population.
Se, as selenocysteine, is at the active centre of the selenoproteins(Reference Rayman5). The iodothyronine deiodinases DIO1 and DIO2 are selenoproteins that convert thyroxine (T4) to the active hormone, triiodothyronine (T3); the deiodinases DIO1 and DIO3 are also able to convert T4 to inactive, reverse T3(Reference Rayman5). A further important role for the glutathione peroxidase selenoprotein (GPX3) is the removal of excess hydrogen peroxide that, together with thyroid peroxidase, is needed for TH synthesis(Reference Rayman5). Low Se status has been associated with newly diagnosed Graves’ disease and Hashimoto’s thyroiditis(Reference Wu, Rayman and Lv12,Reference Winther, Rayman and Bonnema13) . Zn is also involved in thyroid hormone metabolism including regulation of deiodinase activity, thyroid releasing hormone and TSH (thyrotrophin) synthesis(Reference Severo, Morais and de Freitas14). Low plasma Zn concentration has been associated with hypothyroidism and high concentration with hyperthyroidism(Reference Winther, Rayman and Bonnema13). Especially relevant in pregnancy is the effect of Zn deficiency on the offspring’s neuro-psychological functioning, activity and motor development(Reference Adamo and Oteiza15). Previously, a correlation between serum Se, Zn and Cu concentrations and thyroid function was reported in the general US population(Reference Jain16). In females, Cu was significantly associated with log-transformed total thyroxine (logTT4), while Se was associated with logFT3 at the 90 % significance level. No data were reported for pregnant women; women with elevated thyroid peroxidase antibodies (TPO-Ab) were excluded from the analyses(Reference Jain16). Because pregnancy is characterised by fundamental changes in immune function and increasing demands for maternal TH to ensure proper fetal development, it is important to evaluate the possible role of these trace elements on gestational thyroid function.
Up to 8–15 % of women of fertile age have elevated titres of TPO-Ab+(Reference Korevaar, Medici and Visser1). TPO-Ab+ titres during early gestation definitely imply elevated titres before conception and are one of the most obvious signs of a thyroid that is compromised by an autoimmune process; these women are at high risk for having unidentified (sub)clinical thyroid dysfunction or developing thyroid dysfunction(Reference Korevaar, Medici and Visser1). The relationship between plasma mineral (Se, Zn and Cu) concentrations during pregnancy and TPO-Ab status has scarcely been addressed.
The primary aim of the present study was to evaluate first-trimester standard reference ranges of plasma mineral (Se, Zn and Cu) concentrations in a large cohort of healthy pregnant women, adjusting for supplement intake. The secondary aim was to investigate possible cross-sectional relationships between plasma mineral (Se, Zn and Cu) concentrations in subgroups of women with different forms of thyroid dysfunction and TPO-Ab status. A tertiary aim was to compare plasma mineral (Se, Zn and Cu) concentrations with TH concentrations during early gestation, adjusted for important determinants such as smoking, parity, BMI and family history of thyroid dysfunction. Fourth, we aimed to investigate the possible relationship between plasma mineral (Se, Zn and Cu) concentrations and TPO-Ab status.
Materials and methods
The present report is part of the longitudinal prospective HAPPY project (Holistic Approach to Pregnancy and the first Postpartum Year), the details of which have been described elsewhere(Reference Truijens, Meems and Kuppens17,Reference Pop, Broeren and Wiersinga18) .
Participants and procedure
From January 2013 to September 2014, pregnant women who had their first antenatal visit at one of the seventeen participating community midwife offices in the South-East of the Netherlands were invited to participate in the HAPPY project. During the 18 months of the recruitment period, approximately 4150 women visited the participating midwife offices. Only Dutch speaking women (n 3475) were invited to participate. Women (n 3159) were eligible of whom 2275 provided written informed consent (response rate = 72 %)(Reference Jain16). Of these, all obstetric and biological data were available in 2041 women. The study was approved by the Psychology Ethics Committee of Tilburg University (protocol number EC-2012.25) and additionally evaluated by the Medical Ethical Committee of the Máxima Medical Center in Veldhoven.
Assessments
This study is reported according to the STROBE guidelines(Reference Von Elm, Altman and Egger19). At 12 weeks of gestation, as part of the standardised nationwide early-pregnancy blood assessment, non-fasting blood samples were collected in BD Vacutainer PST-tubes and women completed a set of questionnaires that asked about demographic and obstetric features and lifestyle habits.
Thyroid function assessment
At the 12th week of gestation, TSH, free thyroxine (FT4) and TPO-Ab concentrations were determined in heparinised plasma using electrochemiluminescence assays (Cobas_e 601; Roche Diagnostics). The non-pregnant reference range of TSH is 0·40–4·0 mU/l, of FT4 10·0–24·0 pmol/l and of TPO-Abs <35 IU/l. According to the American Thyroid Association guidelines, the reference ranges of TSH and FT4 during pregnancy were defined in TPO-Ab-negative women at the 12th gestational week using the 2·5th and 97·5th percentiles to define the lower and upper limits of normal thyroid function(Reference Alexander, Pearce and Brent20). The following sub-groups of thyroid function were defined: (i) euthyroid (TSH and FT4 within the reference limit); (ii) overt thyroid dysfunction (TSH and FT4 outside the reference limits); (iii) sub-clinical thyroid dysfunction (TSH outside the reference limit with normal FT4); (iv) hypothyroxinaemia (FT4 <2·5th or <5th percentile with normal TSH) and finally, (v) hyperthyroxinaemia (FT4 >95th, or >97·5th percentile with normal TSH concentration)(Reference Alexander, Pearce and Brent20).
Selenium, zinc and copper
Plasma Se, Zn and Cu concentrations were measured at the 12th gestational week in heparinised plasma samples by inductively coupled plasma MS using a Nexion 300X instrument (Perkin-Elmer) in the kinetic energy discrimination mode. Samples were diluted 30 times in 0·5 % HNO3 and 0·01 % triton in MiliQ AquaDest. Se, Cu and Zn were measured employing helium at a flow rate of 1·0 ml/min as the kinetic energy discrimination gas to remove polyatomic interferences. Within- and between-run variation were assessed by CLSI guideline EP5-A2 and found to be <3 % for low and high concentrations of Zn and Cu in plasma and <5 % for Se. According to CLSI guideline EP 17-A2, the lower limit of quantitation was calculated at 0·15, 0·30 and 0·27 µmol/l for Se, Zn and Cu, respectively. Certified reference materials Seronorm L1 or L2 (Nycomed) were used to monitor accuracy. During measurement of the samples of this study, for L1 (lot 1309438), values for Se, Zn and Cu were 0·99 µmol/l (reported analytical value 1·10 µmol/l with a range 0·96–1·25 µmol/l), 17·4 µmol/l (reported analytical value 16·8 µmol/l and range 14·6–19·0 µmol/l) and 17·9 µmol/l (reported analytical value 17·1 µmol/l and range 15·7–18·5 µmol/l), respectively. For L2 (lot 1309416), values for Se, Zn and Cu were 1·58 µmol/l (reported certified mean 1·75 and 1·52–1·98 µmol/l), 25·7 µmol/l (reported certified mean 24·7 µmol/l range 21·5–28·0 µmol/l) and 29·4 µmol/l (reported certified mean 29·1 µmol/l and range 26·7–31·5 µmol/l), respectively. Moreover, pooled sera were measured as well in order to monitor within-run variation at low and high concentrations.
Supplement intake
At 12 weeks’ gestation, supplement intake was carefully investigated. Women were asked whether they used any supplement on a daily basis. In the Netherlands, most supplement tablets including Se/Zn/Cu belong to 2–3 widely used products in which the daily dose is carefully reported (as requested by law). These different tablets were all recorded, including the dose of the trace elements.
Statistics
The most recent guidelines of the Clinical Laboratory Standard Institute (vol. 28 no. 30, 2012) for defining reference ranges in the clinical laboratory advise that an adequate sample size for reliable numbers should at least contain 120 samples(21). The current sample size of 2041 amply meets this criterion.
Statistical analysis was performed using the IBM SPSS Statistics for Windows version 25.0 (IBM Corp.). The skewness and kurtosis of Se data were 0·62 and 1·59, those of Zn 0·51 and 0·71 and those of Cu 0·35 and 0·31, respectively; therefore, parametric testing was used to compare means between subgroups. Descriptive statistics (t test: T (df) = value, P), χ 2 (χ 2 (df) = value, P and ANOVA: F (df) = value, P) were used to analyse the prevalence of abnormal thyroid dysfunction in relation to plasma mineral (Se, Zn and Cu) concentrations. We then compared plasma mineral (Se, Zn and Cu) concentrations with log-transformed FT4 and TSH values adjusted for confounders, using linear regression. Finally, within the TPO-Ab+ group, we compared plasma mineral (Se, Zn and Cu) concentrations and the relationship between Se/Zn/Cu supplement intake and TPO-Ab status, using logistic regression analysis.
Results
Table 1 shows the characteristics of 2041 women with thyroid parameters at 12 weeks’ gestation.
Table 1. Characteristics of 2041 women with thyroid-hormone parameters assessed at 12 weeks’ gestation
(Numbers and percentages; mean values and standard deviations; median values and ranges)

Low, primary education or secondary pre-vocational education; medium, secondary education or vocational education; high, Bachelor or Master’s degree; TSH, thyrotropin; FT4, free thyroxine; TPO-Ab, thyroid peroxidase antibodies.
Almost all women were Caucasian, the majority had a partner and two-thirds were highly educated. Up to 9 % had an elevated titre of TPO-Ab (>35 IU/l) and 10 % reported a history of thyroid dysfunction in the parents. In the entire group, 25 % reported a previous miscarriage; this was 18·6 % in the 1014 primiparous women and 32·7 % in the 1027 multiparous women (χ2 (1) = 52, P < 0·001).
In clinical chemistry, the commonly used cut-offs to determine reference ranges are the 2·5th–97·5th percentiles, which in this cohort of 2041 women, for the plasma mineral (Se, Zn and Cu) were: 0·75–1·31, 17·59–36·37 and 9·42–16·44 µmol/l, respectively. However, in the group of 544 women not taking Se/Zn/Cu supplements, these reference ranges were: 0·72–1·25, 9·57–16·41 and 17·15–35·98 µmol/l, respectively. In many population-based studies of trace minerals, the 5th–95th percentiles are reported. In the present study of the total sample of 2041 women, for plasma mineral (Se, Zn and Cu), the 5th–95th percentiles were 0·79–1·25, 9·97–15·6 and 18·8–34·3 µmol/l, respectively, and in those not taking these supplements, they were: 0·76–1·17, 10·0–15·4 and 18·4–34·2 µmol/l, respectively. In Table 2, different subgroups are shown with the corresponding plasma mineral (Se, Zn and Cu) concentrations according to supplement intake.
Table 2. Plasma concentration of selenium, zinc and copper (μmol/l) in healthy pregnant women, categorised by supplement use during early gestation*
(Numbers and percentages; mean values and standard deviations; median values and ranges)
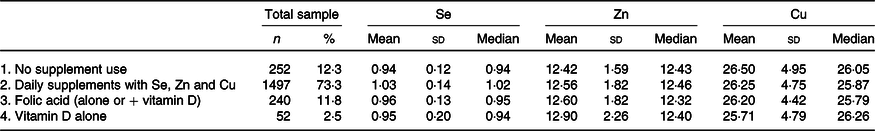
* On daily base intake of Se: 27·5–55 μg; of Zn: 5–10 mg and of Cu: 1 mg.
The women not taking supplements containing Se (groups 1, 3, 4) showed significantly lower mean plasma Se concentrations than those who did (group 2): ANOVA (2037) F = 34·7, P < 0·001, while the mean plasma Cu and Zn concentrations were almost identical in these sub-groups. Based on these figures, we defined two groups: those taking supplements with Se/Zn/Cu (group 2, n 1479, 73·3 %) and those who did not (groups 1 + 3 + 4: n 544, 26·7 %). Women not taking Se/Zn/Cu supplements had significantly lower mean Se concentration (0·96 (sd 0·14) µmol/l) than those with supplement intake (1·03 (sd 0·14) µmol/l), T (2039): 9·9, P < 0·001), while mean concentrations of Zn (12·55 v 12·57 µmol/l) and Cu (26·25 v 26·29 µmol/l) were almost identical in both groups. There were no significant differences in demographics, parity, educational level, smoking habits, alcohol intake, age and BMI between these two groups.
Based on the guidelines of the American Thyroid Association(Reference Alexander, Pearce and Brent20), the different sub-groups of women with thyroid dysfunction are shown with their TPO-Ab status and corresponding mean and standard deviation Se/Zn/Cu values for those sub-groups, regardless of TPO-Ab status (Table 3).
Table 3. Different sub-groups of first-trimester thyroid (dys)function in 2041 pregnant women and their concentrations of plasma mineral selenium, zinc and copper
(Numbers and percentages; mean values and standard deviations)
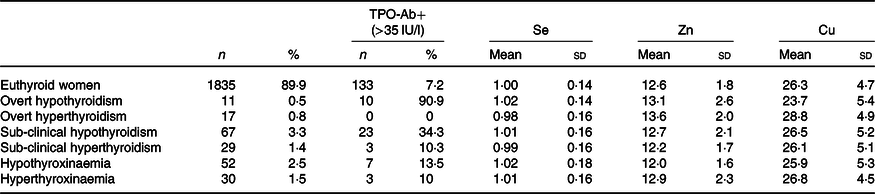
TPO-Ab, thyroid peroxidase antibodies; TSH, thyrotropin; FT4, free thyroxine.
* Reference range for TSH: 0·23–4·0 IU/l; for FT4: 11·5–18 pmol/l, 2·5th and 97·5th percentile in TPO-Ab (<35 IU/l)-negative women. Euthyroid: normal TSH and FT4. Overt thyroid dysfunction: TSH and FT4 outside reference range. Sub-clinical thyroid dysfunction: normal FT4 with TSH outside reference range. Hypothyroxinaemia: FT4 < 2·5th percentile, normal TSH; hyperthyroxinaemia: FT4 > 97·5th percentile, normal TSH.
With regard to plasma Se concentration, using the euthyroid group as the reference group, there was no significant difference between the different sub-groups and the reference group (t test). With regard to plasma Zn concentration, the lowest mean concentration, that is, 11·97 (sd 1·58) µmol/l, was found in hypothyroxinaemic women, which was significantly different from that of the euthyroid group (T (1885) = 2·3, P = 0·019). In the present study, the highest plasma Zn concentrations were found in women with clinical (overt) thyroid dysfunction (hypothyroidism n 11, and hyperthyroidism n 17). Taking these women together (n 28), women with overt thyroid dysfunction had significantly higher plasma Zn concentrations than the euthyroid group (T (1861) = 2·2, P = 0·026). Comparing the women with normal TSH but FT4 at the upper and lower ends of the spectrum, women with hypothyroxinaemia (n 52) had significantly lower plasma Zn concentrations than those with hyperthyroxinaemia (n 32, T (80) = 2·3, P = 0·023). With regard to plasma Cu, women with clinical hypothyroidism had the lowest mean concentrations and those with clinical hyperthyroidism the highest, and these were significantly different: T (26) = 2·6, P = 0·016. Also, women with clinical hyperthyroidism had significantly higher plasma Cu concentrations than the euthyroid group: T (1850) = 2·1, P = 0·027. There were no differences in the occurrence of thyroid dysfunction sub-groups between the groups with and without Se/Zn/Cu supplement intake.
Table 4A shows several linear regression analyses with logFT4 and logTSH as dependent variables and plasma Se/Zn/Cu concentrations separately as dependent variables, adjusted for parity, BMI, smoking, family history of thyroid dysfunction and age. We also adjusted for intake of Se/Zn/Cu supplements.
Table 4. Linear regression with log free thyroxine (logFT4) and log thyrotropin (logTSH) as dependent variables, adjusted for intake of selenium, zinc and copper supplements, parity, BMI, smoking, age and family history of thyroid dysfunction
(Standardised β values and P values; n 2041)
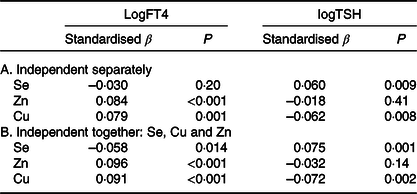
Plasma Se was not related to logFT4 but significantly, independently and positively related to logTSH; higher plasma Se concentration was related to higher TSH. Plasma Zn and Cu concentrations were both positively and independently related to logFT4; higher plasma Zn and Cu concentrations were associated with higher FT4. Plasma Cu (but not Zn) concentration was negatively associated with logTSH. Because of a possible interaction between the trace elements, we subsequently entered Se, Zn and Cu together into the regression (Table 4B) adjusting for the same covariates. Plasma Se (negatively), Zn and Cu (both positively) concentrations were all related to logFT4; plasma Se and Cu (but not Zn) concentrations were also related to logTSH. The Cu/Zn plasma concentration ratio was not related to logFT4 nor to logTSH (data not shown).
We finally looked at the TPO-Ab status. Mean plasma mineral Se/Zn/Cu concentrations did not differ between TPO-Ab+ and TPO-Ab− women. However, the number of women with elevated TPO-Ab titre at 12 weeks’ gestation in those not taking Se/Zn/Cu supplements was 61 (11·2 %) compared with 118 (7·9 %) in those taking these supplements (χ 2 (1) = 5·5, P = 0·019). Women taking additional Se/Zn/Cu supplements were 1·46 times less likely (95 % CI 1·09, 2·04) to have an elevated titre of TPO-Ab at 12 weeks’ gestation.
Discussion
The present study reports reference ranges (2·5th–97·5th percentiles), means and median concentrations of plasma mineral (Se, Zn and Cu) in an appropriately large sample of healthy pregnant women not taking supplements of these trace elements. It also shows that intake of Se/Zn/Cu supplements significantly affects plasma Se concentration but not plasma Zn and Cu concentrations. Moreover, it shows that, at 12 weeks’ gestation, different sub-groups of women with thyroid dysfunction showed different concentrations of plasma Zn and Cu, though not Se concentration, than did euthyroid women. Plasma Zn concentration was significantly lower in women with hypothyroxinaemia than in euthyroid women. Women with overt hyperthyroidism showed the highest plasma Cu concentrations. After adjustment for covariates (including the intake of Se/Zn/Cu supplements), linear regression showed that plasma Cu and Zn concentrations separately and in combination were positively related to FT4. Plasma Cu concentration was inversely related to TSH, while plasma Se concentration was positively correlated with TSH. TPO-Ab status at 12 weeks’ gestation was not related to plasma mineral Se/Zn/Cu concentrations. However, women taking Se/Zn/Cu supplements were less likely to have elevated titres of TPO-Ab at 12 weeks’ gestation.
The reference range of plasma Se concentration in the present study of the total sample (0·75–1·31 µmol/l) is similar to that in a previous study in the same area in a sample of 1000 women, although assessed in serum: 0·77–1·32 µmol/l(Reference Rayman, Wijnen and Vader22). However, in the sub-group without trace element intake in the present study, the reference range was lower (0·72–1·25 µmol/l) than in the previous study, which was performed in 2003–2004. At that time, supplement intake was not carefully assessed, so there are no comparable data available. The median of 0·94 µmol/l plasma Se in the sub-group of the present study not taking these trace elements is low, but the Netherlands (as is Europe in general) is known as a (mildly) Se-deficient area(Reference Stoffaneller and Morse23). Another possibility is that during the first trimester, there is an increased turnover of Se due to immune tolerance processes or increased thyroid hormone production. In the group of women with Se supplement intake, the median was much higher (1·02 µmol/l) suggesting that a daily dose of 27·5–55 µg Se is able to increase plasma Se concentrations (there were no differences in baseline characteristics between the two groups). In the USA National Health and Nutrition Examination Survey (NHANES) 2011–2014, the plasma Se reference range in women was 1·83–3·10 µmol/l (144·2–244·6 µg/l) but without correction for possible supplement intake(Reference Jain16). It is obvious that in the present study, the reference ranges are sex- and age-specific, that is, they relate to women of fertile age. In the NHANES study, the Se reference range increased with age and was higher in males(Reference Jain16). A recent study from Australia reported a mean serum Se concentration of 0·73 µmol/l at 15 weeks’ gestation in 894 primiparous women without fertility problems(Reference Grieger, Grzeskowiak and Wilson24). As in the present study, over 90 % were white Caucasian women but the mean age (23·4 (sd 5·0) years) was substantially lower. There were no data on women not taking Se-containing supplements. The study of Alvarez et al. in Spanish pregnant women showed a much higher mean serum Se at first trimester, that is, 1·38 µmol/l, which decreased significantly to 1·08 µmol/l at term(Reference Alvarez, Castanon and Ruata25). However, no intake of supplements was mentioned in that study either.
The reference range for plasma Zn concentration (9·6–16·4 µmol/l) in women not taking Zn supplements is comparable to that in another study in adult non-pregnant females, that is, 10–18 µmol/l(Reference Hennigar, Lieberman and Fuolgoni26). In the USA (NHANES 2011–2014 data), the 2·5th–97·5th percentiles of serum Zn concentration in 659 non-pregnant women between 19 and 30 years of age were 8·5–16·5 µmol/l (55·7–107·9 µg/dl) which is also close to that of the present study(Reference Hennigar, Lieberman and Fuolgoni26). Again, intake of Zn-containing supplements in the present study did not result in higher mean or median plasma Zn concentration than in women not taking Zn. This seems to be in line with the US study where serum Zn concentrations were not related to dietary or supplemental Zn intake(Reference Hennigar, Lieberman and Fuolgoni26). The mean serum Zn concentration in the Australian study was 9·4 (sd 2·2) µmol/l, which is comparable with the value found in our study(Reference Grieger, Grzeskowiak and Wilson24).
Interestingly, the reference range for plasma Cu concentrations of the total group in the present study, 17·6–36·4 µmol/l, was almost the same as that of the sub-group that was not taking Cu supplements that is, 17·2–36·0 µmol/l. The standard dose of Cu in almost all pregnancy supplements in the Netherlands is 1 mg/d. There are several explanations for not finding a change with supplement taking: the Netherlands is Cu sufficient, which explains why further Cu intake will not increase plasma Cu concentrations. But a recent review showed that serum Cu seems to respond to supplementation only at a higher dose(Reference Bost, Houdart and Oberli7). During the first 3 months of pregnancy, the modest dose of the Cu supplements of 1 mg/d does not seem to meet the substantial physiological drop in serum Cu concentration. It should be noted that the plasma Cu concentration reference range in the present study is much higher than in the reference population of adult females (10–24 µmol/l)(Reference Bost, Houdart and Oberli7). In the Australian study mentioned above, the mean serum Cu concentration was 30·3 (sd 5·4) µmol/l which is higher than that in the present study (26·5 µmol/l)(Reference Grieger, Grzeskowiak and Wilson24). In the Chinese study, the reference range of serum Cu concentrations in the first trimester was substantially lower: 9·5–25·8 µmol/l(Reference Zhang, Yuan and Liu9). These comparisons illustrate the differences between countries in reference ranges of several trace elements, which are mostly to be explained by eating habits and probably by ethnic differences.
Several studies demonstrated a decrease in the concentration of serum Zn concentration and, as found in other studies, an increase in the concentration of plasma and serum Cu concentrations with progression of pregnancy(Reference Liu, Yang and Shi27–Reference Khoushabi, Shadan and Miri29). The decrease in serum Zn concentration can be explained by maternal–fetal transfer and disproportionate increase of plasma volume, while the increase in copper binding proteins, that is, ceruloplasmin, the secretion of which is stimulated by maternal oestrogen as well as decreased biliary Cu excretion, might explain the increase in Cu concentration throughout the pregnancy(Reference Zhang, Yuan and Liu9).
Plasma Se concentration was positively (and independently) associated with TSH – the higher the Se, the higher the TSH – while it was not correlated with FT4. This is difficult to explain because Se is important for the synthesis of thyroid hormone(Reference Rayman5). The most likely explanation is that human chorionic gonadotropin (hCG) at 10–12 weeks’ gestation is highly elevated for several reasons, including the transformation of the post-ovulatory ovary into the gravid corpus luteum for the production of progesterone and oestradiol as well as for the augmentation of immune tolerance by promoting regulatory T-cell recruitment(Reference Schumacher30). The glycoprotein, hCG, has an α-subunit displaying homology with TSH; therefore, during early gestation, the ‘classical’ negative feedback loop between TSH and FT4 is partly overwhelmed by hCG(Reference Korevaar, Medici and Visser1,Reference Nwabuobi, Arlier and Schatz31) . This will result in lower TSH and higher FT4 concentrations ‘contaminating’ the definition of hyperthyroidism. Thus, high Se would normally result in appropriate amounts of FT4, downsizing TSH, but during early gestation, the TSH action of hCG might interfere with Se-dependent thyroid hormone synthesis.
In the NHANES 2011–2014 study, serum Cu concentration was also positively associated with TT4 and TT3 in non-pregnant females with no association for either serum Zn or Se (with TSH) concentrations(Reference Jain16). To the best of our knowledge, there are no previous reports suggesting that plasma Zn concentrations in pregnant women with hypothyroxinaemia and overt thyroid dysfunction (hypo- as well as hyperthyroidism) differ from those in euthyroid women. This finding is intriguing but somewhat contrary to what would be expected. A ‘direct’ effect was suggested in a recent review that suggested that Zn had a role in thyroid-hormone metabolism by regulating deiodinase activity and the synthesis of thyroid releasing hormone and TSH(Reference Severo, Morais and de Freitas14); that could explain the association found in the present study between hypothyroxinaemia and relatively low plasma Zn concentrations. A recent meta-analysis showed that patients with autoimmune disorders (including thyroid disorders) predominantly show lower serum and plasma Zn concentrations than in healthy controls(Reference Sanna, Firinu and Zavattari32). In Table 3, women with (sub)clinical hypothyroidism had the highest incidence of elevated TPO-Ab titre, suggesting an autoimmune origin of poor thyroid function. In the NHANES 2011–2014 study, the low serum Zn concentration cut-off for females >10 years of age was between 9·2 and 10·7 µmol/l (60–70 µg/dl), depending on the time of day when the assessment was carried out(Reference Hennigar, Lieberman and Fuolgoni26). In the present study, there were twenty-eight women with plasma Zn concentrations below the cut-off of 9·2 µmol/l. Those women were 3·6 times (95 % CI 1·12, 12·4, P = 0·032) more likely to have subclinical hypothyroidism at 12 weeks’ gestation. It could be hypothesised that low Zn concentrations enhance (autoimmune) destruction of thyroid cells, as is the case in both Hashimoto’s thyroiditis and Graves’ disease(Reference Korevaar, Medici and Visser1,Reference Saeed, Nadeem and Ahmed3) . However, the highest mean plasma Zn concentrations were found in women with overt hyperthyroidism (Table 3) though none of these women showed elevated concentrations of TPO-Ab, suggesting another origin of thyroid dysfunction than auto-immunity. However, a possible relationship between plasma Zn concentrations and hCG (both of which are important immune modulators) remains to be established. This also seems to be the case for Cu: the highest mean plasma Cu concentration was also found in these hyperthyroid women without elevated TPO-Ab titres.
The mean concentration of the plasma mineral Se/Zn/Cu was not related to TPO-Ab status. However, women with Se/Zn/Cu supplement intake were 1·4 times less likely to have elevated TPO-Ab titre at 12 weeks’ gestation. A recent review highlighted the effect of serum/plasma Zn concentrations on the maturation of T cells and the balance between different T-cell subsets(Reference Elmadfa and Meyer33). Similarly, Se and Cu are involved in the stimulation of T-reg cell formation, crucial for pregnancy immune tolerance(Reference Wessels, Maywald and Rink4,Reference Elmadfa and Meyer33) . It might be hypothesised that the intake of these trace elements will benefit pregnancy immune tolerance to the fetal allograft. It is also known that an epi-phenomenon of immune tolerance is the decrease in antibodies (markers of auto-immune syndromes), including TPO-Ab, which might explain the lower prevalence of an elevated titre in women taking these trace elements(Reference Korevaar, Medici and Visser1).
The present study has several strengths and limitations. A major strength is the relatively high number of pregnant women enabling detection of a group of 544 women not taking Se/Zn/Cu supplements and that is sufficiently large to define accurately the 2·5th–97·5th reference ranges. Also the total number of women in the sample enabled us to define first-trimester-specific reference ranges of TSH and FT4, and sub-groups of hypo- and hyperthyroxinaemia with appropriate power.
A major limitation of the study is that pre-pregnancy trace element concentrations were not available; however, previous reports showed that first-trimester serum/plasma Se concentrations accurately reflect pre-pregnancy concentrations(Reference Rayman, Bath and Westaway34). In the Chinese study, serum Cu and Zn concentrations were compared with those of a matched non-pregnant control group(Reference Zhang, Yuan and Liu9). Throughout pregnancy, serum Cu concentrations were significantly higher than in non-pregnant controls but the intake of pregnancy supplements was not assessed. Serum Zn concentrations were significantly lower during mid-gestation than in non-pregnant controls(Reference Zhang, Yuan and Liu9). With increasing gestation, serum Cu concentrations increased but there was no correlation between serum Zn concentrations and gestational age(Reference Zhang, Yuan and Liu9). During pregnancy, there is a substantial expansion of blood volume but predominantly after the first trimester. During the first 10–12 weeks, the average weight gain is about 0·5–2 kg, which will not explain a possible drop of trace element concentrations due to substantial blood volume expansion(Reference Kominiarek and Peaceman35). Also, in the present study, non-fasting assessments of Zn were analysed but, as shown in the NHANES 2011–2014 study, meal consumption results in a decrease in serum Zn concentrations, which is cumulative following repeated meals, whereas overnight and daytime fasting result in increased circulating Zn concentrations(Reference Hennigar, Lieberman and Fuolgoni26). In the Netherlands, there is a nationwide blood assessment at 12 weeks’ gestation. Therefore, standardised tubes are used which do not meet the guidelines of the International Zinc Nutrition Consultative Group to avoid contamination. Another limitation is that ferritin concentrations were not assessed. Low ferritin levels are common during pregnancy, and some research has suggested an opposite effect on Zn and Cu concentrations. However, in a Norwegian study of 2982 pregnant women, low plasma ferritin concentration (<12 μg/l, assessed at 18 weeks’ gestation) was significantly associated with lower plasma Se concentration but not significantly with (higher) plasma Zn and Cu concentrations(Reference Caspersen, Thomsen and Haug10). Also, the cross-sectional design of the present study does not enable us to draw conclusions with regard to the direction of associations that were found. Our sample consisted primarily of white Caucasian women, which prohibits the generalisability of the findings to the 20 % of Dutch pregnant women of non-Caucasian origin. Studies in the USA show significant differences in trace element concentrations by ethnic background(Reference Jain16). Against this background, comparisons of the present study with US and Chinese studies should be interpreted with caution. Another limitation is that iodine intake, the most important determinant of adequate thyroid function, was not assessed. However, the Netherlands, unlike the UK, for example, is known to be an iodine-sufficient country(Reference Pop, Broeren and Wiersinga18,Reference Bath36) . Because both iodine and Se are crucial for adequate thyroid function(Reference Rayman5), it is reasonable to suggest that in iodine-deficient countries, poor iodine status might affect the status of other trace elements such as Se. The subgroups of women with thyroid dysfunction were rather small which means that several (non) existing associations between these sub-groups and plasma Se/Zn/Cu mineral concentrations should be interpreted with caution. A final limitation that must be mentioned is that all studies of plasma/serum mineral Se/Zn/Cu concentrations are subject to the systemic inflammatory response where serum/plasma Se and Zn concentrations fall and Cu concentration rises so that values do not reflect the true trace element status(Reference Oakes, Lyon and Duncan37); even normal pregnancy is an inflammatory state, as evidenced by high cytokine concentrations(Reference Kristensen, Wide-Swensson and Lindstrom38,Reference Buxton, Meraz-Cruz and Sánchez39) .
In conclusion, the first-trimester reference range (2·5th–97·5th percentiles) for plasma Se is largely dependent on supplement intake which is not the case for Cu and Zn reference ranges. Plasma Zn concentrations were particularly related to several categories of thyroid dysfunction. Women taking Se/Zn/Cu supplements were less likely to have an elevated TPO-Ab titre in the first trimester.
Acknowledgements
We thank the pregnant women who participated into the study and the midwives for their important role of recruitment of the pregnant women.
No funding was received.
V. P. was responsible for the design of the study. J. K. was responsible for the analyses of trace elements. V. P. – and the department of statistics of Tilburg University – and J. K. were responsible for data analyses. V. P., J. K., W. M. and M. R. were responsible for the writing of the manuscript.
The authors declare that there are no conflicts of interest.









